Abstract
Given the assumption that all methods of exercise, e.g., endurance (ET), resistance (RT), or combination of both (E+R), can induce a beneficial effect size (ES) for changes in body composition and health status of individuals who are overfat. Thus the aim and purpose of this study is to evaluate the current body of knowledge to address the question as to the impact that the duration of exercise has on its relative effectiveness for inducing health and body compositional changes in individuals who are overfat to assist with developing periodized exercise protocols and establishing short and long term goals. A tiered meta-analysis of 92-studies and 200-exercise groupings were used for establishing pooled ES within and between groupings based on the increments of 4-week of duration and study designs of ≤8, 9-16, 17-23, 24-36, and ≥36 weeks. Analysis based on random-effect of response indicates a continuum of effectiveness within and between ET, RT and E+R based on duration. Where beneficial effectiveness is not indicated for any measures until after 8-weeks of continuous training with progressive effectiveness being noted in changes to cardiorespiratory fitness, inflammatory cytokines, and alteration of metabolic status from 12-weeks through 32-weeks of continuous training. Results indicate a greater ES for RT and E+R versus ET early in intervention that equalizes with longer durations. Supporting the use of RT and E+R within a periodized program. And secondarily, goals should be established first on performance gains and second body composition or health status modifications for the individual who is overfat.
Keywords: Exercise Duration, Fitness, Health Status, Obesity, Overfatness
INTRODUCTION
Over the past century, the use of exercise, e.g., endurance exercise (ET), resistance exercise (RT), or endurance and resistance combined (E+R), has become a common treatment method for improving body composition and health status of individual who are overfat, i.e. the individual with health issues arising from excessive body fat and not from body mass index (BMI) classification of obese or overweight, or for those individuals with metabolic issues, e.g., type 2 diabetes mellitus or MetS [1, 2]. Resulting in a multitude of institutional position stands and exercise programs (with or without a concurrent change in diet) in an attempt to improve the overall health status and body composition for individuals who are overfat or with metabolic syndrome (T2DM) [3–11]. Each developed in the global effort to deal with the epidemic rise in the rate of overfatness (and the associated health issues) within the global population, universally based on the premise that doing any type of activity is better than doing no activity at all [7–9, 12]. That is furthered by the postulate that increasing overall level of physical activity for the individual who is overfat will ultimately produce a change in behavior that alters an unhealthy lifestyle into a healthy lifestyle [5, 10].
Abbreviations
- ES
– effect size as determined by pooled effect size via random effect computation
- ET
– Endurance Training
- RT
– Resistance Training
- E+R
– combination of ET and RT
- BM
– body mass (kg)
- BMI
– (kg·m-2)
- FM
– fat mass (kg, or as determined by %body fat)
- FFM
– fat-free mass (kg, or as determined by %fat-free mass)
- T2DM
– type 2 diabetes mellitus or metabolic syndrome
- CRP
– C-reactive protein
- TNF-α
– tumor necrotic factor-alpha
- IL-6
– interleukin-6
- VO2max
– maximal aerobic capacity
- HRmax
– maximal sustainable heart rate
- 1RM
– maximal level of resistance for a single repetition (i.e. maximal strength)
- SBP
– systolic blood pressure
- DBP
– diastolic blood pressure
However, issues arise related to the ideal of exercise incorporation into treatment for the individual who is overfat. Principally, given the known value of exercise, why does exercise have such a poor attrition rate in the utilization and compliance with the recommendation for exercise by the population as a whole [13–15]? An answer to which most likely stems from multiple factors, notably the application bias by health professionals coupled to the social stigma individuals who are overfat face in the development of exercise programs [14, 16–19]. Which combine with the limited appreciation for the complexity of exercise, or responses to exercise, that impact the individual’s physiology leading to the changes in overall health status of the individual who is overfat [1, 2, 20]. The former may limit the draw that the individual has to exercise while the latter limits the ability to design exercise programs based on a periodization model [21, 22] that would allow for modification of the level of stimulus across the duration of training. A model of application that is necessary for continual physiological, and morphological, adaptations and improvements in overall health status to take place [2, 14]. Ultimately, poor exercise design and application limits the ability to modify long-term health behaviors required to encourage the modifications necessary to convert the overfat individual from a diseased health status to a non-diseased health status [1, 5, 8]. Thereby, producing the poor attrition and compliance with exercise programs.
While a recent meta analysis has indicated differences in effectiveness between methods of exercise, based on level of total muscle stimulation that favors use of higher levels of training stimulus over lower-levels [23]. In which, higher level stimulus from resistance training (e.g., hypertrophic patterning) shows as being the most effective pattern of training stimulus for inducing changes in morphology and health status of the individual who is overfat [23]. Yet, there appears to be a bias in the promotion of methods of exercise at low overall stimulus versus those of higher level of stimulus. Along with a premise that any increase in activity has benefit and that exercise methods generate a similar level of adaptation, if we just give it enough time [9, 19, 24–26]. Generating a mismatch of unrealistic expectations for outcome from exercise, as stimulus generally promoted is not high enough to produce changes generally desired, along with a lack of modification within the training stimulus to provide for continual and progressive modifications to both body composition and health status. Thus, leading to the poor adherence and lower attrition rate to multiple methods of exercise for individuals who are overfat or at risk for metabolic issues [13–15]. And may explain the cyclic behavior of repetitive attempts of, and withdrawals from, various exercise methods to fulfill the want of improved overall health of the individual who is overfat.
Thus we are left with questions about the means by which to prescribe exercise based on how long must one engages in these methods of exercise to stipulate that a program works? And if it takes disproportionately longer for one program to work, is it actually as effective as another program? Where answers can allow us to determine the time course for overall effectiveness from different methods of exercise and therefore develop first a time course for goal setting and secondly, a method for the periodization of training. That will allow for us to provide an exercise program that provides the greatest degree of benefit and the changes in long-term patterns of physical activity and exercise behaviors to alter the overall health status of the individual who is overfat, or susceptible to metabolic issues.
Therefore, the purpose here is to expand upon previous reports of effectiveness [23] related to changes in body composition and issue of overall health status of the individual who is overfat based on the duration of exercise. Testing the hypothesis that the relative benefit obtained from exercise will become more effective with prolonged exposure to any of the exercise modalities, e.g., endurance, resistance, or combination of endurance and resistance training. And secondarily, inclusion of resistance exercise within training program will continue to show a greater level of effectiveness for eliciting responses across all durations examined for each measures of interest. Where the aim this study is to evaluate observational, tracking and random-control trials to address the impact that the duration of exercise has on its relative effectiveness for inducing health and body compositional changes in individuals who are overfat. So as to provide an understanding as to what, if any, differences exist across the duration of exercise that is required for development of a periodized program of exercise prescription. While also providing the information necessary for developing both the short and long-term goals for the exercise protocols for individuals who are overfat.
MATERIALS AND METHODS
The meta-analysis is a continuation of analysis that has been previously detailed [23] examining the random-effect pooled effect size (ES) for responses in adults who are overfat, clustered for the duration of exercise used in treatment. In which relevant studies (e.g., studies only involving human volunteers within population based research models) were retrieved from electronic database search engines (PubMed, SPORTDiscus, Scopus) through the end of February 2014. Where the searches were conducted based on the key word human, with a combination of any (or all) of the following: obesity, type 2 diabetes, metabolic syndrome, exercise, resistance training or resistance exercise, endurance training or aerobic exercise, strength training, diet, insulin, obesity, weight loss, fat mass, and fat-free mass. From the 3,500 total journal articles, see PRISMA information in supplemental figure 1, returned by the search engines and following the initial screening a total of 92 studies were included based on the following criteria into the meta-analysis:
Inclusion criteria
Published original research from January 1980-February 2014
Published in English or translation of article available
Utilized only human participants with reported average age () for volunteers ranging from 18 and 65 years of age during the duration for the experiment
Study population was either identified as either “overweight” or “obese” by authors or was indicated within the study as meeting at least 1 of the classification metrics for being overweight or obese and indicated elevated fat-mass as cause for classification
Studies involved observational and tracking of changes following intervention, involved a comparison at least two conditions (either within subject cross-over design or comparison to a control or basal/baseline) with possible random assignment to training group(s) or control and to the order or method of training
Study designs examined chronic adaptations (i.e. multiple training sessions, or interventions lasting at least 4 weeks in duration)
Study reported average values and standard deviations for measures observed from intervention for both the pre-test and post-test values
Main purpose was to examine hormonal or cellular responses to exercise
Main purpose was to examine changes in body mass in response to exercise
Main purpose was to examine chronic responses to either modes of exercise (e.g., resistance exercise or endurance exercise) or combination of one of the exercise modes with hypocaloric diet, or combination of both modes of exercise (e.g., combination of resistance and endurance exercise within training regimen) with or without a hypocaloric diet
Exclusion criteria
Publication was a review article
Not published in English or no translation available
Study design utilized an animal model for the problem
Population age could be classified as adolescent, or juvenile, ( < 18 years of age) and/or elderly ( > 65 years of age)
Study population either failed to meet metrics for classification as “obese” or “overweight”, or was indicated to have secondary disease (e.g., cancer, osteoporosis, cardiovascular disease) or had populations indicated to have history of metabolic variables and concurrent treatments (e.g., smoking, pharmacologically controlled type-2 diabetes mellitus (T2DM), cardiovascular diseases) that might confound the response to exercise and/or diet treatment
Study design examined strictly acute responses (i.e., single exercise bout, or intervention lastly fewer than 4 weeks in duration)
Study reported percentage of changes without indication of averages and standard deviation of pre- and post-test values
Main purpose did not involve measure of hormonal or cellular response to exercise or diet
Results did not report absolute changes in hormones or body mass following intervention
Indication of use of dietary supplement, or pharmacological dosing of anabolic or androgenic hormones
Due to the inability to blind participants to whether or not the individual has been exposed to exercise, studies were not excluded by the criteria of random-control blinding to treatment.
From the 92 studies, 200 study-groupings were developed for comparison of responses within the review across the duration of studies and then chronological time of continuous training. These groupings were based on study demographics, e.g., age, gender, overfatness and disease state, followed by the method of exercise indicated, e.g., endurance (ET), resistance (RT), or endurance and resistance combined (E+R) and then by indication of relative intensity of training. Classification of exercise was performed, regardless of additional dietary intervention to the exercise regimen, as it was previously noted that diet (except for lower carbohydrate diet) had a minimal overall impact on the exercise’s level of effectiveness for adaptations in measures of interest analyzed here [23]. After entry of study demographics, each study was identified by the duration of exercise intervention, by total number of weeks of training, and the group size for human volunteers within each group for that study. After which the reported averages (pre-, and post-, test) and standard deviations of each test for all measures of interest (e.g., body mass (BM), fat mass (FM), fat-free mass (FFM), body mass index (BMI), systolic (SBP) and diastolic (DBP) blood pressure, aerobic fitness (VO2max), [insulin]pl, [glucose]pl, [glycated hemoglobin (A1c)]pl, [adiponectin]pl, [leptin]pl, [c-reactive protein (CRP)]pl, and [tumor necrotic factor- α (TNF-α)]pl) were entered into the data table. To determine the progressive change in ES within and between exercise methods for eliciting changes in these measures studies were clustered based on the length of investigation for each study included at ≤8-weeks (19-studies [27–45], group size: 19±6.4, age: 45±10.2, female:male ratio: 1.6), 9-15 weeks (29-studies [46–73], group size: 19±22, age: 47±10.4 , female:male ratio: 1.3), 16-23 weeks (16-studies [44, 74–88], group size: 15±11.4, age: 52±9.5, female:male ratio: 1.3), 24-36 weeks (14-studies [35, 71, 77, 89–99], group size: 41±27.9, age: 55±6.3, female:male ratio 0.9) and >36-weeks (15-studies [77, 89, 91, 100–111], group size: 53±46.4, age: 46±9.2, female:male ratio: 1.14), see supplemental table 1, and then grouped within these clusters by 4-week increments of continuous training for examination of within grouping changes.
Analysis of tabulated results was performed to determine the degree of skew (that is only due to publication bias toward only reporting the positive findings and dissimilar exercise parameters within studies) within the responses to assess the likelihood of continuing analysis utilizing aggregate pooled effect based on the method previously utilized [23, 112]. There was an active attempt to limit the selection bias of studies included by examining all study methods used to evaluate intervention programs that used exercise, beyond the classically desired random-controlled and clinical trails. In this effort, population demographics were also evaluated to ensure that populations within each study was not so restrictive as to limit the generalizability of the findings provided by the authors of that study. Based on such analysis, bias in the data indicated that relative effectiveness for eliciting changes must be conducted utilizing a non-parametric, non-uniform method for the determination of ES across and between methods of exercise for the durations performed. Where all grouped analysis is based on the assumption of random-effect pooled effect size, similar to what this author previously performed and reported on [23, 112, 113]. The determined pooled ES, and resultant confidence intervals (CI.95) of ES was determined, to examine the overall effect relative to a case of no change (i.e. ES=0.00 or a CI.95 for ES crossing zero within range for expected responses) as previously described [23, 112].
The comparisons analyzed here are based on each of the following 1) within and across all the various exercise modalities, e.g., all exercise (All Ex), endurance exercise (ET), resistance exercise (RT), or combination of endurance and resistance (E+R), based on clustering of groups within 4-week increments of training duration from 4-weeks to 40-weeks and then at 52-weeks of exercise training; 2) within all the various exercise modalities, e.g., All Ex, ET, RT, or E+R, based on clustering of groups of study durations (≤8-weeks, 9-15 weeks, 16-23 weeks, 24-36 weeks and 52-weeks); and 3) differences between exercise modality (e.g., ET versus RT, ET versus E+R, RT versus E+R) based on clustering of groups of study durations (≤8-weeks, 9-15 weeks, 16-23 weeks, 24-36 weeks and 52-weeks). With results indicated as ES (CI.95 low value, CI.95 high value) when significant differences were noted, i.e. ES ≠ 0, and where CI.95 did not include 0 within the range for ES.
In an effort to establish a secondary directionality for difference between exercise treatments, the within study treatment ES, (μpost-treatment-μpre-treatment)/(σpre-treatment), were then clustered for 4x4 χ2 analysis to determine if there was any difference in the level of response between methods of exercise. In which the χ2 analysis was utilized through identification of number of greater effectiveness and number of lesser effectiveness within each exercise and duration group based comparison to study’s ability to elicit a greater, or lesser, level of effectiveness relative to the pooled effectiveness of comparison (i.e. ET versus RT, ET versus E+R, RT versus E+R) and a diet-only intervention that has been previously reported [23].
RESULTS
As indicated in figure 1-5 and supplemental figures 2-4, the responses for effectiveness (ES) in eliciting changes follows the trend of continuum for a differential level of ES toward eliciting beneficial responses based on the duration of training both within and between the methods of exercise, table 1. The spectrum of overall beneficial effect (i.e. ES≠0, or CI.95 of ES not including 0.00) both within and between methods of exercise appears to wane and plateau as training progresses into ever-longer durations. As comparisons show that shorter duration training appear to be more effective than the moderate and longer durations and moderate duration training more effective than the longer durations.
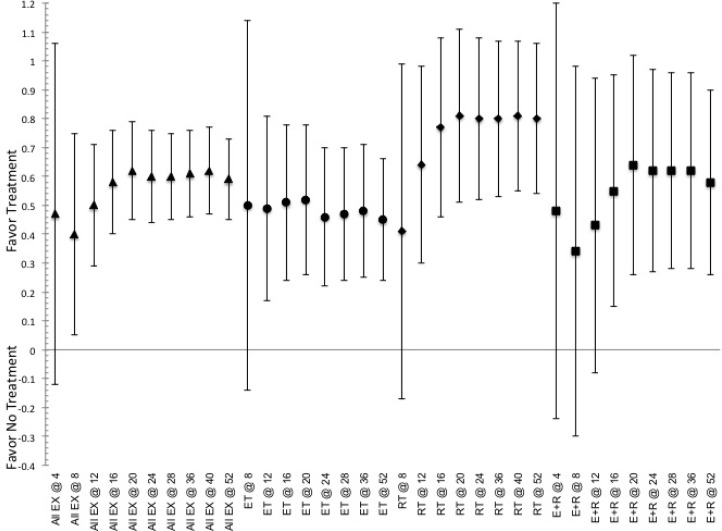
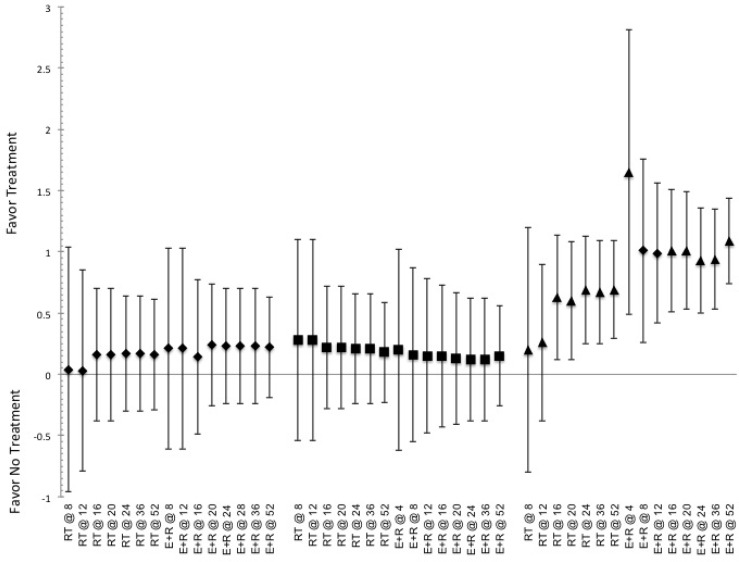
FIG. 1.
Indicated pooled ES and CI.95 for effective to elicit responses in measures of body morphology (fat mass) for the duration of training (indicated by @ and then number of weeks of training) based on exposure to stimulus of the various methods of exercise. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
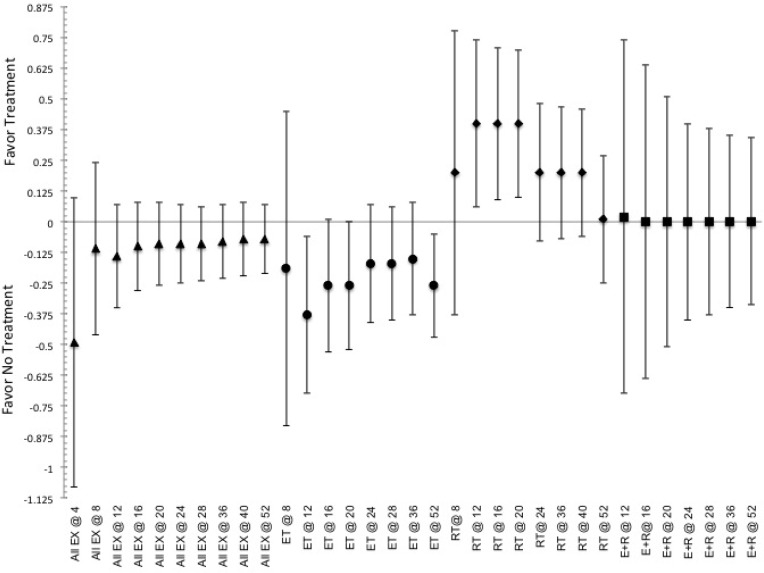
FIG. 5.
Indicated pooled ES and CI.95 for effectiveness to induce improvements in aerobic fitness, VO2max, based on the various training durations (indicated by @ and then number of weeks of training) of exercise. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
TABLE 1.
Summary of the effectiveness, ES (low CI.95, high CI.95), for eliciting changes in response of measure of interest between the various methods of exercise based on the durations of training.
| BM | FM | FFM | BMI | SBP | DBP | VO2 | Insulin | Glu | A1c | OB | Adip | IL-6 | CRP | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within <8-weeks of training | |||||||||||||||
| ET v. RT | -0.11 (-0.49, 0.27) |
-0.39 (-0.80, 0.02) |
-0.26 (-0.84, 0.01) |
-0.28 (-0.73, 0.17) |
0.00 (-0.71, 0.71) |
-2.45 (-3.77, -1.13) |
-0.69 (-1.33, -0.04) |
0.04 (-0.46, 0.54) |
-0.16 (-0.63, 0.31) |
-0.15 (-0.69, 0.39) |
0.07 (-0.87, 0.14) |
-0.37 (-0.87, 0.14) |
-0.26 (-1.26, 0.75) |
-0.94 (-1.99, 0.12) |
-0.38 (-1.20, 0.45) |
| ET v. E+R | -0.26 (-0.62, 0.11) |
-0.40 (-0.83, 0.03) |
-0.18 (-0.89, 0.53) |
0.09 (-0.28, 0.45) |
--- | --- | 0.60 (-0.12, 1.09) |
0.32 (-0.63, 0.90) |
0.07 (-0.44, 0.57) |
0.22 (-0.28, 0.72) |
-0.19 (-0.63, 0.26) |
0.21 (-0.19, 0.64) |
-0.19 (-0.82, 0.44) |
-0.23 (-0.94, 0.48) |
-0.55 (-1.27, 0.17) |
| E+R v. RT | 0.02 (-0.37, 0.42) |
-0.03 (-0.46, 0.39) |
-0.08 (-0.78, 0.63) |
-0.21 (-0.68, 0.27) |
--- | --- | -1.26 (-1.35, -0.54) |
-0.23 (-0.86, 0.41) |
-0.17 (-0.71, 0.37) |
-0.30 (-0.88, 0.28) |
0.20 (-0.25, 0.65) |
-1.02 (-1.64, -0.41) |
0.22 (-0.42, 0.85) |
-0.55 (-1.27, 0.17) |
0.21 (-0.61, 1.03) |
| Within 9-15 weeks of training | |||||||||||||||
| ET v. RT | -0.05 (-0.31, 0.20) |
-0.34 (-0.59, -0.08) |
-0.18 (-0.48, 0.13) |
-0.08 (-0.36, 0.20) |
0.41 (0.13, 0.95) |
0.13 (-0.41, 0.66) |
0.24 (-0.06, 0.53) |
-0.17 (-0.67, 0.34) |
-0.10 (-0.61, 0.40) |
-0.22 (-0.65, 0.21) |
0.02 (-0.62, 0.65) |
-0.13 (-0.52, 0.26) |
-0.29 (-0.83, 0.24) |
0.05 (-0.59, 0.68) |
-1.10 (-1.78, -0.42) |
| ET v. E+R | -0.12 (-0.41, 0.18) |
-0.19 (-0.51, 0.12) |
-0.25 (-0.62, 0.11) |
-0.07 (-0.37, 0.25) |
-0.73 (-1.33, -0.14) |
0.24 (-0.34, 0.82) |
0.41 (0.08, 0.73) |
-0.15 (-0.63, 0.32) |
0.09 (-0.46, 0.48) |
0.06 (-0.32, 0.44) |
--- | -0.20 (-0.65, 0.25) |
0.04 (-0.60, 0.67) |
0.02 (-0.56, 0.59) |
0.23 (-0.59, 1.05) |
| E+R v. RT | 0.03 (-0.39, 0.46) |
-0.34 (-0.70, 0.03) |
0.11 (-0.32, 0.53) |
-0.08 (-0.51, 0.35) |
1.35 (0.45, 2.26 |
-0.13 (-0.95, 0.68) |
-0.10 (-0.55, 0.43) |
0.21 (-0.61, 1.02) |
-0.11 (-0.92, 0.71) |
-0.54 (-1.13, 0.05) |
--- | 0.13 (-0.45, 0.71) |
-0.30 (-1.12, 0.53) |
0.05 (-0.66, 0.76) |
-1.09 (-1.97, -0.22) |
| Within 16-23 weeks of training | |||||||||||||||
| ET v. RT | 0.07 (-0.26, 0.40) |
0.23 (-0.16, 0.62) |
0.02 (-0.43, 0.46) |
0.04 (-0.35, 0.44) |
0.25 (-0.23, 0.72) |
0.13 (-0.34, 0.61) |
0.23 (-0.20, 0.66) |
0.63 (0.17, 1.08) |
0.38 (-0.03, 0.79) |
0.11 (-0.32, 0.53) |
--- | -1.06 (-1.93, -0.18) |
--- | -0.50 (-1.07, 0.10) |
--- |
| ET v. E+R | -0.26 (-0.59, 0.08) |
-0.08 (-0.48, 0.31) |
0.02 (-0.43, 0.46) |
-0.55 (-1.00, -0.09) |
-0.13 (-0.63, 0.34) |
-0.30 (-0.80, 0.20) |
0.43 (-0.03, 0.88) |
0.58 (0.10, 1.06) |
0.07 (-0.38, 0.52) |
-0.77 (-1.26, -0.29) |
--- | 0.39 (-0.25, 1.03) |
--- | 0.03 (-0.67, 0.70) |
--- |
| E+R v. RT | 0.32 (-0.02, 0.65) |
0.33 (-0.05, 0.71) |
0.00 (-0.37, 0.38) |
0.65 (0.21, 1.09) |
0.35 (-0.29, 0.99) |
0.45 (-0.19, 1.09) |
-0.15 (-0.79, 0.48) |
-0.01 (-0.49, 0.46) |
0.31 (-0.20, 0.81) |
0.82 (0.30, 1.34) |
-1.61 (-2.55, -0.87) |
-0.86 (-1.91, -0.18) |
--- | --- | --- |
| Within 24-36 weeks of training | |||||||||||||||
| ET v. RT | 0.28 (-0.06, 0.61) |
-0.77 (-1.13, -0.40) |
-0.07 (-0.46, 0.33) |
0.70 (0.25, 1.13) |
--- | --- | 0.55 (0.13, 0.96) |
0.21 (-0.27, 0.68) |
0.03 (-0.47, 0.53) |
0.57 (0.09, 1.05) |
--- | --- | --- | --- | --- |
| ET v. E+R | -0.13 (-0.51, 0.24) |
-0.12 (-0.53, 0.29) |
-0.03 (-0.50, 0.44) |
-0.11 (-0.56, 0.33) |
--- | --- | 0.09 (-0.32, 0.50) |
--- | 0.03 (-0.47, 0.53) |
0.41 (-0.06, 0.89) |
--- | --- | --- | --- | --- |
| E+R v. RT | 0.49 (0.07, 0.90) |
-0.72 (-1.19, -0.26) |
-0.04 (-0.49, 0.41) |
0.67 (0.12, 1.22) |
1.00 (-0.06, 2.06) |
0.41 (-0.60, 1.42) |
1.09 (0.02, 2.16) |
--- | -0.01 (-0.82, 0.81) |
0.15 (-0.43, 0.72) |
--- | --- | --- | --- | --- |
| Within >36-weeks of training | |||||||||||||||
| ET v. RT | -0.33 (-0.72, 0.07) |
-0.21 (-0.66, 0.24) |
-0.44 (-1.08, 0.20) |
-0.54 (-0.99, -0.08) |
0.58 (-0.07, 1.23) |
0.48 (-0.17, 1.12) |
0.00 (-0.54, 0.54) |
-0.09 (-0.80, 0.62) |
0.07 (-0.47, 0.60) |
-0.48 (-1.12, 0.17) |
--- | --- | --- | --- | --- |
| ET v. E+R | -0.34 (-0.75, 0.07) |
-0.15 (-0.60, 0.29) |
-0.44 (-1.07, 0.21) |
-0.27 (-0.67, 0.12) |
0.43 (-0.04, 0.91) |
-0.18 (-0.65, 0.30) |
-0.20 (-0.63, 0.23) |
0.08 (-0.49, 0.66) |
0.11 (-0.47, 0.69) |
-0.06 (-0.59, 0.48) |
--- | --- | --- | (7.14) (5.57, 8.70) |
--- |
| E+R v. RT | 0.03 (-0.60, 0.66) |
-0.09 (-0.80, 062) |
-0.13 (-1.13, 0.88) |
-0.38 (-0.86, 0.10) |
0.43 (-0.11, 0.90) |
0.67 (0.16, 1.28) |
0.24 (-0.34, 0.82) |
-0.15 (-0.85, 0.56) |
-0.05 (-0.68, 0.59) |
-0.42 (-0.96, 0.12) |
--- | --- | --- | --- | --- |
Note: Note that comparisons based on endurance versus resistance training methods (El v. RT), endurance versus combination of endurance and resistance training methods (ET v. E+R), combination of endurance and resistance versus resistance training methods (E + R v RT). Negative (-) values indicate favor toward the second exercise group in the comparison between the methods of exercise, while ‘---‘ indicates indicates not enough studies in the group to determine pooled effect size and CI.95.
Comparing the elicited responses within each study analyzed indications for differential ES favoring a specific type of exercise based on the duration of the exercise within the individual study relative to the ES from diet (previously reported [23]) or in comparison to the pooled ES for the methods of exercise being compared. One such indication is RT’s ability to elicit a larger ES for altering DBP and glucose at longer durations (beyond 36-weeks) relative to ET (χ2=3.48, p=0.02 and χ2=3.49, p=0.04, respectively). Along with trends favoring RT for altering glucose through the moderate duration (up to 24-weeks) of training relative to ET, χ2=1.28 (p=0.12), and E+R (up to16-weeks), χ2=1.49 (p=0.13), and for changes in fasting insulin levels relative to ET, χ2=1.77 (p=0.14). Moreover, RT has a trend for being more effective at shorter durations (up to 8-weeks) for altering FFM and through the moderate durations (up to 24-weeks) for altering FM than ET, χ2=1.24 (p=0.15) and χ2=1.38 (p=0.12) respectively. Whereas, E+R trends toward being more effective than ET for altering FM through the moderate duration (up to16-weeks), χ2=1.31 (p=0.12), and after longer durations (beyond 36-weeks), χ2=1.77 (p=0.12), along with eliciting alterations in insulin, χ2=2.11 (p=0.11), after longer durations (beyond 36-weeks).
Effectiveness related to ability to elicit changes in morphology
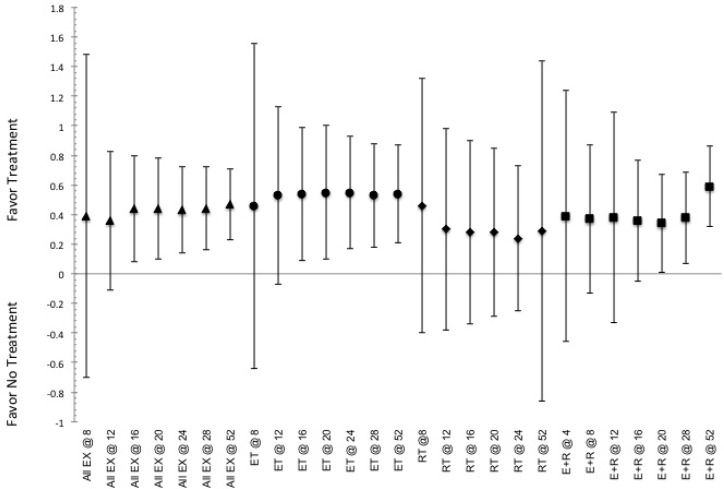
Exercise has a greater ES at shorter and moderate durations, than at longer durations, with no indication for possible beneficial effects in changes of body morphology (BM, FM or FFM) occurring for each method of exercise until after 12-weeks of continuous training with changes in BMI not indicating a total beneficial effect until after 16-weeks, figure 1 and supplemental figure 2. The greatest ES for altering any measure of body morphology, with the exception of FFM, occurring at a training duration of 16-23 weeks, with the greatest ES for altering BM continues through 36-weeks of training, ES=0.47 (0.17, 0.78). Within the grouping analysis for each type of exercise notes that the use of RT and E+R appear to be more effective than ET, however the CI.95 can have an ES of 0 and thus differences may not be significantly more effective for all duration, figure 1 and supplemental figure 2. RT becomes effective (i.e., CI.95 always favoring treatment) at altering FM within the shorter training duration, ≤8-weeks, and all other measures of morphology at 9-15 weeks, while ET and E+R develop similar beneficial effectiveness after moderate and longer duration training periods, e.g., beyond 16-weeks of continuous training, regardless of the measures of body morphology being examined, figure 1 and supplemental figure 2.
FIG. 2.
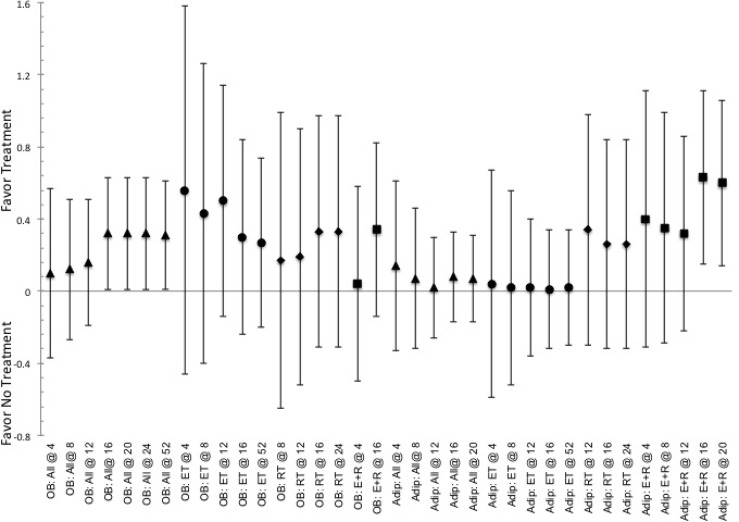
Indicated pooled ES and CI.95 for effective to elicit responses for altering adipokines (leptin, OB, and adiponectin, Adip) associated with health issues related to overfatness obtained from the various training durations (indicated by @ and then number of weeks of training) for the various modes of exercise. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
The comparison between methods, see table 1, indicates that E+R and ET were more effective than RT for altering BM at moderate durations (24-36 weeks). RT is more effective than both ET and E+R at altering FM at 9-15 weeks and 24-36 weeks of training. Additionally, RT was more effective than ET at altering FFM at training duration ≤ 8-weeks, 0.27 (0.01, 0.53), and at 52-weeks, 0.44 (0.04, 0.84), but shows no absolute difference (i.e. CI.95 crosses ES=0) in effectiveness relative to E+R for effectiveness at altering FFM. Furthermore, E+R does not show any absolute difference (i.e. CI.95 crosses ES=0) relative to ET alone for effectiveness at altering FFM. In addition to changes in body composition, the effectiveness for altering BMI following exercise indicates that ET and E+R is more effective than RT at 24-36 weeks, whereas E+R appears more effective than either ET or RT at 16-23 weeks of continuous training, yet RT is more effective than ET at 52-weeks, 0.54 (0.09, 0.99), table 1.
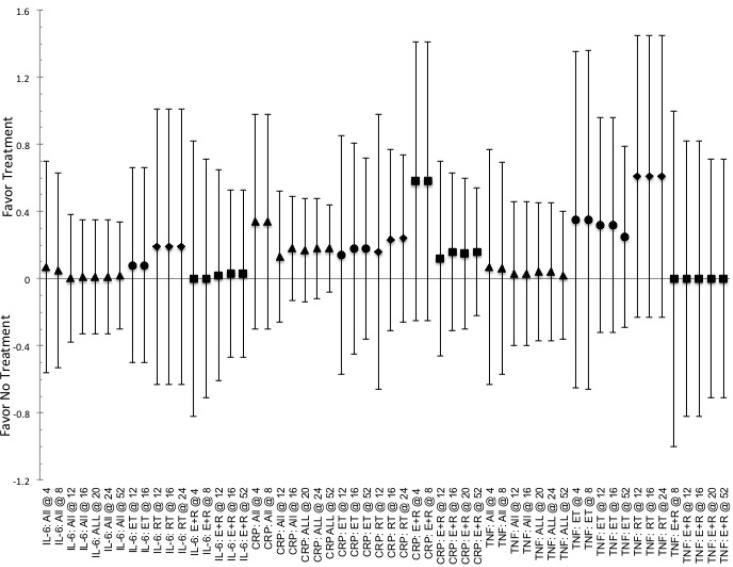
Effectiveness related to changes in cytokines associated with health status
There appears to be little overall difference in the ES for altering cytokines associated with overfatness (e.g., leptin, adiponectin, IL-6, CRP, TNF- α) between the methods of exercise relative to the duration of training. Where each method of exercise indicates that a short-to-moderate duration of training, i.e. ≤23-weeks, is more effective relative to the longer durations of training. Within the ability to elicit changes in leptin or adiponectin, figure 2, RT appears to be more effective than ET and E+R at 16-23 weeks of training and E+R at ≤8-weeks of training for altering adiponectin, only. RT also appears to be more effective than ET, or E+R, for altering TNF-α levels at 9-15 weeks, supplemental figure 3. Moreover, RT was the only method of exercise to indicate differences between durations for altering leptin levels, where durations of ≤8-weeks was more effective than 16-23 weeks of training, 0.56 (0.04, 1.08). Unlike RT, ET and E+R indicated differences in effectiveness based on the duration of training for altering adiponectin, as each were more effective at 16- 23 weeks than at 9-15 weeks of training, 0.95 (0.62, 1.28) and 1.55 (1.01, 2.09) for ET and E+R respectively. Further E+R shows that a training duration of 16-23 weeks of training was more effective than any of the other training durations, 1.55 (1.03, 2.08) for altering adiponectin. Yet the duration of 9-15 weeks of training was the least effective relative to than any of the other training durations, -0.64 (-1.00, -0.16); especially when compared to the duration of ≤8-weeks of training, where the shortest duration is favored, 0.93 (0.37, 1.49). Additionally, no differences in effectiveness were seen in any of the methods of exercise (ET, RT, or E+R) to alter IL-6 based on the duration of training, supplemental figure 3. While on a limited basis for comparison, due to limited reports, RT at 9-15 weeks indicates being more effective than ≤8-weeks for altering CRP levels, 0.99 (0.12, 1.86), while ≤8-weeks is more effective than 16-23 weeks of training for altering TNF-α, 0.59 (0.06, 1.12), supplemental figure 3. Lastly, for exercise in general a training duration ≤8 weeks is more effective 24-36 weeks, 0.99 (0.37, 1.61), for altering TNF-α.
FIG. 3.
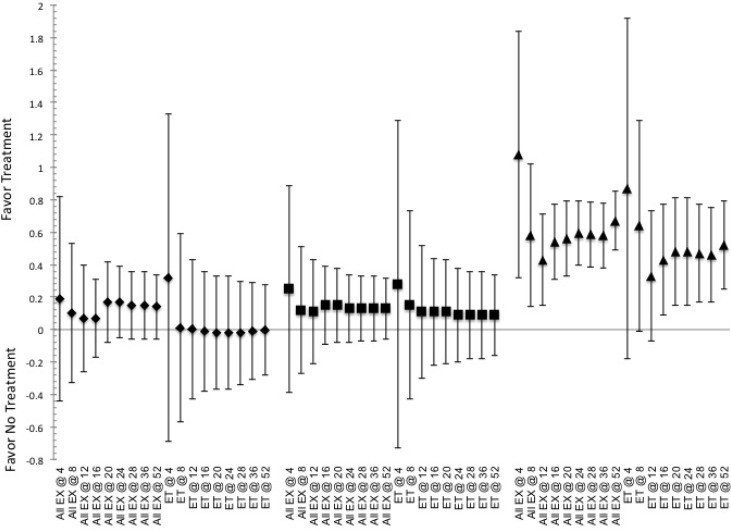
Indicated pooled ES and CI.95 for effective to elicit responses for altering markers of T2DM, fasting insulin, glucose and A1c levels, associated with overfatness obtained following the various training durations (indicated by @ and then number of weeks of training) for all exercise (ALL Ex) or endurance training ET. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. Note that ♦ indicates Insulin, ■ indicates glucose and ▲ indicates A1c.
Effectiveness related to changes in markers of T2DM
Duration of training appears to have limited impact on the effectiveness for the specific type of exercise to elicit changes in the markers for T2DM, e.g. fasting levels of glucose and insulin, figures 3-4. That is except for E+R, where a training duration of 24-36 weeks is more effective than 9-15 weeks, 1.48 (0.36, 2.60) and at the duration of 9-15 weeks being more effective than the duration of 52-weeks, 0.59 (0.14, 1.04), for altering glucose and insulin concentrations respectively.
Even so, differences are noted between exercise based on the durations of training, table 1 and figures 3-4. As the use of RT and E+R are both more effective than ET at durations of 16-23 weeks of training for both altering glucose and insulin levels, table 1. While no other directional selection for effectiveness being noted between the RT and E+R for altering the levels of glucose of insulin, based on the duration of training. Yet E+R is more effective than RT at durations of 9-15 week training but less effective at 16-23 weeks, table 1.
FIG. 4.
Indicated pooled ES and CI.95 for effectiveness to elicit responses for altering markers of T2DM, fasting insulin, glucose and A1c levels, associated with overfatness elicited within the various training durations (indicated by @ and then number of weeks of training) for resistance training RT or combination of endurance and resistance training E+R. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. Note that ♦ indicates Insulin, ■ indicates glucose and ▲ indicates A1c.
Yet duration may have an impact on the effectiveness to elicit changes in A1c levels, figures 3-4. It appears that longer duration (52-week) of training is less effective than any other time frame, -0.71 (-1.04, -0.38), especially in comparison to training durations of ≤8-weeks, -0.48 (-0.93, -0.02), 9-15 week, -0.67 (-1.15, -0.18), 16-23 weeks, -0.77 (-1.24, -0.38) and the 24-36 weeks, -0.68 (-1.16, -0.20), with moderate durations of 9-15 weeks and 24-36 weeks being the most effective. Based on the method of exercise both ET and E+R indicate having a lower level of effectiveness at 52-weeks of training relative to all other shorter duration training, -0.51 (-0.77, -0.25) and -0.64 (-0.99, -0.29) respectively. Additionally, E+R is more effective than ET at moderate duration (16-23 week) of training, table 1. Whereas, RT at ≤8-weeks and 9-15 weeks are more effective than any of the longer duration studies, 0.46 (0.07, 0.86) and 0.74 (0.33, 1.15) respectively, with a duration ≤8-weeks being more effective than 16-23 weeks, or 52-weeks of training, 1.13 (0.60, 1.66) or 0.90 (0.01, 1.89) respectively, yet 16-23 weeks is less effective than the 52-weeks of training, -0.62 (-1.21, -0.03). Just as RT, E+R indicates that short and moderate durations are more effective than the 52-week duration of training, 0.46 (0.01, 0.99), 0.82 (0.33, 1.31) and 1.13 (0.47, 1.99), for ≤8-weeks, 9-15 weeks and 16-23 weeks respectively and ET indicates being more effective at ≤8-weeks and 9-15 weeks than 52-weeks of training, 0.67 (0.12, 1.12) and 0.52 (0.03, 1.01).
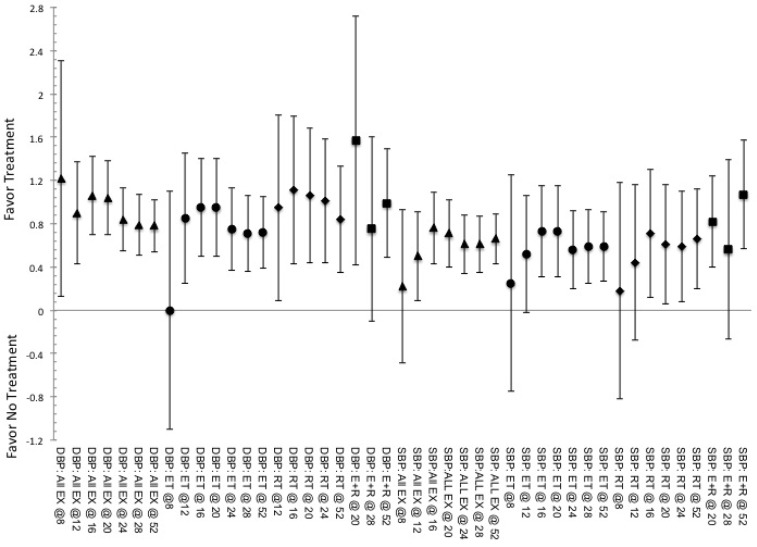
Effectiveness related to ability to alter measures of cardiorespiratory fitness
There were limited differences in effectiveness related to the changes in cardiovascular measures both between and within the methods of exercise based on the duration, table 1 and supplemental figure 4. Yet, the maximal effectiveness occurs between 8 and 16 weeks of continuous training followed by a plateau in the ES for both ET and RT. In regard to E+R it appears that shorter duration (≤ 8-weeks) training is more effective than moderate to long duration 9-15 weeks, 2.09 (1.08, 3.10) and 1.2 (0.98, 1.92), 16-23 weeks, 1.33 (0.60,1.93) and 1.17 (0.32, 1.90), or 52-weeks, 1.63 (0.48, 2.78) and 0.76 (0.41, 1.11), of training for eliciting changes in SBP and DBP respectively. Furthermore, for SBP, E+R indicated 52-weeks of training being less effective than all other shorter durations, -0.54 (-1.04, -0.06) while training for 9-15 and 16-23 weeks was more effective than any longer duration, 0.55 (0.24, 0.86) and 1.13 (0.82, 1.44) respectively. For ET, where a training duration of 16-23 weeks is the least effective, -0.64 (-1.03, -0.13), relative to all other durations with ≤8-weeks showing the greatest effectiveness relative to the measures at 16-23 weeks of training, 0.50 (0.01, 0.99). For the use of RT the greatest effectiveness occurs at durations of 24-36 weeks of training, 1.13 (0.65, 1.62) versus all other durations while 9-15 weeks is more effective relative to any of the longer durations, 0.55 (0.08,1.02), and specifically to 52-weeks of training, 0.77 (0.06, 1.53).
This pattern was also seen with effectiveness for altering DBP, supplemental figure 4. Where, comparisons within ET responses based on duration show a greater effectiveness at ≤8-weeks of training relative to any other longer training durations, 0.77 (0.43, 1.10), specifically being more effective versus training durations of 9-15 weeks, 1.02 (0.42, 1.86), 16-23 weeks, 1.17 (0.55, 1.92), and 52-weeks, 0.97 (0.15, 1.62). While E+R was less effective at the 9-15 week of training, 1.03 (0.53, 1.53), than any other duration and specifically less effective than the 52-weeks of training, 1.57 (0.85, 2.12). And RT showed greatest effectiveness at the 24-36 week training versus all other durations, 0.52 (0.05, 0.98) and was more effective at ≤8-weeks versus 52-weeks, 0.50 (0.03, 0.97), of training.
Aerobic fitness (VO2max) shows a continuous increase in ES throughout the duration of exercise, figure 5, yet effectiveness appears to be variable based on the duration of exercise, or the type of exercise program utilized, table 1 and figure 8. Exercise in general, only indicates an overall ES favoring for altering VO2max with duration of ≤8-weeks or 52-weeks of exercise intervention, 0.42 (0.00, 0.79) and 0.93 (0.50, 1.33) respectively, within the continuum shorter duration (≤8-weeks) exhibited a greater effectiveness relative to any longer duration, 1.00 (0.44, 1.56), and specifically versus 52-weeks, 1.23 (0.07, 2.30), or 9-15 weeks, 0.91 (0.18, 1.63), of training. As far as within exercise choices, ET is the only exercise that indicates a progressive increase in ES for eliciting changes inVO2max, figure 8, yet no clear evidence for greater ES based on a specific duration of training. While for RT ≤8-weeks of training was more effective than any of the longer duration training, 0.69 (0.29, 1.09), and specifically more effective than 52-weeks, 1.23 (0.80,1.66), or 9-15 weeks, 0.63 (0.15, 1.11) of training and for E+R 52-weeks of training was more effective than either 9-15 weeks, 0.75 (0.36, 1.14), or 16-23 weeks, 0.72 (0.17, 1.27). Comparison between exercise methods show that RT is also more effective than both ET and E+R at duration of ≤8-weeks, table 1 and figure 8, that reverses to the point of indicating no difference in effective as the training duration progresses into longer program designs, table 1.
DISCUSSION
Given the acceptance and recommendation that exercise, or an increase in the level of physical activity (with or without dietary modificaiton), [1, 8–10, 12, 24] while often ignoring the implication that not all exercise or physical activity are equal to each other, can produce positive benefits for any individual who is overfat; it behooves us to assess the impact that acute program variables (e.g., training method and duration) have on the effectiveness in eliciting these reported positive benefits. In particular, it becomes necessary to ascertain the impact and effect (e.g., therapeutic, the alteration of physiological functions, or cheerleader, the small physiological changes and psychological rewards that increase adherence and lead obtaining therapeutic effects) being imparted by exercise within physiological and pathophysiological functions leading to the change in health status of the individual who is overfat. As such, analysis here is a continuation of previous reports on the topic [23] which shows exercise imparts both cheerleader and therapeutic effects, coupling together to allow adaptations in the short duration to encourage the progressive improvement that only occurs from prolonged exposure to exercise ultimately generating the improvements in overall health status expected from exercise.
Within these effects, a continuum of effectiveness for eliciting beneficial responses from exercise develops from both the method and duration of exercise training. Where a lower limit for an effectiveness being completely beneficial, i.e. CI.95 for ES>0, occurs at 8-weeks of training and an upper limit for continual gain in effect (where a plateau of ES) at 32-weeks of training exist for any type of exercise exposure. Thus indicating that the duration of the exposure to stimulus from exercise is a factor for inducing changes not only in morphology, but also in the overall health status of the individual who is overfat. With the implication for a delay in the onset of effectiveness for inducing modifications from exercise that will also reach a point where no further benefit is achievable. Something to take into account not only when developing the short-term and long-term goals for exercise, but also in establishing the timing for when to modify the training stimulus within a continuous training regimen.
Furthermore, the implications for the timing of initial differences in effectiveness may help shed some light on why previous reports have indicated no difference between exercise methods in treating health issued for individuals who are overfat [24, 42, 114, 115]. As the delay in a distinct type of exercise exhibiting greater effectiveness versus other methods of exercise does not become evident until at least 8-weeks of continuous training is within the time frame that these reports are indicating for study durations. Thus the implication that any exercise has equal benefit is partially corrupted, as training durations being compared could simply be too short to have differences seen, rather than an implication and recommendation that equality between methods of exercise truly exists. Additionally, the continuum of responsiveness here indicates differences in effectiveness for the eliciting changes in various measures of interest are both duration and exercise dependent. Supporting the idea of a continuum of effectiveness noted previously for exercise (with and without diet modification) due to the level of muscle stimulation, without regard to duration [23]. And provides support to the idea of variability within responses that are due to the complex interactions between a multitude of factors, where exercise is one of a number of regulatory factors, in developing and resolving the diseased health status affecting the individual who is overfat [2, 8, 14].
As we parse through this continuum a distinct, and slightly perplexing, trend arises in relation to the difference in effectiveness previously noted as well as to the readily recommended methods of exercise, e.g., endurance (aerobic) or lower-intensity training, for the individual who is overfat to utilize [3, 6, 11, 23, 116–118]. Where after 8-weeks of continuous training, RT exhibits a greater effectiveness than ET across all measures of interest, with the exception of SBP and VO2max, and indicates limited differences to the responses elicited from E+R, with the exception of RT being more effective for altering positive changes in fat-free mass within overall body composition. Interestingly, given the dominance of recommendation, ET appears to have an effectiveness that favors not preforming that type of exercise as the duration of training progresses past 16-weeks of training for altering insulin concentrations. A finding that hints to difference in the metabolic stimulus between RT versus ET, or E+R, leading to the difference in responses and deserves much more attention [2, 14].
This conflict with the readily recommended method of exercise, i.e. reliance on ET [11, 117, 119], for individuals who are overfat, or susceptible to metabolic issues, may begin to help to explain why there is such low attrition for programs previously recommended and prescribed [13, 14]. As the effectiveness to alter outcome measures are not matching the implied desires for responses, or the goals developed, for the individual based on the recommendations for using exercise, i.e. ET, that does not provide the sufficient level of stimulus to effectively induce the desired outcome [2, 14, 23, 26, 120, 121]. However, the underlying physiological rationale for the implication of the total training stimulus intensity has on the responses to exercise from individuals who are overfat is incomplete, and deserves much more research. In particular, understanding of how the training stimulus provided from RT instills differences in effectiveness, beyond a moderate duration of training not provided from either ET or E+R [23, 115, 118]. Which is especially important given that we need to have a prolonged engagement with exercise to instill constant improvements in health status of the individual who is overfat.
Although there are trends in conflict with previous reports, some trends support the connotation to the previously touted choice for ET as the selected method of exercise [7, 11, 26, 117–122]. Specifically related to its effectiveness for altering aerobic fitness (VO2max), a finding that might be due to the ET induced alterations in adipose metabolism and the subsequent changes in hormonal and metabolite signals [59, 119, 122–124]. Moreover, while ET indicates being more effective than RT, it shows a high degree of similarity in effectiveness relative to E+R. With the differences between all of the training methods only seen early in training that wane as the duration of training progresses in length. Therefore, indicating that the alteration in acute metabolic responses to the exposure of ET do not occur until later in the duration of training from the inclusion of RT within the exercise training regimen [59, 119, 122–124]. The indication of difference also supports a contention [26] that E+R maybe more effective than either RT or ET alone, as E+R could possibly provide a combination of the stimulus that may induce acute changes similar to ET that do not exist for RT until the exercise duration becomes prolonged. In which E+R may be inducing a metabolic stress that are in fact different from RT or ET, based on the intensity and duration of exercise, an issue that deserves more attention in future research, yet additional speculation falls outside the scope of this analysis.
Nonetheless, the differences indicated here are most likely the results of linking modifications in metabolic state and cardiovascular fitness to the ability to effectively alter inflammatory biomarkers associated with overfatness. As modifications develop through the combination of these cytokines coupled with the difference in the metabolic and mechanical stimulus provided by exercise impacts the overall level of response [8, 23, 112, 125, 126]. Where the trend for effectiveness continues to show agreement with a previous discussion related to the importance of inclusion of RT, while also showing disagreement with recent reviews touting the use of E+R versus either ET or RT [23, 26, 42, 115, 125, 126]. In addition to this agreement, the comparison between the types of exercise show that both RT and E+R are more effective than ET, yet show no difference between each other at the various durations examined. An implication which would mean while seeing differential cardiorespiratory responses absolutely is due to the overall duration of training, the level of effectiveness to elicit the response supports the indication for the importance of the stimulus of training [23]. Yet, just as with the indication of differences in altering aerobic fitness and metabolism the interactions are an issue that needs further investigation.
Notwithstanding the number of questions remaining unanswered regarding the underlying physiological differences between methods of exercise, indications from the findings here allow extrapolation to the designing and developing of exercise programs. Most important is an indication of effectiveness waning as training becomes prolonged, especially when compared to shorter and moderate duration training regimens. Thus, indicating that the differences in absolute values that may support the use of longer duration training programs may simply be the indication of total time being a factor in establishing absolute differences, not for the overall effectiveness. Which is interesting given the desire that exercise becomes a continued pattern of behavior throughout life for improved health [5, 8]. However, if you were to couple the impact that shorter duration training has to overall effectiveness, i.e. utilize intermittent and varying intensities of training through a periodization of the exercise program, with the prolonged use of exercise we may be able to maximize the overall impact that exercise can have within a treatment regimen.
From the perspective of coupling the shorter duration effectiveness over the long duration use of exercise, a series of recommendations are available to establish a periodization of exercise in the treatment of health issues for the individual who is overfat. First, given the peak time for greatest effectiveness, training programs may be best when designed around a blocked-periodized schedule of 4-to-12-week durations, using a schedule of 3 days of RT and 3-4 days ET per 7-day training week. Second, when developing exercise prescriptions RT is highly recommended for use as the principal form of exercise utilized intermittently in shorter durations, e.g., 4 to 8 weeks, or through a concurrent pattern of exercise with ET in both shorter and moderate duration regimens. With the training intensity of RT being most effective when utilizing a level of exercise stimulus associated with muscle hypertrophy (e.g., >75% 1RM at a training volume of 3-sets of 10-repetitions with 60-seconds of rest) [23]. While ET should be rarely implemented alone, and when utilized should be incorporated into a training regimen through a combination of concurrent training with RT for short to moderate durations of training, i.e. 8-to-12 weeks, at the previously indicated [23] levels for greatest effectiveness (e.g., intervals of variable intensities for 30-40 minutes, or continuously at a heart rate intensity >75% HRmax (VO2max) for 40-60 minutes). Yet more investigation into the patterning and programming of periodized exercise for individuals who are overfat is necessary before these recommendations can become definitive, especially related to the impact of the method (e.g., linear, blocked, or undulating) for periodization on physiological responses seen within the mesocycle and the macrocycle development of the periodized training regimen.
Furthermore, understanding the pattern of effectiveness based on the duration of training also allows for the establishment of properly organized and segmented goals, e.g., short-term, moderate-term, and long-term goals. Which is ever important, as there is the given need to have a psychological attraction to, and reward for completing, the individual exercise session that eventually allows for long-term positive adaptations in health status to develop for the individual who is overfat. While it may be true that body compositional changes are a key cheerleader effect to continuation of exercise for individuals who are overfat reliance on body composition early can be a hindrance to comply with using exercise. This may be due to the apparent delay in the onset of effectiveness, at least 8-weeks before CI.95 of ES is always >0.00, and the over-reliance on such modifications, in the short-term, where the inability to meet unrealistic goals may be detrimental to the psychological adherence necessary for continual application of exercise. Thereby, short-term (<8-weeks) goals reflect behavior changes, i.e. finding a “workout partner” or “getting into the gym”, and performance gains, i.e. increase in strength or endurance measures for a given exercise or pattern of activity, more than improvements in either body composition or health status. As these changes in behavior and performance will act as the cheerleader effects necessary early in any behavior intervention that leads to the continuation in the intervention, e.g., exercise, when self-selection for continuation is required. Stemming from these performance goals should be moderate-term (8-12 week) and long-term (i.e. >12-week) goals related to modifications in outcome measures, e.g., changes in body composition, diabetic indices, aerobic fitness, that should be reachable within a beneficial therapeutic effect (i.e. favoring use of treatment). Additionally, intermediate goals should be a reflection of a combination of performance, body compositional and health status modifications that are necessary to ensure the continual use of exercise throughout the lifetime of the individual.
Even with these implications and indications, the ability to formally aver these statements is limited. Namely, the formalization of these implications is limited by the current state of publication bias toward reporting only positive findings along with any study published after the end of inclusion and beginning of analysis. Secondly, limitations to the over-arching similarity of exercise prescription utilized and responses from participants in the various studies included within the analysis here. Thirdly, while the general rule for meta-analysis and regression analysis is to examine random-controlled studies only, a methodological hindrance in blinding subjects to use of exercise studies for human participants exists and therefore studies analyzed here included observational, tracking and peer-grouped controlled studies along with the random-controlled studies. To combat these limitations, analysis was performed based on the assumption of random-effect in all calculations. However, even the use of the assumption for random-effect will not eliminate all limitations to this study and therefore, we must continue to review and analyze findings on a pooled-effect to further ascertain the level of effectiveness that different methods of exercise acts to elicit beneficial effect for individuals who are overfat. Additionally, the implications for training intensities and development of periodization of training for individuals who are overfat has gone completely unanswered, outside of this report and earlier from this author and leaves a large hole in the body of knowledge to fill if we wish to continue to stipulate the use of exercise in the medical treatment of overfatness.
CONCLUSIONS
Analysis of effectiveness based on the duration of exercise training indicates a continuum of effectiveness for both the type of exercise and the overall duration of training. In which exercise does not provide an overall level of beneficial effect (i.e. CI.95 of ES not including 0.00) until the completion of at least 8-weeks of continuous training. And where differences between types of exercise do not become evident until 8-weeks for changes in body morphology and 12-weeks for modifying measures of either cardiorespiratory or metabolic characteristics. As such, it needs to be stressed that for individuals who are overfat even with anecdotal reports for rapid results from the inclusion of exercise, the effectiveness of exercise for eliciting modifications in both body composition and health status may in fact be a delayed. From which it is possible to make distinct clinical recommendations. First, early goals must focus on behavioral changes and performance (e.g., starting the pattern of exercise, making improvements in the within session training intensities) modifications versus clinical (e.g., morphological, or cardiovascular and metabolic fitness) modifications early in treatment. While clinical modification becomes important for goal setting once training has become prolonged. Secondly, since the beneficial effectiveness of exercise wanes over time, where we see differences in effectiveness at very short durations that plateaus as durations become longer that parallels the reduction in the difference of effectiveness between the methods of exercise as the duration of the training reaches prolonged duration periods of continuous training. Specifically, the waning of effectiveness indicates that 32-weeks of continuous training may be a point where adaptations of benefit have been optimally reached. As such, long-term exercise regimens must be periodized so as to maximize the short-term benefits while minimizing the impact that the 32-week plateau has on continued effectiveness and responses to exercise.
When parsed into the distinct methods of exercise, there is an indication of differences between the methods of exercise within these continuums of effectiveness based on duration. In which RT, or E+R, is more effective for eliciting beneficial alterations to measures of body composition (e.g., FM and FFM), DBP, markers of inflammation and T2DM than ET across the various timeframes. With the greatest difference between methods of exercise seen at shorter and moderate duration lengths of training (8-to-24 weeks in duration). While ET is more effective at altering SBP and VO2 than RT or E+R and equally effective for altering BM, only seen at longer duration lengths of training (>24-weeks in duration). Furthermore, even though recently touted for greater effectiveness, E+R appears to have little difference in overall effectiveness related to the changing the measures of interest in comparison to that of either RT or ET based on the duration of training.
Acknowledgements
The author would like to thank GSC, BAC, and CD for there assistance in reviewing studying included in the analysis and the mathematical models established for ascertaining effectiveness of exercise methods presented here.
Supplemental figures
SUPPLEMENTAL FIG. 1.
Summary of the evaluation methods and search engine returns of studies leading to the inclusion and subsequent meta-analysis based on the PRISMA checklist.
SUPPLEMENTAL FIG. 2.
Indicated pooled ES and CI.95 for effective to elicit responses in measures of body morphology (fat-free mass) for the various training duration utilized (indicated by @ and then number of weeks of training) for the exercise stimuli. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
SUPPLEMENTAL FIG. 3.
Indicated pooled ES and CI.95 for effective to elicit responses for altering cytokines (C-reactive protein as CRP, interleukin-6 as IL-6 and tumor necrotic factor-α as TNF-α) associated with health issues related to overfatness based on the various training durations (indicated by @ and then number of weeks of training) for the stimulus from the various modes of exercise. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
SUPPLEMENTAL FIG. 4.
Indicated pooled ES and CI.95 for effectiveness to elicit responses in measures of cardiovascular function, systolic (SBP) and diastolic (DBP) blood pressures, obtained based on the various training durations (indicated by @ and then number of weeks of training) for the stimulus of exercise. Indication of a positive ES shows favor for the use of intervention, while negative ES shows favor toward not utilizing said intervention. ET indicated by ●, RT indicated by ♦, E+R indicated by ■, and All Ex indicated by ▲.
SUPPLEMENTAL TABLE 1.
Studies utilized for analysis of differences in effectiveness for eliciting alternations in measures of interest based on the duration of training and the modality of exercise.
| Study | Exercise & Duration of Intervention | Summary Description of Exercise | Measures of Interest for comparison | |||
|---|---|---|---|---|---|---|
| ≤ 8-weeks | ||||||
| Ahmadi* [27] | ET: 5x’s/wk for 4-wk | ET‡: cycle 40 min/session @ 50-60% VO2max for 5 sessions/wk | Morph, Adip, T2DM | |||
| Ara [28] | RT: 3x’s/wk for 6-wk | RT‡: 1-3 x 3-12 @ Progressive 1RM (range 50-90%) for Squats, Leg Press, Leg Curl/Ext, Hip Flexion w/ 90 s rest @ total expenditure of 220-300 kcal/session | Morph, Ob | |||
| Ballor [29] | RT: 3x’s/wk for 8-wk | RT: 3x10-12 @ 10RM for: Chest Press, Leg Press, Lateral Pull-down, Arm Curl/Ext, Leg Curl/Ext, Calf Raise | Morph | |||
| Boudou [30] | ET: 3x’s/wk for 8-wk | ET: 2-sessions continuous @ 45-min/session @ 75% VO2peak, 1-session interval @ 5x2-min @85% VO2peak, 3-min rest interval @ 50% VO2peak | Morph, Ob, Adip | |||
| Fisher [31] | ET or RT: 3x’s/wk for 8-wk | ET‡: 20-40 min @ 65-80% MHR (progressive) RT‡: 1-2x10 @ 60-80% 1RM (progressive) for Leg Press, Squats, Leg Ext/Curl, Arm Curl, Lateral Pull-down, Bench Press, Military Press, Trunk Exercises |
CRP, Il-6, TNF-a | |||
| Halle [32] | ET: Daily for 4-wk | ET‡: 5-session/wk @30-min/session @ 70% HRmax, 2-sessions/wk general PA @ self-selected intensity & duration | Morph, Ob, Adip | |||
| Hallsworth [33] | ET: 3x’s/wk for 8-wk | RT: 3 set of 8-exercise CRT @ unknown repetition for bicep curl, calf press, triceps press, chest press, hamstring curl, shoulder press, leg extension, lateral pull-down @ 50-70% 1RM (progressive) with unknown rest interval | Morph, T2DM | |||
| Hammer [34] | ET: 5x’s/wk for 6-wk | ET‡: distance of 1.6-4.8 km/session (progressive) @ 60-85% HRM (progressive) | Morph, T2DM | |||
| Hansen* [35] | ET: 3x’s/wk for 8-wk | ET: 55-min/session @ 50%VO2peak on ergometer OR 40-min/session @ 75% VO2peak on ergometer with training equivalent energetics (1.3±0.05 MJ/session) | Morph, VO2, Met S | |||
| Hill [36] | ET: Daily for 5-wk | ET‡: distance of 1.6-5.6 km/session (progressive) @ unknown intensity | Morph | |||
| Ishii [37] | ET: 5x’s/wk for 6-wk | ET‡: 60-min/session @ 50% VO2max (adjusted intensity per week) | Morph, Ob, VO2 | |||
| Kanaley [38] | RT: 3x’s/wk for 6-wk | RT: 3 sets x 8-12 reps @ 80% 1RM (progressive) for chest press, leg press, shoulder press, lateral pull-down, leg extension, leg curl, bicep curl, triceps press-down, abdominal exercise (3x15) | Morph, Ob | |||
| Kempen [39] | ET: 3x’s/wk for 8-wk | ET‡: 90-min group exercise sessions @ 50-60% VO2max | Morph | |||
| Lucotti [40] | ET or E+R: 5x’s/wk for 4-wk | ET‡: 15-min rowing erg/session; 15-min cycle erg/ session @ 70% APHRmax
E+R‡: 45-min/session with RT @1 set x 10 rep (for arm curls, military press, push-ups, upright row, back extension) & 1 set x 20 rep (for squats, knee extensions, heel raises, bent-knee sit-ups) 40-50% 1RM with rest < 60 sec between exercises and ET: 15-min rowing erg/session; 15-min cycle erg/session @ 70% APHRmax |
Morph, Ob, Adip, CRP, TNF-α, SBP, DBP, VO2 | |||
| Maiorana [41] | E+R: 3x’s/wk for 8-wk | E+R: CRT for RT @ 45 s of RT @ 55-65 % MVC (progressive) w/ 15 s rest between RT followed by 5-min ET @ 70-85 % PHR (progressive) intermittent to RT-exercises | Morph, Ob, Adip, VO2 | |||
| Ng [42] [2] | ET or RT: 2-3x’s/wk for 8-wks | ET & RT equated to 3.5 METs per session per exercise ET‡: 50-min/session ergometer @ 65-70% APHRmax (progressive) RT‡: CRT 3-circuits of 1x10 for leg press, leg raises, hamstring curls, bicep curls, triceps extension, anterior shoulder raises, lateral shoulder raises, hip abduction, hip extension @ 65-70% 1RM (progressive), unknown rest |
Morph, SBP, DBP, VO2 | |||
| Oberbach [43] | ET: 4x’s/wk for 4-wks | ET: 3-days: 60-min unknown intensity (20-min calisthenics/20-min steady state/20-min “power-training” & 1-day: 60-min swimming | Morph, Adip, OB, Il-6, CRP | |||
| Tokmakidis* [44] | E+R: 4x’s/wk (2 ET, 2 RT) for 4-wk |
ET: 40-45 min/session treadmill @ 60-80% HRmax (progressive) RT: 3 set x 12 rep @ 60% 1RM for bench press, seated row, leg extension, lateral pull-down, pec-deck, leg curl and 45-60 sec rest per set and 180-240 sec rest per exercise |
Morph, T2DM | |||
| Touvra [45] | E+R: 4x’s/wk for 8-wk | ET: 30 min/session @ 70-80% HRmax
RT: 3 set x 15 rep for leg press, knee extension, abduction, bench press, pec-deck, rows@ 60% 1RM with 60-sec rest between set and 120-sec between exercise |
Morph, CRP, TNF-α, Il-6 | |||
| 9-15 Weeks | ||||||
| Ahmadizad [46] | ET or RT: 3x’s/wk for 12-wk | ET: 75–85% of MHR for 20-30-min (progressive), RT: 4x12 CRT of 11 exercises @ 50–60% 1RM |
Morph, Adip | |||
| Ballor [47] | ET or RT: 3x’s/wk for 12-wk | ET: 50% VO2max x 20-60 min (progressive) RT: 3x8 @ 50-80% 1RM (progressive) Squat, Bench, Leg Ext/Curl, Arm Ext/Curl, Lateral Pulldown |
Morph, SBP, DBP, VO2 | |||
| Bouchard [48] | RT: 3x’s/wk for 12-wk | RT‡: 3x8 @ 80% 1RM for (leg press, chest press, leg extension, shoulder press, sit-up, seated row, triceps extension, arm curl, and calf extension) w/ 60-90 s rest | Morph | |||
| Bryner [49] | ET: 4x’s/wk RT: 3x’s/wk for 12-wk |
ET‡: 20-60 min (progressive) @ self-paced RT‡: 2-4x15-12 @ 15RM-to-8-RM (progressive) for 10-exercise CRT w/ 60-s rest |
Morph | |||
| Bweir [50] | RT: 3x’s/wk for 10-wk | ET: 20-30-min/session (progressive) @ 60-75% HRmax
RT: 3 set x 8-10 rep 7-exercise CRT knee and hip flexion/extension, hip abduction/adduction, elbow flexion/extension, chest press @ unknown intensity with 120-sec rest intervals |
Morph, T2DM | |||
| Christiansen [51] | ET: 3x’s/wk for 12-wk | ET‡: 60-75 min @ unknown intensity to equate to 500- 600 kcal/session | Morph, Ob, Adip, Il-6, TNF-α, SBP, DBP, VO2 | |||
| Donnelly [52] | ET, RT, or E+R: 4x’s/wk for 12-wk | ET‡: 20-60 min (progressive) @ 70% HRR RT‡: 2-3 x 6-8 @ 70-80% 1RM (progressive) on CRT exercises unknown, rest unknown |
Morph, VO2 | |||
| Donnelly [53] | RT: 3x’s/wk for 12-wk | RT‡: 3 sets 8,6,6 @ 70% 1RM, progress to 4 sets 8.6.6.4 @ 80% 1RM for Bench Press, Latissimus Pull-down, Leg Ext/Curl, Shoulder Press, Arm Pullover, Arm Curl/Ext | Morph, VO2 | |||
| Giannopoulou [54] | ET: 3x’s/wk for 14-wk | ET‡: 50-min/session @ 65-70% VO2peak (equated to 250-300 kcal/session) | Morph, VO2 | |||
| Hill [55] | ET: 5x’s/wk for 12-wk | ET‡: 20-50 min (progressive) @ 60-70% HRM | Morph | |||
| Ho [56] | ET, RT, or E+R: 5x’s/wk for 12-wks |
ET‡: 30-min @ 60% HRR RT‡: 4x12 @ 10RM for Leg Press, Leg Curl/Ext, Bench Press, Seated Row w/ 60 s rest E+R‡: ET for 15-min @ 60% HRR & RT for 2x12 @75%1RM |
Morph, Ob, Adip, VO2 | |||
| Jorge [57] | ET, RT, or E+R: 3x’s/wk for 12-wk | 60-min/session: ET: cycle @ LAT RT: unknown volume of CRT for leg press, bench press, lateral pull-down, seated row, shoulder press, abdominal curls, leg flexion @ unknown intensity (%1RM), or rest intervals E+R: cycle @ LAT ½ time & RT @ ½ training volume |
Morph, Adip, CRP, TNF-α, SBP, DBP, VO2 | |||
| Jung [58] | ET: 5x’s/wk for 12-wk | ET: 30-min/session @ >5.3 METs OR 60-min/session @ 3.5-5.2 METs with training equated to 500 kcal/session |
Morph | |||
| Kadoglou [59] | ET: 4x’s/wk for 12-wk | ET: 30-60 min/session (progressive) @ 60-75% HRmax (progressive) | Morph, SBP, DBP, VO2 | |||
| Kang [60] | ET or E+R: 3x’s/wk for 12-wk | ET‡: 60-min/session @ 60% HRR E+R‡: CRT 60-min/session @ 60 % HRR with RT @ 3 set x 12 rep for lateral pull-down, abdominal curls, leg curls, leg extension, bicep curls & ET @ 20-min |
Morph, Adip, CRP, VO2 | |||
| Kempf [61] | ET: 7x’s/wk for 12-wk | ET: 30-min/session of WiiFit Plus program @ self-selected intensity | Morph | |||
| Kerksick [62] | E+R: 3x’s/wk for 14-wk | E+R: @ HR of 60-80% MHR using CRT of 14 exercises either paired: Arm Ext/Curl, Leg Ext/Curl, Shoulder Press/Lateral Pulldown, Hip Abd/Add, Chest Press/Seated Row, Abdominal Crunch/Back Extension, Shoulder Shrug/ Dip; or unpaired: Leg Press, Squat, Pec-Deck, Oblique, Hip Ext, side bends, stepping) x 30 s @ unknown %1RM w/ callisthenic 30 s between sets/paired exercise |
Morph | |||
| Klimcakova [63] | RT: 3x’s/wk for 12-wk | RT‡: 1x12-15 @ 60-70% for 17-exercise CRT | Morph, Ob, Adip, Il-6, TNF-a, CRP, SBP, DBP, VO2 | |||
| Kwon [64] | RT: 3x’s/wk for 12-wk | RT: 3 sets x 10-15 reps (elastic resistance) @ 40-50% 1RM (equivalent) for bicep curl, triceps extension, upright row, shoulder press, chest press, squat/deadlift, hip flexion, leg flexion, leg extension | Morph, T2DM | |||
| Lee [65] | ET: 5x’s/wk for 13-wk | ET: 60-min/session @ 60% VO2peak | Morph, VO2 | |||
| Moreira [66] | ET: 3x’s/wk for 12-wks | ET: 20-60 min (progressive) @ 10% of Anaerobic Threshold Interval ET 20-60 min (progressive) total time @ 2:1 ratio of 120% Anaerobic Threshold to Rest time |
Morph | |||
| Poirier [67] | ET: 3x’s/wk for 12-wk | ET: 30 to 60-min/session (progressive) @ 60% VO2peak | Morph, VO2 | |||
| Polak [68] | ET: 5x’s/wk for 12-wk | ET: 45-min @ 50-65% VO2max (progressive) for 2x’s/wk group exercise class, 3x’s/wk cycle ergometer | Morph, Ob, Adip, Il-6, TNF-α, VO2 | |||
| Racette [69] | ET: 3x’s/wk for 12-wk | ET‡: 35-min @ 65% VO2max | Morph | |||
| Schjerve [70] | ET or RT: 3x’s/wk for 12-wk | ET: Intervals @ 10-min @ 50-60% MHR followed by 4 cycles of 4-min: 3-min ratio of 85-95% MHR then 50-60% MHR followed by 5-min @ 50-60% MHR, or 47-min @60-70% MHR RT: 4x5 @ 90% 1RM (progressive) for Leg Press or Squats, trunk exercises @ 3x30 w/ 30 s rest |
Morph, SBP, DBP, VO2 | |||
| Sigal* [71] | ET, RT, or E+R: 3x’s/wk for 12-wk | ET‡: 15-min/session @60% HRmax & 45-min/session @ 75% HRmax
RT‡: 2-3 sets x 7-9 reps for 7-whole body exercises @ unknown intensity or rest interval E+R: full version of both ET and RT |
Morph, T2DM | |||
| Trapp [72] | ET: 3x’s/wk for 15-wks | ET: Interval cycle ergometer @ 8-sec sprint: 12-sec recover intervals progress from 5-min to 20-min total time or 10-40 min @ 60% VO2peak (progressive) | Morph, Ob, Adip, VO2 | |||
| Wycherley [73] | ET: 4-5x’s/wk for 12-wk | ET‡: 25-60 min/session (progressive) @ 60-80% baseline-HRmax (progressive) | Morph, SBP, DBP, VO2 | |||
| 16-23 weeks | ||||||
| Cauza [74] | RT or ET: 3x’s/wk for 16-wk | ET: 15-90 min/session (progressive @ 5-min/session per week after 4th week) @ 60% VO2max
RT: 1-3 sets (progressive) x 10-15 reps @ 10-15RM (progressive) for bench press, shoulder press, chest flies, lateral pull-down, bicep curls, triceps extension, leg press, calf press, leg extension, and abdominal exercises |
Morph, SBP, DBP, VO2 | |||
| Cuff [75] | ET or E+R: 3x’s/wk for 16-wk | E+R: 75-min @ 60-75% HRR w/ RT@ 2x12 for Leg Press, Leg Curl, Hip Ext, Chest Press, Latissimus Pulldown @ unknown intensity or rest ET: 75 min @ 60-75% HRR |
Morph, VO2 | |||
| De Feyter [76] | E+R: Unknown sessions/wk For 20-wk |
ET: Interval @ 4-8 sets (progressive) x 30-sec or 60- sec (50-60% Wmax) RT: whole body (unknown exercises) @ 2-sets x 10-repetitions @ 50-80% 1 RM (progressive) and unknown rest intervals |
Morph, Adip, CRP, TNF-α, SBP, DBP, VO2 | |||
| Donnelly* [77] | ET: 5x’s/wk for 16-wk | ET: 20-45 min @ 60%-75% HRR for ≈2000 kcal/wk (400 kcal/session) | Morph, VO2 | |||
| Honkola [78] | RT: 3x’s/wk for 20-wk | RT: 2-set x 12-15 rep CRT for 8-10 whole body exercise (unknown exercises) @unknown intensity w/ <60-sec rest | Morph, SBP, DBP | |||
| Ibanez [79] | RT: 2-3x’s/wk for 16-wk | RT‡: 3-4x10-15 @ 50-80% 1RM (progressive) CRT for 8-wks & 3-5x10-12@60-80% or 3-5x 4-6@80-90% alternate for 8-wks | Morph, Ob, Adip, Met S | |||
| Irving [80] | ET: 3-5x’s/wk for 16-wk | ET: unknown time @ RPE of 10-12 equate to 300-400 kcal/session OR unknown time @ RPE of 15-17 to equate to 300-400 kcal/session | Morph, Ob, Adip, SBP, DBP, VO2 | |||
| Josse [81] | E+R: 7x’s/wk ET @ 7x’s RT @ 2x’s for 16-wk |
ET: 7x’s/wk @ total expenditure of 250 kcal unknown duration or intensity RT: 3x10 unknown intensity & rest interval |
Morph, Il-6, TNF-α | |||
| Layman [82] | E+R: 5x’s/wk ET @ 5x’s RT @ 2x’s for 16-wk |
ET‡: 30-min @ unknown intensity RT‡: 1x12 @ unknown resistance intensity for 7 exercise in CRT |
Morph, Ob, Adip | |||
| Marks [83] | E+R or RT: 3x’s/wk for 20-wk | ET‡: 12-36 min (progressive) @ 70-85% HRM RT‡: 2x8-12 @ 70-90% 1RM for: Leg Ext/Curl, Seated Row, Chest Press, Arm Ext/Curl, and abdominal curls, with unknown rest ET&RT: 12-24 min of ET and 1 set of RT |
Morph | |||
| Rice [84] | ET: 5x’s/wk RT: 3x’s/wk for 16-wk |
ET‡: 20-60 min @ 50-85% MHR (progressive) RT‡: 1x8-12 @ 8-12RM (progressive) for Leg Ext/Curl, Latissimus pull-over, Bench Press, Should Press, Arm Ext/Curl |
Morph, T2DM | |||
| Snel [85] | ET: 5x’s/wk for 16-wk | ET‡: @70% VO2max with 4 sessions @ 30-min/session cyclergometer & 1 session @ 60-min/session (aerobic exercise session) | Morph, CRP, SBP, DBP, VO2 | |||
| Tjønna+ [86] | ET: 3x’s/wk for 16-wk | Interval ET: 10-min @ 70% MHR followed by 4-cyles of 4-min: 3-min @ 90% MHR and 70% MHR, then 5-min @ 50-60% MHR ET: 47-min @ 70% MHR |
Morph, Adip, SBP, DBP, VO2 | |||
| Tokmakidis* [44] | E+R: 4x’s/wk (2 ET, 2 RT) for 16-wk |
ET: 40-45 min/session treadmill @ 60-80% HRmax (progressive) RT: 3 set x 12 rep @ 60% 1RM for bench press, seated row, leg extension, lateral pull-down, pec-deck, leg curl and 45-60 sec rest per set and 180-240 sec rest per exercise |
Morph, T2DM | |||
| Toledo [87] | ET: 6x’s/wk for 20-wk | ET‡: 30-40 min/session (progressive) @ 60-70% HRmax | Morph, VO2 | |||
| Wycherley [88] | RT: 3x’s/wk for 16-wk | RT‡: 2x8-12@70-85% 1RM for Leg Press, Leg Ext, Chest Press, Latissimus Pull-down, Seated Row, Arm Ext w 60 s rest | Morph, CRP, SBP, DBP, VO2 | |||
| 24-36 weeks | ||||||
| Borg* [89] | ET: 3x’s/wk for 24-wk | ET‡: 45 min @ 60-70% VO2max
RT‡: 3x8 @ 60-80% 1RM CRT |
Morph, T2DM | |||
| Brochu [90] | RT: 3x’s/wk for 24-wk | RT‡: 3-4 x 8-12 @ 65-80% 1RM (progressive) for (Leg Press, Chest Press, Lateral Pulldown, Shoulder Press, Arm Curl/Ext) w/ 60-90 s rest | Morph, CRP, SBP, DBP | |||
| Carr* [91] | ET: 3x’s/wk for 24-wk | ET‡: 60-min/session @ 70% HRR | Morph, VO2 | |||
| Church [92] | ET, RT, or E+R: 3x’s/wk for 36-wk | ET: 50-min/session @ 50-80% VO2max equated to 12 kcal/kg body mass per wk RT: 2 set x 10-12 rep for bench press, seated row, shoulder press, lateral pull-down; 3 set x 10-12 rep for leg press, leg extension, leg flexion @ 12 RM E+R: Same as ET (limited to 10 kcal/kg per wk) and RT (limited to 1 set for all exercises) |
Morph, T2DM | |||
| Dobrosielski [93] | E+R: 3x’s/wk for 26-wk | ET: 45-min/session @ 60-90% HRmax
RT: 2-sets x 10-15 reps for 7 exercises (unknown whole body) @ 50% 1RM |
Morph, SBP, DBP, VO2 | |||
| Dobrosielski+ [94] | E+R: 3x’s/wk for 26-wk | ET: unspecified RT: unspecified Both in accordance with American College Sports Medicine (ACSM) guidelines |
Morph, VO2 | |||
| Donnelly* [77] | ET: 5x’s/wk for 36-wk | ET: 20-45 min @ 60%-75% HRR for 1st 24-wks then 55%-70% of HRM (progressive) for ≈2000 kcal/wk (400 kcal/session) | Morph, VO2 | |||
| Dunstan [95] | RT: 3-4x’s/ wk for 24-wk | RT‡: 3x8-10 @ 50-85% 1RM (progressive) for Bench Press, Leg Ext/Curl, Upright Row, Lateral Pull-down, Shoulder Press, Arm Curl/Ext, Abdominal exercises | Morph | |||
| Hansen* [35] | ET: 3x’s/wk for 24-wk | Equivalent energetics (1.3±0.05 MJ/session) ET: Low: 55-min/session @ 50%VO2peak on ergometer; High: 40-min/session @ 75% VO2peak on ergometer |
Morph, VO2 | |||
| Karstoft [96] | ET: 5x’s/wk for 24-wk | ET @ equated energetic demand for 60-min/session @ 55% VO2peak, or Interval @ 3-min intervals for 60-min/ session @ 1:1 ratio of high (>70% VO2peak) and low (self-paced <70% VO2peak) | Morph, SBP, DBP, VO2 | |||
| Ryan [97] | RT or E+R: 3x’s/wk for 24-wk | ET‡: 45-min @ 50-75% HRR (progressive) RT‡: variable resistance for 15-rep (3RM to 15 RM) 2-3 sets for Leg Press, Chest Press, Chest Flies, Latissimus Pull-down, Leg Curl/Ext, Arm Curl/Ext w/ 30 s rest |
Morph | |||
| Sigal* [71] | ET, RT, or E+R: 3x’s/wk for 24-wk | ET‡: 15-min/session @60% HRmax & 45-min/session @ 75% HRmax
RT‡: 2-3 sets x 7-9 reps for 7-whole body exercises @ unknown intensity or rest interval E+R‡: full version of both ET and RT |
Morph, T2DM | |||
| Volpe [98] | ET: 3x’s/wk for 36-wk | ET‡: 15-30 min for 3-5 x’s/wk (progressive) @ unknown intensity via ski-ergometer | Morph, Ob, SBP, DBP, VO2 | |||
| Watkins [99] | ET: 3-4x’s/ wk for 26-wk | ET‡: 30-35 min @ 70-80% HRR | Morph, SBP, DBP, VO2 | |||
| >36 weeks | ||||||
| Albu [100] | ET: Unknown for 52-wk | ET‡: general PA @ >175 min/wk of unknown intensity | Morph, T2DM | |||
| Anderssen [101] | ET: 3x’s/wk for 52-wk | ET‡: 60–80% of PHR for 60-min | Morph, Ob, Adip, VO2 | |||
| Balducci+ [102] | ET or E+R: 2x’s/wk for 52-wk | Equal energetics for ET & E+R progressive ET: unknown duration or intensity OR 60-min @ 70- 80% VO2max E+R: 40-min ET @ 70-80% VO2max, RT of 4 exercises (chest press, lateral pull-down, squat, abdominal exercise) @ 80% 1 RM with unknown set x rep and rest interval |
Morph, Ob, Adip, Il-6, TNF-α, CRP, SBP, DBP, VO2, T2DM | |||
| Balducci+ [103] | E+R: 2x’s/ wk for 52-wk | E+R progressive ET‡: unknown duration, unknown intensity RT‡: 4 exercises (chest press, lateral pull-down, squat, abdominal exercises) @ unknown intensity, rep or set count and rest intervals |
Morph, CRP, SBP, DBP, VO2, T2DM | |||
| Balducci+ [104] | E+R: 2-3x’s/ wk for 52-wk | E+R progressive ET: 55-70% VO2max on ergometer (stair, treadmill, cycle) for unknown duration/session RT: 3 sets x 10 reps of 4 exercises (chest press, lateral pull-down, squat, abdominal exercises) @ 60-80% 1RM with unknown rest |
Morph, CRP, SBP, DBP, VO2, T2DM | |||
| Balducci+ [105] | E+R: 2-3x’s/ wk for 52-wk | E+R progressive ET: unknown duration RT: 4 exercises (chest press, lateral pull-down, squat, abdominal exercises) @ unknown volume and rest interval E+R: Low @ 55% VO2max & 60% 1RM High @ 70% VO2max & 60% 1RM |
Morph, CRP, SBP, DBP, VO2, T2DM | |||
| Borg* [89] | ET: 3x’s/wk for 40-wk | ET‡: 45 min @ 60-70% VO2max
RT‡: 3x8 @ 60-80% 1RM CRT |
Morph, T2DM | |||
| Carr* [91] | ET: 3x’s/wk for 104-wk | ET‡: 60-min/session @ 70% HRR | Morph, VO2, T2DM | |||
| Donnelly* [77] | ET: 5x’s/wk for 52-wk and 68-wk | ET: 20-45 min @ 60%-75% HRR for 1st 24-wks then 55%-70% of HRM (progressive) for ≈2000 kcal/wk (400 kcal/session) | Morph, VO2 | |||
| Hawkins [106] | ET: 6x’s/wk for 52-wk | ET: 60 min/session @ 60-85% HRmax either on ergometer or via community walking | Morph, VO2 | |||
| Heufelder [107] | E+R: 3x’s/wk for 52-wk | ET‡: 30 min/session @ unspecified intensity RT‡: unspecified |
Adip, CRP, SBP, DBP | |||
| Olson [108] | RT: 2x’s/wk for 52-wk | RT: 3x8–10 @ 8-10RM (Progressive) for unknown exercises indicated as isotonic variable resistance machines and free weights targeting the following major muscle groups: quadriceps, hamstrings, gluteals, pectorals, latissimus dorsi, rhomboids, deltoids, biceps and triceps | Morph, Adip, Il-6, CRP, SBP, DBP, T2DM | |||
| Pritchard [109] | ET: 5x’s/wk for 52-wk | ET: 30-45 min @ 65-75% HRM | Morph, T2DM | |||
| Rokling-Andersen [110] | ET: 3x’s/wk for 52-wk | ET‡: 60-min/session of self-selected “aerobic” exercise or running @ unknown intensity | Morph, Adip, CRP, TNF-α, Il-6 | |||
| Yavari [111] | ET, RT, or E+R: 3x’s/wk for 52-wk | ET: 20-60 min/session (progressive) @ 60-70% HRmax (progressive) on treadmill or cyclergometer RT: 3 set x 8-10 rep for bench press, seated row, shoulder press, lateral pull-down, abdominal crunch, leg press, leg extension, triceps pushdown, seated bicep curl @ 60-80% 1RM (progressive) with 90-120 sec rest intervals E+R: ET @ 20-30 min/session @ 60-70% HRmax (progressive) on treadmill or cyclergometer & RT @ 2 set x 8-10 rep for bench press, seated row, shoulder press, lateral pull-down, abdominal crunch, leg press, leg extension, triceps pushdown, seated bicep curl @ 60-80% 1RM (progressive) with 90-120 sec rest intervals |
Morph, SBP, DBP, VO2, T2DM | |||
Note: Morph indicates any measure of body morphology/composition (e.g., body mass, fat mass or fat-free mass) and T2DM (e.g., fasting levels of glucose, insulin or HbA1c); and D indicates a dietary component to the intervention. All other abbreviations are noted within the list of abbreviations and generally agreed upon abbreviations for hormones.
Indicates study has data for analysis at multiple time points.
Indicates some results mirror previous reports from author and only utilized once within a time period for analysis.
Indicates a hypocaloric, or diabetic/low carbohydrate, dietary intervention used in conjunction with exercise.
Funding Source
None
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Clark JE. An overview of the contribution of fatness and fitness factors, and the role of exercise, in the formation of health status for individuals who are overweight. J Diabetes Metab Disord. 2012;11:19. doi: 10.1186/2251-6581-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark JE, Goon DT. The roles of resistance training for treatment of obesity related health issue and for changing helath status of the individual who is overfat or obese: A review. J Sports Med Phys Fit. 2015;55:205–22. [PubMed] [Google Scholar]
- 3.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 4.Foster-Schubert KE, Cummings DE. Emerging therapeutic strategies for obesity. Endocr Rev. 2006;27:779–93. doi: 10.1210/er.2006-0041. [DOI] [PubMed] [Google Scholar]
- 5.Fogelholm M. How physical activity can work? Int J Pediatr Obes. 2008;3(Suppl 1):10–4. doi: 10.1080/17477160801896481. [DOI] [PubMed] [Google Scholar]
- 6.Gebel E. The science of sweat. Is exercise the best medicine? Diabetes Forecast. 2010;63:47–51. [PubMed] [Google Scholar]
- 7.Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, et al. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33:2145–56. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hainer V, Toplak H, Mitrakou A. Treatment modalities of obesity: what fits whom? Diabetes Care. 2008;31(Suppl 2):S269–77. doi: 10.2337/dc08-s265. [DOI] [PubMed] [Google Scholar]
- 10.Matheson EM, King DE, Everett CJ. Healthy lifestyle habits and mortality in overweight and obese individuals. J Am Board Fam Med. 2012;25:9–15. doi: 10.3122/jabfm.2012.01.110164. [DOI] [PubMed] [Google Scholar]
- 11.Praet SFE, van Loon LJC. Exercise therapy in type 2 diabetes. Acta Diabetol. 2009;46:263–78. doi: 10.1007/s00592-009-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin L, Knol MJ, Corpeleijn E, Stolk RP. Does physical activity modify the risk of obesity for type 2 diabetes: a review of epidemiological data. Eur J Epidemiol. 2010;25:5–12. doi: 10.1007/s10654-009-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson JG. Exercise and the treatment of type 2 diabetes mellitus. An update. Sports Med. 1999;27:381–91. doi: 10.2165/00007256-199927060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Clark JE. Role of resistance training in obesity treatments. In: Ther JOWL, editor. 1st International Conference on Obesity. Philadelphia, PA: OMICS; 2012. [Google Scholar]
- 15.Gilson TA, Chow GM, Ewing ME. Using goal orientations to understand motivation in strength training. J Strength Cond Res. 2008;22:1169–75. doi: 10.1519/JSC.0b013e318173c566. [DOI] [PubMed] [Google Scholar]
- 16.Duncan DT, Wolin KY, Scharoun-Lee M, Ding EL, Warner ET, Bennett GG. Does perception equal reality? Weight misperception in relation to weight-related attitudes and behaviors among overweight and obese US adults. International Journal of Behavioral Nutrition and Physical Activity. 2011;8:20. doi: 10.1186/1479-5868-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011;10:9. doi: 10.1186/1475-2891-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hafekost K, Lawrence D, Mitrou F, O’sullivan TA, Zubrick SR. Tackling overweight and obesity: does the public health message match the science? BMC Med. 2013;11:41. doi: 10.1186/1741-7015-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall KD. Diet versus exercise in „the biggest loser” weight loss competition. Obesity. 2013;21:957–9. doi: 10.1002/oby.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise’s complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol (Lond) 2009;587:5527–39. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36:674–88. doi: 10.1249/01.mss.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- 22.Bird SP, Tarpenning KM, Marino FE. Designing resistance training programmes to enhance muscular fitness: a review of the acute programme variables. Sports Med. 2005;35:841–51. doi: 10.2165/00007256-200535100-00002. [DOI] [PubMed] [Google Scholar]
- 23.Clark JE. Diet, exercise or diet with exercise: comparing the effectiveness of treatment options for weight-loss and changes in fitness for adults (18–65 years old) who are overfat, or obese; systematic review and meta-analysis. J Diabetes Metab Disord. 2015;14:31. doi: 10.1186/s40200-015-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SL, Hyde J, Karunaratne A, Kausman R, Komesaroff PA. „They all work…when you stick to them”: a qualitative investigation of dieting, weight loss, and physical exercise, in obese individuals. Nutr J. 2008;7:34. doi: 10.1186/1475-2891-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barakat A, Williams KM, Prevost AT, Kinmonth A-L, Wareham NJ, Griffin SJ, et al. Changes in physical activity and modelled cardiovascular risk following diagnosis of diabetes: 1-year results from the ADDITION-Cambridge trial cohort. Diabetic Med. 2013;30:233–8. doi: 10.1111/j.1464-5491.2012.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/ obese subjects: a systematic review and network meta-analysis. PLoS One. 2013;8:e82853. doi: 10.1371/journal.pone.0082853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadi I, Shalmzary HQ. Effect Aerobic Training in Hypertension and Blood Glucose Middle Age People Given To Hypertension and Type II Diabetes. International Conference on Humanities, Geography and Economics. 2011 Dec;:280–3. [Google Scholar]
- 28.Ara I, Perez-Gomez J, Vicente-Rodriguez G, Chavarren J, Dorado C, Calbet JAL. Serum free testosterone, leptin and soluble leptin receptor changes in a 6-week strength-training programme. Br J Nutr. 2006;96:1053–9. doi: 10.1017/bjn20061956. [DOI] [PubMed] [Google Scholar]
- 29.Ballor DL, Katch VL, Becque MD, Marks CR. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am J Clin Nutr. 1988;47:19–25. doi: 10.1093/ajcn/47.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier J-F. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. Euro J Endocrinol. 2003;149:421–4. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 31.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity. 2011;19:1131–6. doi: 10.1038/oby.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halle M, Berg A, Garwers U, Grathwohl D, Knisel W, Keul J. Concurrent reductions of serum leptin and lipids during weight loss in obese men with type II diabetes. Am J Physiol. 1999;277:E277–82. doi: 10.1152/ajpendo.1999.277.2.E277. [DOI] [PubMed] [Google Scholar]
- 33.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–83. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammer RL, Barrier CA, Roundy ES, Bradford JM, Fisher AG. Calorie-restricted low-fat diet and exercise in obese women. Am J Clin Nutr. 1989;49:77–85. doi: 10.1093/ajcn/49.1.77. [DOI] [PubMed] [Google Scholar]
- 35.Hansen D, Dendale P, Jonkers RAM, Beelen M, Manders RJF, Corluy L, et al. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia. 2009;52:1789–97. doi: 10.1007/s00125-009-1354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JO, Sparling PB, Shields TW, Heller PA. Effects of exercise and food restriction on body composition and metabolic rate in obese women. Am J Clin Nutr. 1987;46:622–30. doi: 10.1093/ajcn/46.4.622. [DOI] [PubMed] [Google Scholar]
- 37.Ishii T, Yamakita T, Yamagami K, Yamamoto T, Miyamoto M, Kawasaki K, et al. Effect of exercise training on serum leptin levels in type 2 diabetic patients. Metab Clin Exp. 2001;50:1136–40. doi: 10.1053/meta.2001.26745. [DOI] [PubMed] [Google Scholar]
- 38.Kanaley JA, Fenicchia LM, Miller CS, Ploutz-Synder LL, Weinstock RS, Carhart R, et al. Resting leptin responses to acute and chronic resistance training in type 2 diabetic men and women. Int J Obes Relat Metab Disord. 2001;25:1474–80. doi: 10.1038/sj.ijo.0801797. [DOI] [PubMed] [Google Scholar]
- 39.Kempen KP, Saris WH, Westerterp KR. Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women. Am J Clin Nutr. 1995;62:722–9. doi: 10.1093/ajcn/62.4.722. [DOI] [PubMed] [Google Scholar]
- 40.Lucotti P, Monti LD, Setola E, Galluccio E, Gatti R, Bosi E, et al. Aerobic and resistance training effects compared to aerobic training alone in obese type 2 diabetic patients on diet treatment. Diabetes Res Clin Pract. 2011;94:395–403. doi: 10.1016/j.diabres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56:115–23. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- 42.Ng CLW, Goh S-Y, Malhotra R, Østbye T, Tai ES. Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: a randomised trial. J Physiother. 2010;56:163–70. doi: 10.1016/s1836-9553(10)70021-7. [DOI] [PubMed] [Google Scholar]
- 43.Oberbach A, Tönjes A, Klöting N, Fasshauer M, Kratzsch J, Busse MW, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Euro J Endocrinol. 2006;154:577–85. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 44.Tokmakidis SP, Zois CE, Volaklis KA, Kotsa K, Touvra A-M. The effects of a combined strength and aerobic exercise program on glucose control and insulin action in women with type 2 diabetes. Euro J Appl Physiol. 2004;92:437–42. doi: 10.1007/s00421-004-1174-6. [DOI] [PubMed] [Google Scholar]
- 45.Touvra A-M, Volaklis KA, Spassis AT, Zois CE, Douda HD, Kotsa K, et al. Combined strength and aerobic training increases transforming growth factor-β1 in patients with type 2 diabetes. Hormones. 2011;10:125–30. doi: 10.14310/horm.2002.1302. [DOI] [PubMed] [Google Scholar]
- 46.Ahmadizad S, Haghighi AH, Hamedinia MR. Effects of resistance versus endurance training on serum adiponectin and insulin resistance index. Eur J Endocrinol. 2007;157:625–31. doi: 10.1530/EJE-07-0223. [DOI] [PubMed] [Google Scholar]
- 47.Ballor DL, Harvey-Berino JR, Ades PA, Cryan J, Calles-Escandon J. Contrasting effects of resistance and aerobic training on body composition and metabolism after diet-induced weight loss. Metab Clin Exp. 1996;45:179–83. doi: 10.1016/s0026-0495(96)90050-5. [DOI] [PubMed] [Google Scholar]
- 48.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16:66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- 49.Bryner RW, Ullrich IH, Sauers J, Donley D, Hornsby G, Kolar M, et al. Effects of resistance vs. aerobic training combined with an 800 calorie liquid diet on lean body mass and resting metabolic rate. J Am Coll Nutr. 1999;18:115–21. doi: 10.1080/07315724.1999.10718838. [DOI] [PubMed] [Google Scholar]
- 50.Bweir S, Al-Jarrah M, Almalty A-M, Maayah M, Smirnova IV, Novikova L, et al. Resistance exercise training lowers HbA1c more than aerobic training in adults with type 2 diabetes. Diabetol Metab Syndr. 2009;1:27. doi: 10.1186/1758-5996-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298:E824–31. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 52.Donnelly JE, Pronk NP, Jacobsen DJ, Pronk SJ, Jakicic JM. Effects of a very-low-calorie diet and physical-training regimens on body composition and resting metabolic rate in obese females. Am J Clin Nutr. 1991;54:56–61. doi: 10.1093/ajcn/54.1.56. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly JE, Sharp T, Houmard J, Carlson MG, Hill JO, Whatley JE, et al. Muscle hypertrophy with large-scale weight loss and resistance training. Am J Clin Nutr. 1993;58:561–5. doi: 10.1093/ajcn/58.4.561. [DOI] [PubMed] [Google Scholar]
- 54.Giannopoulou I, Ploutz-Snyder LL, Carhart R, Weinstock RS, Fernhall B, Goulopoulou S, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1511–8. doi: 10.1210/jc.2004-1782. [DOI] [PubMed] [Google Scholar]
- 55.Hill JO, Schlundt DG, Sbrocco T, Sharp T, Pope-Cordle J, Stetson B, et al. Evaluation of an alternating-calorie diet with and without exercise in the treatment of obesity. Am J Clin Nutr. 1989;50:248–54. doi: 10.1093/ajcn/50.2.248. [DOI] [PubMed] [Google Scholar]
- 56.Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. doi: 10.1186/1471-2458-12-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jorge MLMP, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz ALD, et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60:1244–52. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Jung JY, Han KA, Ahn HJ, Kwon HR, Lee JH, Park KS, et al. Effects of aerobic exercise intensity on abdominal and thigh adipose tissue and skeletal muscle attenuation in overweight women with type 2 diabetes mellitus. Diabetes Metab J. 2012;36:211–21. doi: 10.4093/dmj.2012.36.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadoglou NPE, Vrabas IS, Kapelouzou A, Lampropoulos S, Sailer N, Kostakis A, et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit. 2012;18:CR290–5. doi: 10.12659/MSM.882734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang S, Woo JH, Shin KO, Kim D, Lee H-J, Kim YJ, et al. Circuit resistance exercise improves glycemic control and adipokines in females with type 2 diabetes mellitus. J Sports Sci Med. 2009;8:682–8. [PMC free article] [PubMed] [Google Scholar]
- 61.Kempf K, Martin S. Autonomous exercise game use improves metabolic control and quality of life in type 2 diabetes patients - a randomized controlled trial. BMC Endocr Disord. 2013;13:57. doi: 10.1186/1472-6823-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerksick C, Thomas A, Campbell B, Taylor L, Wilborn C, Marcello B, et al. Effects of a popular exercise and weight loss program on weight loss, body composition, energy expenditure and health in obese women. Nutr Metab. 2009;6:23. doi: 10.1186/1743-7075-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klimcakova E, Polak J, Moro C, Hejnova J, Majercik M, Viguerie N, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–12. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 64.Kwon HR, Han KA, Ku YH, Ahn HJ, Koo B-K, Kim HC, et al. The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean Diabetes J. 2010;34:101–10. doi: 10.4093/kdj.2010.34.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee S, Kuk JL, Davidson LE, Hudson R, Kilpatrick K, Graham TE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol. 2005;99:1220–5. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 66.Moreira MM, Souza HPCd, Schwingel PA, Sá CKCd, Zoppi CC. Effects of aerobic and anaerobic exercise on cardiac risk variables in overweight adults. Arq Bras Cardiol. 2008;91:200–6. doi: 10.1590/s0066-782x2008001600003. 19-26. [DOI] [PubMed] [Google Scholar]
- 67.Poirier P, Tremblay A, Broderick T, Catellier C, Tancrède G, Nadeau A. Impact of moderate aerobic exercise training on insulin sensitivity in type 2 diabetic men treated with oral hypoglycemic agents: is insulin sensitivity enhanced only in nonobese subjects? Med Sci Monit. 2002;8:CR59. [PubMed] [Google Scholar]
- 68.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metab Clin Exp. 2006;55:1375–81. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Racette SB, Schoeller DA, Kushner RF, Neil KM. Exercise enhances dietary compliance during moderate energy restriction in obese women. Am J Clin Nutr. 1995;62:345–9. doi: 10.1093/ajcn/62.2.345. [DOI] [PubMed] [Google Scholar]
- 70.Schjerve IE, Tyldum GA, Tjønna AE, Stølen T, Loennechen JP, Hansen HEM, et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin Sci. 2008;115:283–93. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- 71.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 72.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes. 2008;32:684–91. doi: 10.1038/sj.ijo.0803781. [DOI] [PubMed] [Google Scholar]
- 73.Wycherley TP, Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Effect of caloric restriction with and without exercise training on oxidative stress and endothelial function in obese subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10:1062–73. doi: 10.1111/j.1463-1326.2008.00863.x. [DOI] [PubMed] [Google Scholar]
- 74.Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehab. 2005;86:1527–33. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–82. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 76.De Feyter HM, Praet SF, van den Broek NM, Kuipers H, Stehouwer CD, Nicolay K, et al. Exercise training improves glycemic control in long-standing insulin-treated type 2 diabetic patients. Diabetes Care. 2007;30:2511–3. doi: 10.2337/dc07-0183. [DOI] [PubMed] [Google Scholar]
- 77.Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–50. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 78.Honkola A, Forsén T, Eriksson J. Resistance training improves the metabolic profile in individuals with type 2 diabetes. Acta Diabetol. 1997;34:245–8. doi: 10.1007/s005920050082. [DOI] [PubMed] [Google Scholar]
- 79.Ibáñez J, Izquierdo M, Martínez-Labari C, Ortega F, Grijalba A, Forga L, et al. Resistance training improves cardiovascular risk factors in obese women despite a significative decrease in serum adiponectin levels. Obesity. 2010;18:535–41. doi: 10.1038/oby.2009.277. [DOI] [PubMed] [Google Scholar]
- 80.Irving BA, Weltman JY, Patrie JT, Davis CK, Brock DW, Swift D, et al. Effects of exercise training intensity on nocturnal growth hormone secretion in obese adults with the metabolic syndrome. J Clin Endocrinol Metab. 2009;94:1979–86. doi: 10.1210/jc.2008-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J Nutr. 2011;141:1626–34. doi: 10.3945/jn.111.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–10. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 83.Marks BL, Ward A, Morris DH, Castellani J, Rippe JM. Fat-free mass is maintained in women following a moderate diet and exercise program. Med Sci Sports Exerc. 1995;27:1243–51. [PubMed] [Google Scholar]
- 84.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–91. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 85.Snel M, Gastaldelli A, Ouwens DM, Hesselink MKC, Schaart G, Buzzigoli E, et al. Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2012;97:2512–20. doi: 10.1210/jc.2011-3178. [DOI] [PubMed] [Google Scholar]
- 86.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–54. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toledo FGS, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–7. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 88.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care. 2010;33:969–76. doi: 10.2337/dc09-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borg P, Kukkonen-Harjula K, Fogelholm M, Pasanen M. Effects of walking or resistance training on weight loss maintenance in obese, middle-aged men: a randomized trial. Int J Obes Relat Metab Disord. 2002;26:676–83. doi: 10.1038/sj.ijo.0801962. [DOI] [PubMed] [Google Scholar]
- 90.Brochu M, Malita MF, Messier V, Doucet E, Strychar I, Lavoie J-M, et al. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. Journal Clin Endocrinol Metab. 2009;94:3226–33. doi: 10.1210/jc.2008-2706. [DOI] [PubMed] [Google Scholar]
- 91.Carr DB, Utzschneider KM, Boyko EJ, Asberry PJ, Hull RL, Kodama K, et al. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not beta-cell function. Diabetes. 2005;54:340–7. doi: 10.2337/diabetes.54.2.340. [DOI] [PubMed] [Google Scholar]
- 92.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. J Am Med Assoc. 2010;304:2253–62. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobrosielski DA, Gibbs BB, Ouyang P, Bonekamp S, Clark JM, Wang N-Y, et al. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med. 2012;27:1453–9. doi: 10.1007/s11606-012-2103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dobrosielski DA, Barone Gibbs B, Chaudhari S, Ouyang P, Silber HA, Stewart KJ. Effect of exercise on abdominal fat loss in men and women with and without type 2 diabetes. BMJ Open. 2013;3:e003897. doi: 10.1136/bmjopen-2013-003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–36. doi: 10.2337/diacare.25.10.1729. [DOI] [PubMed] [Google Scholar]
- 96.Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36:228–36. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1066–71. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 98.Volpe SL, Kobusingye H, Bailur S, Stanek E. Effect of diet and exercise on body composition, energy intake and leptin levels in overweight women and men. J Am Coll Nutr. 2008;27:195–208. doi: 10.1080/07315724.2008.10719691. [DOI] [PubMed] [Google Scholar]
- 99.Watkins LL, Sherwood A, Feinglos M, Hinderliter A, Babyak M, Gullette E, et al. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Arch Intern Med. 2003;163:1889–95. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- 100.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, et al. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes. 2010;59:627–33. doi: 10.2337/db09-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 2007;17:687–95. doi: 10.1111/j.1600-0838.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 102.Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr Metab Cardiovasc Dis. 2009;20:608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 103.Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 104.Balducci S, Zanuso S, Cardelli P, Salvi L, Bazuro A, Pugliese L, et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES) PLoS One. 2012;7:e49297. doi: 10.1371/journal.pone.0049297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balducci S, Zanuso S, Cardelli P, Salerno G, Fallucca S, Nicolucci A, et al. Supervised exercise training counterbalances the adverse effects of insulin therapy in overweight/obese subjects with type 2 diabetes. Diabetes Care. 2012;35:39–41. doi: 10.2337/dc11-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hawkins VN, Foster-Schubert K, Chubak J, Sorensen B, Ulrich CM, Stancyzk FZ, et al. Effect of exercise on serum sex hormones in men: a 12-month randomized clinical trial. Med Sci Sports Exerc. 2008;40:223–33. doi: 10.1249/mss.0b013e31815bbba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 108.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes. 2007;31:996–1003. doi: 10.1038/sj.ijo.0803534. [DOI] [PubMed] [Google Scholar]
- 109.Pritchard JE, Nowson CA, Wark JD. A worksite program for overweight middle-aged men achieves lesser weight loss with exercise than with dietary change. J Am Diet Assoc. 1997;97:37–42. doi: 10.1016/S0002-8223(97)00015-1. [DOI] [PubMed] [Google Scholar]
- 110.Rokling-Andersen MH, Reseland JE, Veierød MB, Anderssen SA, Jacobs DR, Urdal P, et al. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr. 2007;86:1293–301. doi: 10.1093/ajcn/86.5.1293. [DOI] [PubMed] [Google Scholar]
- 111.Yavari A, Najafipoor F, Aliasgarzadeh A, Niafar M, Mobasseri M. Effect of aerobic exercise, resistance training or combined trainingin on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport. 2012;29:135. [Google Scholar]
- 112.Clark JE. Does the type of intervention method really matter for combating childhood obesity? A systematci review and meta-analysis. J Sports Med Phys Fit. 2015;55:1524–43. [PubMed] [Google Scholar]
- 113.Crespi CM, Wong WK, Mishra SI. Using second-order generalized estimating equations to model heterogeneous intraclass correlation in cluster-randomized trials. Stat Med. 2009;28:814–27. doi: 10.1002/sim.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weeks DL. Exercise interventions for diabetes control: do we really know that strength training is better than endurance training? Arch Phys Med Rehab. 2007;88:397. doi: 10.1016/j.apmr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 115.Hills AP, Shultz SP, Soares MJ, Byrne NM, Hunter GR, King NA, et al. Resistance training for obese, type 2 diabetic adults: a review of the evidence. Obes Rev. 2010;11:740–9. doi: 10.1111/j.1467-789X.2009.00692.x. [DOI] [PubMed] [Google Scholar]
- 116.Grandy S, Fox KM, Hardy E, Group SS. Association of Weight Loss and Medication Adherence Among Adults With Type 2 Diabetes Mellitus: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes) Current Therapeutic Research. 2013;75:77–82. doi: 10.1016/j.curtheres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Latouche C, Jowett JBM, Carey AL, Bertovic DA, Owen N, Dunstan DW, et al. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol. 2013;114:453–60. doi: 10.1152/japplphysiol.00978.2012. [DOI] [PubMed] [Google Scholar]
- 118.Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013;1281:141–59. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ribisl PM, Gaussoin SA, Lang W, Bahnson J, Connelly SA, Horton ES, et al. Lifestyle intervention improves heart rate recovery from exercise in adults with type 2 diabetes: results from the Look AHEAD study. J Obes. 2012;2012:309196. doi: 10.1155/2012/309196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. doi: 10.1111/j.1467-789X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 121.Kozey Keadle S, Lyden K, Staudenmayer J, Hickey A, Viskochil R, Braun B, et al. The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors. Appl Physiol Nutr Metab. 2014;39:770–80. doi: 10.1139/apnm-2013-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samjoo IA, Safdar A, Hamadeh MJ, Raha S, Tarnopolsky MA. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutr Diabetes. 2013;3:e88. doi: 10.1038/nutd.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carey DG. Quantifying differences in the „fat burning” zone and the aerobic zone: implications for training. J Strength Cond Res. 2009;23:2090–5. doi: 10.1519/JSC.0b013e3181bac5c5. [DOI] [PubMed] [Google Scholar]
- 124.Sakurai T, Ogasawara J, Kizaki T, Sato S, Ishibashi Y, Takahashi M, et al. The Effects of Exercise Training on Obesity-Induced Dysregulated Expression of Adipokines in White Adipose Tissue. Int J Endocrinol. 2013;2013:801743. doi: 10.1155/2013/801743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Engler D. Hypothesis: Musculin is a hormone secreted by skeletal muscle, the body’s largest endocrine organ. Evidence for actions on the endocrine pancreas to restrain the beta-cell mass and to inhibit insulin secretion and on the hypothalamus to co-ordinate the neuroendocrine and appetite responses to exercise. Acta Biomed. 2007;78(Suppl 1):156–206. [PubMed] [Google Scholar]
- 126.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]