Abstract
Neuroendocrine carcinomas (NEC) of the pancreas are defined by a mitotic count > 20 mitoses/10 high power fields and/or Ki67 index > 20%, and included all the tumors previously classified as poorly differentiated endocrine carcinomas. These latter are aggressive malignancies with a high propensity for distant metastases and poor prognosis, and they can be further divided into small- and large-cell subtypes. However in the NEC category are included also neuroendocrine tumors with a well differentiated morphology but ki67 index > 20%. This category is associated with better prognosis and does not significantly respond to cisplatin-based chemotherapy, which represents the gold standard therapeutic approach for poorly differentiated NEC. In this review, the differences between well differentiated and poorly differentiated NEC are discussed considering both pathology, imaging features, treatment and prognostic implications. Diagnostic and therapeutic flowcharts are proposed. The need for a revision of current classification system is stressed being well differentiated NEC a more indolent disease compared to poorly differentiated tumors.
Keywords: Pancreatic neuroendocrine tumors, Surgery, Neuroendocrine carcinomas, Chemotherapy, Prognosis, Metastases, Morphology, Proliferation
Core tip: In this study, we reviewed the available literature for neuroendocrine carcinomas of the pancreas with a special focus on the differences between morphological poorly-differentiated and well-differentiated tumors. Although the quality of current evidence is suboptimal because of the retrospective design of the available studies, morphological well-differentiated tumors are associated with lower ki67 proliferative index, are less responsive to standard platinum-based chemotherapy and are associated with improved survival. The current category of neuroendocrine carcinomas should be revised taking into account these differences and new diagnostic criteria should be considered in order to clearly define poorly- and well-differentiated tumors.
INTRODUCTION
The 2010 World Health Organization (WHO) classification of pancreatic neuroendocrine tumors (PNEN) introduced a major change compared with the previous ones. In fact the 2010 WHO system identified three categories of PNEN based on mitotic count and ki67 index[1,2]. The category of neuroendocrine carcinoma (NEC) was defined by a mitotic count > 20 mitoses/10 high power fields (HPF) and/or Ki67 index > 20% , and included all the tumors previously classified as poorly differentiated endocrine carcinomas (PDECs) in the 2000 and 2004 WHO classification[1-3]. Pancreatic PDECs are clinically aggressive tumors characterized by poorly differentiation with features suggesting endocrine differentiation, a high-proliferative rate and frequently abundant necrosis with prominent angioinvasion[4-6]. The category of PDECs include two different entities, namely small cell and large cell endocrine carcinomas. Historically, poorly differentiated NECs have been considered as nearly equivalent to small cell lung cancer given the histologic similarities observed between the two diseases[7-10]. As a consequence, some of the treatment recommendations for pancreatic PDEC are based on the small cell lung cancer literature and there are scant clinical data regarding to pancreatic PDEC[4-6].
Moreover, based on the WHO 2010 criteria, a morphological well-differentiated tumor showing > 20 mitoses/10 HPF or Ki67 index > 20%, is classified as NEC. Therefore, the WHO 2010 NEC category likely comprise all PD-NEC (WHO 2000) but also tumors morphologically classified as well-differentiated PNETs (WHO 2000) but with G3 features[3]. This overlap between morphologically well- and poorly-differentiated tumors has strong clinical and therapeutic implications, since their biological behavior may significantly differ.
In the present paper we review the current knowledge on pancreatic NEC (PNEC) analyzing their clinical and pathological characteristics, treatment and prognosis, and evaluating potential pitfalls in their current classification.
EPIDEMIOLOGY AND CLINICAL FEATURES
Pancreatic NEC is rare tumors, accounting for about 5% of all PNENs. They usually arise in adults in the VIth decade of life with a male predominance[11]. Some patients have associated paraneoplastic syndromes such as Cushing’s syndrome, hypercalcemia and carcinoid syndrome. Unlike patients with well-differentiated NETG1/NETG2 who typically present with a relatively indolent disease process, most patients with NEC present with symptoms similar to ductal adenocarcinoma, including back pain, cachexia, weight loss and jaundice[5].
A specific association between NEC and genetic syndromes such as MEN1 syndrome has not been established.
IMAGING AND STAGING
More than 70% of patients with pancreatic NEC present with metastatic disease or with locally-advanced tumors, and only 20% to 30% of patients are amenable of surgical resection[3-5]. Appropriate diagnosis and staging is of paramount importance in order to establish the subsequent treatment. Pancreatic NEC, especially if morphologically PD, may metastasize virtually to every organ of the body, although the most common site of metastases is the liver. For this reason, the whole body should be studied with imaging techniques to rule out distant metastases.
Patients with a pancreatic solid mass should undergo high-resolution imaging techniques including multidetector computed tomography (MDCT) or magnetic resonance imaging (MRI)[12-17]. Current guidelines suggest that total-body contrast-medium MDCT should be the preferred imaging modality[12]. MDCT can give information regarding the local spread of the tumor, the presence of peripancreatic or distant lymphadenopathy, and of distant metastases. For the purpose of local staging, it is important to assess the size of the tumor and localization within the pancreas, its relationship to the MPD and CBD, the major peripancreatic vessels (celiac trunk and its branches, superior mesenteric and splenic artery and vein, portal vein) and other adjacent structures. Pancreatic NET usually present as hyper vascularized lesions. This can be also the presentation of NEC, basically when there is a morphologically WD NEC with a relatively low ki67 (< 50%). On the other hand, morphologically PD NEC with higher proliferative index (ki67 > 50%) may present as a hypo vascularized mass frequently associated with the presence of necrosis (Figure 1)[14-17]. This latter radiological presentation may resemble that of pancreatic ductal adenocarcinoma.
Figure 1.
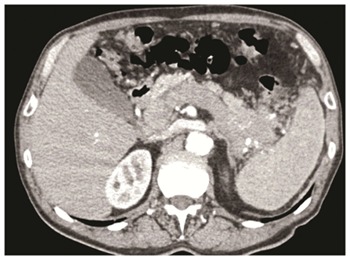
Pancreatic poorly differentiated neuroendocrine carcinomas of the pancreatic body-tail associated with neoplastic thrombosis of the splenic vein/portal vein, and with a lymphadenopathy along the stomach. The patients underwent left pancreatectomy with splenectomy, portal vein resection with portal vein thrombectomy and partial gastric resection.
Nuclear medicine imaging is generally helpful in the imaging work-up of PNENs by using a PET camera. For PNEN imaging, two types of radiotracers are principally used: those related to receptor expression and those reflecting tumor metabolism[13,18,19]. The first category includes somatostatin analogues (SSAs) labeled with the positron emitter 68Ga and the most often used preparations are 68Ga-DOTATOC, 68Ga-DOTANOC and 68Ga-DOTATATE besides somatostatin receptor scintigraphy imaging (Octreoscan®)[18-20]. The use of 68Ga positron emission tomography (PET) or of Octreoscan® is helpful in order to confirm the endocrine nature of the lesion and PET is a useful tool complementary to CT for staging of regional and distant metastases[18-21]. Of note, when a NEC is suspected, it is of paramount importance to perform both a 68Ga PET and 18FDG-PET. In fact 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) is preferred for tumor detection and staging as the somatostatin receptor (SSR) expression of these tumors is generally low or missing[22-24]. Additionally, it has been demonstrated that a significant correlation exists between FDG-PET positivity and both Ki-67 and World Health Organization tumor grade. Particularly when the Ki-67 is greater than 15%, the sensitivity of FDG-PET is greater than 92%[22]. As such, these imaging modalities may be useful in distinguishing low- vs high-grade tumors. As a consequence, most morphologically WD NEC usually show a positivity for both 68Ga PET and 18FDG-PET, while in morphologically PD NEC with high ki67 index, there is almost exclusively a positivity for 18FDG-PET[22-24]. Although PET imaging can be of help in differentiating poorly- and well-differentiated NEC, there are still many situations of uncertainty or of mild positivity of both 68Ga PET and 18FDG-PET; therefore all data from PET imaging should be always carefully integrated with clinical and pathological features.
Tissue biopsy is critical for a number of reasons[5]. First, tissue biopsy confirms that the tumor is of neuroendocrine origin. Second, it provides further data regarding: (1) morphologic differentiation (i.e, WD NEC vs PD NEC, small cells vs large cells PDEC); and (2) ki67 index evaluation. In order to provide these data, it is important to perform a fine-needle ago-biopsy (FNAB) rather than a FNA. These procedures can be carried out with endoscopic ultrasound of the primary pancreatic tumor, but FNAB may be performed on metastases as well. When several metastases are present, 18FDG positivity may be of help in order to select for biopsy those lesions with a higher metabolic activity, that are associated with a higher ki67 index.
PATHOLOGY AND PROGNOSTIC CORRELATION
As previously mentioned, NEC (WHO G3) is currently defined by a mitotic count > 20 mitoses/10 HPF and/or Ki67 index > 20[2,3]. However, these tumors may be reported with a different terminology, including poorly differentiated carcinomas, high-grade neuroendocrine tumors, G3 neuroendocrine tumors, G3 NET, and well-differentiated neuroendocrine tumors with a high proliferative rate. Historically these tumors have been defined as PDEC, but with the 2010 WHO classification, the NEC category has become morphologically and biologically heterogeneous[2-5]. In fact, at present, in the NEC category we may include:
Morphological PD NEC: these tumors were the previously classified PDEC[1-3]. Morphological PD NEC are characterized by a high ki67 index, usually more than 50%-60%. They represent a group of very aggressive malignancies which show morphological and clinical features similar to those of the more frequent pulmonary PD NEC[1-6]. Similarly to the lungs, they have traditionally been divided into the small cell (Figure 2A and B) and large cell (Figure 3A and B) subtypes, based on the morphological features of the neoplastic cells[1,7]; various combinations of both small and large cells can be observed, and the term of “mixed type” has been proposed for this category.
Figure 2.
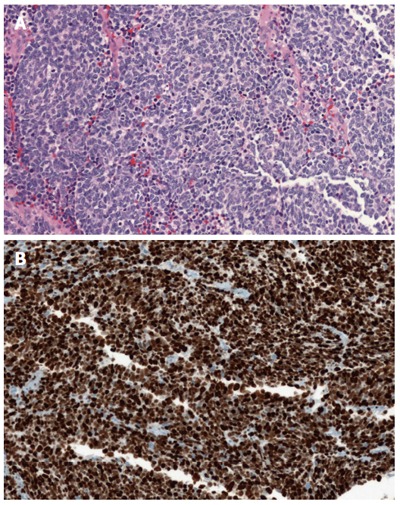
A small cell poorly differentiated neuroendocrine carcinoma of the pancreas (A) (haematoxylin-eosin stain) and a small cell poorly differentiated neuroendocrine carcinoma of the pancreas with high ki67 proliferative index (B, Ki67: 90%).
Figure 3.
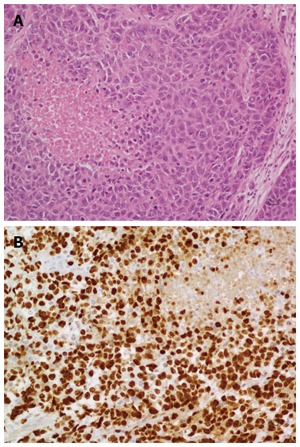
Shows a large cell poorly differentiated neuroendocrine carcinoma of the pancreas (A, haematoxylin-eosin stain) and a large cell poorly differentiated neuroendocrine carcinoma of the pancreas with its Ki67 proliferative index (B, Ki67: 80%).
Patient with morphologically PD NEC have a clinical behavior similar to that of small cell carcinoma or large cell neuroendocrine carcinoma of the lung, which is far worse than that of well-differentiated NETs. In the two largest series of pancreatic poorly differentiated NEC, the vast majority of patients had lymph node or distant metastases at presentation[8]. Basturk et al[8] reported a median survival of 11 mo (range 0 to 104 mo) with a five-year survival of 16% in a cohort of 44 patients. Crippa et al[25] reported a similar survival in a cohort of 49 patients with PD NEC (median DSS: 12 mo). Of note patients with metastatic PD NEC succumb without treatment within weeks after diagnosis, and even with systemic chemotherapy the prognosis still remain severe with an expected survival of less than 6 mo[8,9,25].
Morphological WD NEC: these tumors are well-differentiated NETs by a morphological point of view but they have a mitotic count > 20 mitoses/10 HPF and/or Ki67 index > 20% (Figure 4A and B). Of note, in this category there is also a small subset of patients with morphological well differentiated NET with less than 20 mitoses/10 HPF (G2 by mitotic count), but are associated with Ki-67 > 20%. Recently Basturk et al[26] demonstrated that the clinical behavior of these grade-discordant NET was worse than grade-concordant G2 tumors (median survival 68 mo vs 54 mo), although the difference was not statistically significant.
Figure 4.
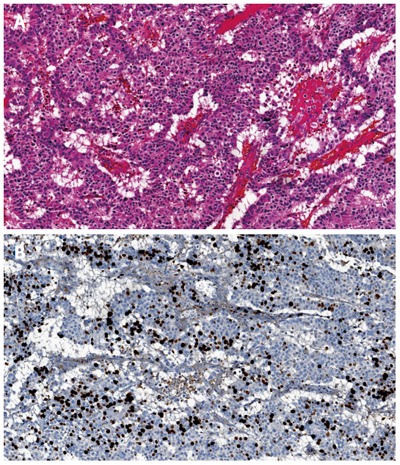
Morphological well differentiated neuroendocrine carcinoma of the pancreas (A, haematoxylin-eosin stain) and morphological well differentiated neuroendocrine carcinoma of the pancreas with Ki67 proliferative index of 30% (B).
Several studies have challenged the assumption that poorly differentiated histology and high tumor grade are equivalent[5,27-29]. In fact when we consider morphological WD NEC, these tumors are associated with a markedly improved survival compared to morphological PD NEC. In a recent publication from our group, we found that patients with WD NEC had a significantly longer survival compared to those with PD NEC (43 mo vs 12 mo, P = 0.004)[25]. Similar results were also reported by other Authors. Vélayoudom-Céphise et al[28] reported a median survival of 41 vs for WD NEC compared to only 17 mo for PD NEC. Basturk et al[26] found a significantly improved survival for 19 patients with WD NEC compared to 43 PD NEC (median survival 54 mo vs 11 mo). Tang and colleagues showed a median disease-specific survival of 55 mo for WD NEC and of 16 mo for PD NEC.
The presence of low-/intermediate-grade and high-grade regions within the same NET is largely interpreted as well-differentiated NETs with progression to a more proliferative state (WD NEC)[30]. Some tumors show features of well-differentiated NETs in some regions, including a low proliferative rate, but other regions or metastatic foci show a much higher proliferative rate along with more atypical cytological features.
Unfortunately definitive histological diagnostic criteria are not clearly defined and accepted. Therefore further and larger studies are needed in order to better define and clarify histological diagnostic criteria and classification of both PD NEC and WD NEC.
MOLECULAR ALTERATIONS
Genomic investigations have found recurrent and mutually exclusive DAXX and ATRX mutations, which culminate in loss of corresponding protein expression in tumor cells, in approximately 44% of pancreatic WD-NETs[31]. This genotype is specific for WD-NET and has not been seen in other pancreatic neoplasms, including PD-NECs[7,30].
In contrast, pancreatic PD-NECs share some of the genotypic alterations of conventional pancreatic ductal adenocarcinoma including frequent gene mutations in TP53 and, less commonly KRAS, p16, and SMAD4, but these alterations were not found in pancreatic WD-NETs in several studies[7,30].
Moreover, RB1 gene mutations and the associated loss of Rb protein expression are commonly observed in high-grade PD-NECs. Specifically, this mutation is found in more than 90% of small cell PD NEC while large cell subtype exhibit RB1 mutation in 50% to 60% of cases[32,33]. On the contrary, RB1 and TP53 mutations have not been identified in WD-NETs[7,30].
DIAGNOSTIC FLOWCHART
Figure 5 shows a diagnostic flowchart. In the suspect of a NEC or when there is a cytological diagnosis of NET with high-grade features, it is of paramount importance to perform a FNAB in order to collect tissue for a proper histological evaluation. The first evaluation should be a morphological one with the aim of classifying NEC in PD NEC or WD NEC. As previously discussed, the performance of a combined 68GaPET and of 18FDG PET may be of help in order to make a distinction between these two entities, although PET cannot fully discriminate between the two forms and data from PET imaging should be carefully integrated with other clinico-pathologic data. When morphological evaluation is uncertain or ambiguous, immunohistochemical studies should be considered. The loss of DAXX and ATRX are diagnostic for a WD NEC while the loss of Rb or an abnormal expression of p53 suggest the diagnosis of PD NEC.
Figure 5.
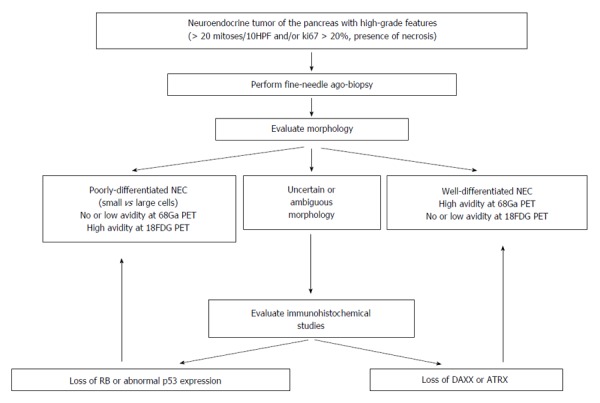
Diagnostic flowchart algorithm in patients with pancreatic neuroendocrine carcinomas.
MANAGEMENT OF POORLY DIFFERENTIATED NEUROENDOCRINE CARCINOMA
Figure 6 shows the potential therapeutic strategies for patients with PD NEC. After an accurate disease-staging, PD NEC can be classified in resectable, locally-advanced or metastatic tumors. Two recent studies have demonstrated that surgical resection of primary pancreatic tumor in resectable PD NEC is an independent predictor of survival[25,34]. However, surgery alone is rarely curative, and the vast majority of patients with PD NEC undergoing resection will develop recurrence, being most recurrences distant and not local. For these reasons, adjuvant chemotherapy after curative resection of PD NEC should be considered, although no prospective studies are available to support this practice[5]. Recent North American NeuroEndocrine Tumor Society guidelines recommend adjuvant therapy with 4 to 6 cycles of cisplatin or carboplatin plus etoposide[6].
Figure 6.
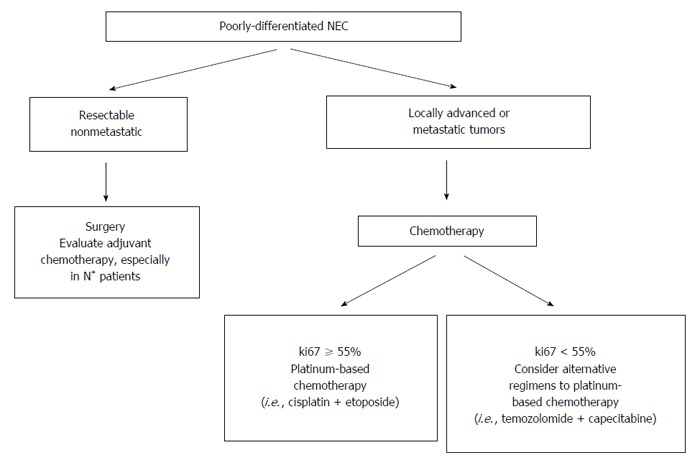
Different therapeutic options in patients with morphological poorly differentiated neuroendocrine carcinomas of the pancreas. 18FDG-PET: 18F-fluorodeoxyglucose positron emission tomography.
Recently the Nordic Neuroendocrine Tumor Group published the results of surgical treatments of patients with pancreatic NEC[34]. They found, in a limited number of patients (n = 14) with localized non-metastatic disease, that surgical resection followed by adjuvant chemotherapy was associated with improved survival. However the median disease-free survival in this group was only 7 mo. Of note 13 out 14 patients developed early metastatic disease after resection, and this may be related to the presence of occult metastatic disease at diagnosis. In view of these results these Authors suggested that neoadjuvant chemotherapy may be also considered, but nowadays there are no evidence to support neoadjuvant chemotherapy in all patients with resectable PD NEC[5,34].
Patients with locally-advanced or metastatic PD NEC should undergo chemotherapy[6]. The role of surgery in the setting of resectable pancreatic PD NEC with metastases limited to the liver is debated. Resection of primary NET in the presence of unresectable hepatic metastases is controversial, and most data come from retrospective and heterogeneous cohorts including mainly NET G1/G2[35,36]. Some of these studies suggest possible benefit of primary tumor resection, but a bias toward more aggressive surgical approach in patients with better performance status or less advanced disease seems likely[37,38]. For all these reasons, palliative resection of the primary pancreatic NEC in the setting of unresectable liver metastases is not recommended[6,35]. Surgical metastasectomy is not recommended as well in the management of NEC[6,39]. In fact in small series the median survival after partial hepatectomy for metastatic NEC from gastrointestinal tract including pancreas was 6 to 15 mo[40,41]. Recently Partelli and coworkers demonstrated in a multicenter retrospective study that the presence of pancreatic neuroendocrine carcinoma G3 was the only factor independently associated with a poorer survival after resection in a cohort of 91 patients who underwent resection of primary NEN with (n = 18) or without (n = 73) hepatic resection[42].
Ki67 index is important to establish the most appropriate chemotherapy regimen. In fact ki67 threshold of 55% was predictive for response to first-line platinum-based chemotherapy in different studies[4-6,43,44]. Patients with PD NEC with ki67 > 55% had a response rate of 42%-67% to treatment with cisplatin/etoposide, while those with ki67 < 55% were less responsive to platinum-based chemotherapy (response rate: 15%). In these latter cases other agents including temozolomide proved to be more effective[39,43,44]. Expected survival in patients with advanced PD NEC is less than one year, and performance status represents a significant prognostic factor[9,25]. Patients with poor performance status does not receive chemotherapy in most cases but only best supportive care, reaching a median survival time of only 2 mo in such cases.
MANAGEMENT OF WELL DIFFERENTIATED NEUROENDOCRINE CARCINOMA
Figure 7 indicates the management flowchart for patients with WD NEC. In this setting the treatment may be more complex than in patients with PD NEC. In patients with resectable disease surgery with curative intent must be considered. In patients with locally-advanced disease there is a wide range of possible therapies including temozolamide-based or streptozocin-based chemotherapy, peptide receptor radionuclide therapy, somatostatin analogues long-acting release and target therapies (i.e., mTOR inhibitor everolimus)[25,35,39]. Unfortunately little evidence-based data are available to guide therapy, and the decision to perform a treatment rather than another one should be individualized considering morphology, ki67, performance status and primary aim of the treatment (downsizing/staging).
Figure 7.
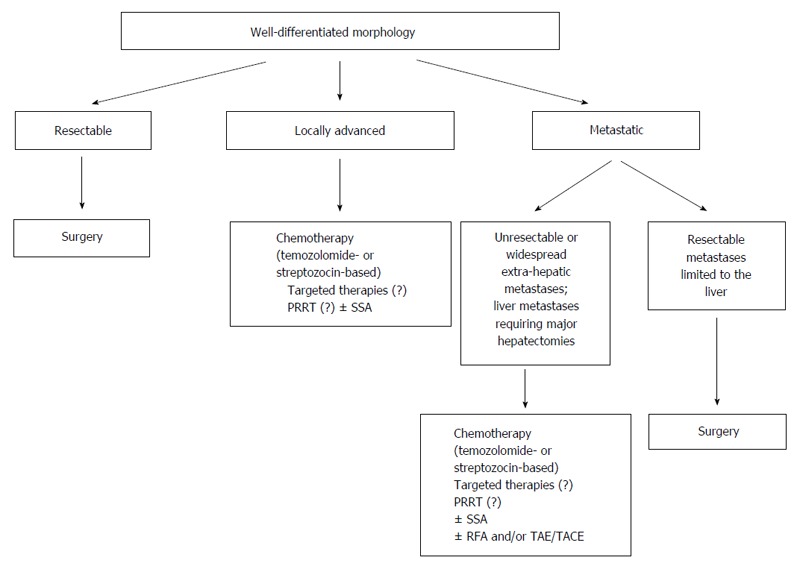
Management flowchart algorithm in patients with morphological well differentiated neuroendocrine carcinomas of the pancreas. PRRT: Peptide receptor radionuclide therapy; TAE: Transarterial embolization; TACE: Transarterial chemoembolization; SSA: Somatostatin analogues.
In patients with resectable primary WD-NEC associated with resectable metastases limited to the liver, surgery can be considered with the aim of obtaining a curative resection, providing that no major hepatectomies are required[35,39]. In patients with widespread metastatic disease and/or unresectable metastases limited to the liver, the benefit of a palliative resection of the primary pancreatic tumor is uncertain. In these patients there is a wide range of therapeutic options, including systemic therapies as well as liver-directed treatments. Again, there is a lack of strong evidence-based information in order to plan the most appropriate treatment or to determine the sequence of treatment to do. However, the less aggressive biological behavior of WD NEC as well as the different therapeutic options available can improve the prognosis of patients with WD NEC even in the metastatic setting.
CONCLUSION
Based on the current data, it is clear that the current WHO high-grade NEC category should be revised. In fact NEC constitute a heterogeneous group of neoplasms including WD NEC and PD NEC. Morphological WD NEC represents a subgroup with markedly improved survival while PD NEC are more aggressive tumors. This difference has significant implications for treatment and prognosis. A new classification of NEC is required considering both morphology and ki67 index. Specific and definite diagnostic criteria for histological diagnosis of PD NEC and WD NEC are also required.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest, no financial support.
Peer-review started: July 25, 2016
First decision: September 28, 2016
Article in press: November 13, 2016
P- Reviewer: Sun Y, Zavras N S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Solcia E, Capella C, Kloppel G, Heitz PU, Sobin LH, Rosai J. Endocrine tumours of the gastrointestinal tract. In: Solcia E, Kloppel G and Sobin LH, editors. Histologic typing of endocrine tumours, WHO international histological classification of tumours. New York: Springer Verlag; 2000. pp. 57–67. [Google Scholar]
- 2.Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In Bosman FT, Carneiro F, Hruban RH and Theise ND. WHO Classification of Tumors of the Digestive System. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 3.Crippa S, Partelli S, Boninsegna L, Falconi M. Implications of the new histological classification (WHO 2010) for pancreatic neuroendocrine neoplasms. Ann Oncol. 2012;23:1928. doi: 10.1093/annonc/mds166. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186–194. doi: 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- 5.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814–2823. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 6.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 10.Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730–2739. doi: 10.1200/JCO.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 11.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2016;469:288–293. doi: 10.1016/j.bbrc.2015.11.102. [DOI] [PubMed] [Google Scholar]
- 12.Sundin A, Vullierme MP, Kaltsas G, Plöckinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90:167–183. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 13.van Essen M, Sundin A, Krenning EP, Kwekkeboom DJ. Neuroendocrine tumours: the role of imaging for diagnosis and therapy. Nat Rev Endocrinol. 2014;10:102–114. doi: 10.1038/nrendo.2013.246. [DOI] [PubMed] [Google Scholar]
- 14.d’Assignies G, Couvelard A, Bahrami S, Vullierme MP, Hammel P, Hentic O, Sauvanet A, Bedossa P, Ruszniewski P, Vilgrain V. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 15.Jang KM, Kim SH, Lee SJ, Choi D. The value of gadoxetic acid-enhanced and diffusion-weighted MRI for prediction of grading of pancreatic neuroendocrine tumors. Acta Radiol. 2014;55:140–148. doi: 10.1177/0284185113494982. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen ZE, Yaghmai V, Nikolaidis P, McCarthy RJ, Merrick L, Miller FH. Diffusion-weighted MR imaging in pancreatic endocrine tumors correlated with histopathologic characteristics. J Magn Reson Imaging. 2011;33:1071–1079. doi: 10.1002/jmri.22541. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Lee JM, Park JH, Kim SC, Joo I, Han JK, Choi BI. Solid pancreatic lesions: characterization by using timing bolus dynamic contrast-enhanced MR imaging assessment--a preliminary study. Radiology. 2013;266:185–196. doi: 10.1148/radiol.12120111. [DOI] [PubMed] [Google Scholar]
- 18.Rufini V, Baum RP, Castaldi P, Treglia G, De Gaetano AM, Carreras C, Kaemmerer D, Hommann M, Hörsch D, Bonomo L, et al. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom Imaging. 2012;37:1004–1020. doi: 10.1007/s00261-012-9871-9. [DOI] [PubMed] [Google Scholar]
- 19.Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A, Vitek P, Sundin A, Plöckinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology. 2009;90:184–189. doi: 10.1159/000225946. [DOI] [PubMed] [Google Scholar]
- 20.Shi W, Johnston CF, Buchanan KD, Ferguson WR, Laird JD, Crothers JG, McIlrath EM. Localization of neuroendocrine tumours with [111In] DTPA-octreotide scintigraphy (Octreoscan): a comparative study with CT and MR imaging. QJM. 1998;91:295–301. doi: 10.1093/qjmed/91.4.295. [DOI] [PubMed] [Google Scholar]
- 21.Schmid-Tannwald C, Schmid-Tannwald CM, Morelli JN, Neumann R, Haug AR, Jansen N, Nikolaou K, Schramm N, Reiser MF, Rist C. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur J Nucl Med Mol Imaging. 2013;40:897–907. doi: 10.1007/s00259-013-2371-5. [DOI] [PubMed] [Google Scholar]
- 22.Binderup T, Knigge U, Loft A, Mortensen J, Pfeifer A, Federspiel B, Hansen CP, Højgaard L, Kjaer A. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51:704–712. doi: 10.2967/jnumed.109.069765. [DOI] [PubMed] [Google Scholar]
- 23.Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, Boucher E, Raoul JL. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858–864. doi: 10.2967/jnumed.108.057505. [DOI] [PubMed] [Google Scholar]
- 24.Partelli S, Rinzivillo M, Maurizi A, Panzuto F, Salgarello M, Polenta V, Delle Fave G, Falconi M. The role of combined Ga-DOTANOC and (18)FDG PET/CT in the management of patients with pancreatic neuroendocrine tumors. Neuroendocrinology. 2014;100:293–299. doi: 10.1159/000368609. [DOI] [PubMed] [Google Scholar]
- 25.Crippa S, Partelli S, Bassi C, Berardi R, Capelli P, Scarpa A, Zamboni G, Falconi M. Long-term outcomes and prognostic factors in neuroendocrine carcinomas of the pancreas: Morphology matters. Surgery. 2016;159:862–871. doi: 10.1016/j.surg.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol. 2011;29:2372–2377. doi: 10.1200/JCO.2010.33.0688. [DOI] [PubMed] [Google Scholar]
- 28.Vélayoudom-Céphise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D, Debaere T, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 29.Jean-Yves S, Anne C, Genevieve M, Emmanuelle L, Genevieve B, Serge G, Pierre D, Marie D, Xavier P, Come L. Well-differentiated grade 3 digestive neuroendocrine tumors: myth or reality? The PRONET Study Group (abstract) J Clin Oncol. 2012;1:30 abstract 4129. Available from: http://meetinglibrary.asco.org/content/100442. [Google Scholar]
- 30.Tang LH, Untch BR, Reidy DL, O’Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011–1017. doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takizawa N, Ohishi Y, Hirahashi M, Takahashi S, Nakamura K, Tanaka M, Oki E, Takayanagi R, Oda Y. Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol. 2015;46:1890–1900. doi: 10.1016/j.humpath.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Li AF, Li AC, Tsay SH, Li WY, Liang WY, Chen JY. Alterations in the p16INK4a/cyclin D1/RB pathway in gastrointestinal tract endocrine tumors. Am J Clin Pathol. 2008;130:535–542. doi: 10.1309/TLLVXK9HVA89CHPE. [DOI] [PubMed] [Google Scholar]
- 34.Haugvik SP, Janson ET, Österlund P, Langer SW, Falk RS, Labori KJ, Vestermark LW, Grønbæk H, Gladhaug IP, Sorbye H. Surgical Treatment as a Principle for Patients with High-Grade Pancreatic Neuroendocrine Carcinoma: A Nordic Multicenter Comparative Study. Ann Surg Oncol. 2016;23:1721–1728. doi: 10.1245/s10434-015-5013-2. [DOI] [PubMed] [Google Scholar]
- 35.Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 36.Capurso G, Bettini R, Rinzivillo M, Boninsegna L, Delle Fave G, Falconi M. Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology. 2011;93:223–229. doi: 10.1159/000324770. [DOI] [PubMed] [Google Scholar]
- 37.Gaujoux S, Gonen M, Tang L, Klimstra D, Brennan MF, D’Angelica M, Dematteo R, Allen PJ, Jarnagin W, Fong Y. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol. 2012;19:4270–4277. doi: 10.1245/s10434-012-2462-8. [DOI] [PubMed] [Google Scholar]
- 38.Sarmiento JM, Que FG, Grant CS, Thompson GB, Farnell MB, Nagorney DM. Concurrent resections of pancreatic islet cell cancers with synchronous hepatic metastases: outcomes of an aggressive approach. Surgery. 2002;132:976–982; discussion 982-983. doi: 10.1067/msy.2002.128615. [DOI] [PubMed] [Google Scholar]
- 39.Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, Krenning E, Knigge U, Salazar R, Pape UF, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103:172–185. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 40.Cho CS, Labow DM, Tang L, Klimstra DS, Loeffler AG, Leverson GE, Fong Y, Jarnagin WR, D’Angelica MI, Weber SM, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer. 2008;113:126–134. doi: 10.1002/cncr.23523. [DOI] [PubMed] [Google Scholar]
- 41.Saxena A, Chua TC, Sarkar A, Chu F, Liauw W, Zhao J, Morris DL. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery. 2011;149:209–220. doi: 10.1016/j.surg.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Partelli S, Inama M, Rinke A, Begum N, Valente R, Fendrich V, Tamburrino D, Keck T, Caplin ME, Bartsch D, et al. Long-Term Outcomes of Surgical Management of Pancreatic Neuroendocrine Tumors with Synchronous Liver Metastases. Neuroendocrinology. 2015;102:68–76. doi: 10.1159/000431379. [DOI] [PubMed] [Google Scholar]
- 43.Mitry E, Baudin E, Ducreux M, Sabourin JC, Rufié P, Aparicio T, Aparicio T, Lasser P, Elias D, Duvillard P, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351–1355. doi: 10.1038/sj.bjc.6690325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]


