Abstract
Background
The ‘smoker's paradox’ refers to the observation of favorable prognosis in current smokers following an acute ST elevation myocardial infarction (STEMI) in the era of fibrinolysis, however, several STEMI studies have demonstrated conflicting results in patients undergoing primary percutaneous coronary intervention (p-PCI).
Objective
Aim of the current study was to evaluate the impact of cigarette smoking on left ventricular function in STEMI patients undergoing p-PCI.
Methods
Our population is represented by 74 first-time anterior STEMI patients undergoing p-PCI, 37 of whom were smokers. We assessed left ventricular function by left ventricular ejection fraction (LVEF) on the second day after admission and at 3-month follow-up. Early predictors of adverse left ventricular remodelling after STEMI treated by p-PCI were examined.
Results
Basal demographics and comorbidities were similar between groups. Although the LVEF during the early phase was higher in smokers compared to non-smokers (44.95 ± 7.93% vs. 40.32 ± 7.28%; p = 0.011); it worsened in smokers at follow-up (mean decrease in LVEF: −2.70 ± 5.95%), whereas it improved in non-smokers (mean recovery of LVEF: +2.97 ± 8.45%). In univariate analysis, diabetes mellitus, peak troponin I, current smoking, and lower TIMI flow grade after p-PCI, pain-to-door time and door-to-balloon times were predictors of adverse left ventricular remodelling. After multivariate logistic regression analysis, smoking at admission, lower TIMI flow grade after p-PCI, the pain-to-door time and door-to-balloon times remained independent predictors of deterioration in LVEF.
Conclusion
True or persistent ‘smoker's paradox’ does not appear to be relevant among STEMI patients undergoing p-PCI. The ‘smoker's paradox’ is in fact a pseudo-paradox. Further studies with larger numbers may be warranted.
Keywords: Smoker's paradox, Cigarette smoking, ST elevation myocardial infarction, Primary percutaneous coronary intervention
1. Introduction
Cigarette smoking (CS) remains the leading cause of preventable cardiovascular morbidity and mortality in the world.1, 2 It impacts all phases of atherosclerosis from endothelial dysfunction to acute thrombotic coronary syndromes.3 Despite these well-known deleterious effect on cardiovascular health, some studies have suggested a “smoker's paradox” meaning neutral or better outcomes in current smokers following an acute ST elevation myocardial infarction (STEMI) in the era of fibrinolysis.4, 5, 6, 7, 8 However, multiple studies in smokers have yielded conflicting results in STEMI patients undergoing primary percutaneous coronary intervention (p-PCI).9, 10, 11, 12, 13, 14, 15, 16 We aim to determine the presence of a ‘smoker's paradox’ in a cohort of first-time anterior STEMI patients undergoing p-PCI.
2. Method
We studied 74 consecutive Caucasian patients with first ever anterior STEMI, who underwent coronary angiography within 12 h after the onset of chest pain. All patients were treated by successful p-PCI for acute total occlusion of single vessel left anterior descending artery. Information about age, gender, body-mass index, family history of premature coronary artery disease (male first degree relatives <55 years old and female first degree relatives <65 years old), dyslipidemia (high LDL-cholestrol based on ATP III or HDL-cholestrol <40 mg/dL, or triglycerides >150 mg/dL), diabetes mellitus (fasting blood glucose ≥126 mg/dL, 2 h postprandial glucose ≥200 mg/dL, or use of hypoglycemic agents or insulin), hypertension (positive past history of hypertension or use of antihypertensive drugs) and, CS consumption were collected.
The patients were categorized as active smokers or non-smokers at the time of hospital admission. The previous smokers, the previous history of STEMI and non-anterior STEMI patients were excluded from the study because of their possible confounding effect on results.
Univariate and multivariate analyses were performed to identify predictors for adverse left ventricular remodelling, defined as a decrease in left ventricular ejection fraction (LVEF) at 3-month follow-up compared with the baseline.
After primary PCI, the global LVEF was measured by an experienced physician unaware of patient identity using transthoracic echocardiography (System V GE Vingmed Ultrasound, Horton, Norway) at second hospitalization days and at 3 months post-discharge period. The LVEF was measured using the modified Simpson's rule.17 All patients discharged with modern pharmacological treatment including beta-blockers, ACE-inhibitors or ARBs, dual antiplatelet and statins. Adherence to evidence-based medications was evaluated at post-discharge 3 months.
Written informed consent was obtained from all patients, and the study protocol was approved by the ethics committee of the hospital.
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows 20 (IBM SPSS Inc., Chicago, IL). We calculated that a sample size of 37 per group would be required to achieve a desired power of 0.80 with an alpha value of 0.05. Normal distributions of variables were evaluated with Kolmogorov–Smirnov test. Numerical variables with a normal distribution were presented as the mean ± standard deviation and numerical variables with a skewed distribution were presented as the median (interquartile range) and categorical variables were presented as percentages (%). Two group comparisons of normally distributed variables were tested by unpaired t test and paired t test. The nonparametric Mann–Whitney U test was used for comparisons of nonnormally distributed variables. Paired comparisons of nonnormally distributed variables were analyzed by the Wilcoxon signed-rank test. Chi square test and Fischer's exact 2 test were used for comparisons of categorical variables. Predictors of left ventricular remodelling were determined by logistic regression analysis. All items with significant results in univariate analysis were included in multivariate analysis, followed by a stepwise forward elimination. The strength of association between variables and the occurrence of adverse left ventricular remodelling were represented by odds ratios and their accompanying 95% confidence intervals. For all tests, a two tailed p-value less than 0.05 was defined statistically significant.
3. Results
Basal demographics and comorbidities were similar between groups (Table 1). Two patients in smokers group were excluded from study because of periprocedural cardiac death. No adverse cardiac adverse events including mortality or recurrence of angina at 3 months post-discharge follow-up were detected.
Table 1.
Basal demographic and clinical characteristics features in first anterior STEMI patients undergoing p-PCI.
| Anterior STEMI patients undergoing p-PCI | Smokers (n: 37) | Non-smokers (n: 37) | p value |
|---|---|---|---|
| Age (year, mean ± SD) | 54.68 ± 8.03 | 58.81 ± 11.05 | 0.070 |
| Smoking pack-years | 28.46 ± 8.19 | – | NA |
| Gender (male, %) | 78% | 65% | 0.197 |
| Hypertension (%) | 41% | 46% | 0.639 |
| Diabetes mellitus (%) | 27% | 30% | 0.797 |
| Hyperlipidemia (%) | 30% | 35% | 0.619 |
| Premature family history for CAD (%) | 22% | 32% | 0.295 |
| Body mass index (mean ± SD) | 26.68 ± 5.13 | 28.08 ± 4.09 | 0.199 |
| Pain-to-door time (h, median, IQR) | 5 (2) | 6 (5) | 0.402 |
| Door-to-balloon time (min, median, IQR) | 38 (15) | 38 (10) | 0.904 |
| TIMI 0–1 flow grade before p-PCI (%) | 100% | 100% | NA |
| TIMI 3 flow grade after p-PCI (%) | 88% | 90% | 0.862 |
| Dual antiplatelet agents at 3 months post-discharge (%) | 100% | 100% | NA |
| Beta blocker at 3 months post-discharge (%) | 92% | 95% | 0.643 |
| ACE-I/ARB at 3 months post-discharge (%) | 100% | 100% | NA |
| Statin at 3 months post-discharge (%) | 92% | 97% | 0.304 |
| Furosemide at 3 months post-discharge (%) | 22% | 34% | 0.295 |
| Spirinolactone at 3 months post-discharge (%) | 11% | 19% | 0.327 |
| Peak troponin I (ưgr/L, median, IQR) | 35 (14) | 44 (20) | 0.016 |
| Killip class at admission (median, IQR) | 1 (0) | 1 (1) | 0.141 |
| Smoking quitting rate at 3 months post-discharge (%) | 84% | – | NA |
STEMI, ST elevation myocardial infarction; p-PCI, primary percutaneous coronary intervention; CAD, coronary artery disease; ACE-I/ARB, angiotensinogen converting enzyme inhibitors/angiotensinogen receptor blockers; SD, standard deviation; IQR, interquartile range; NA, not applicable.
Although the LVEF during the early phase was higher in smokers compared to non-smokers (44.95 ± 7.93% vs. 40.32 ± 7.28%; p = 0.011), it worsened in smokers at follow-up (mean decrease in LVEF: −2.70 ± 5.95%), whereas it improved in non-smokers (mean recovery of LVEF: +2.97 ± 8.45%) (Fig. 1, Table 2, Table 3).
Fig. 1.
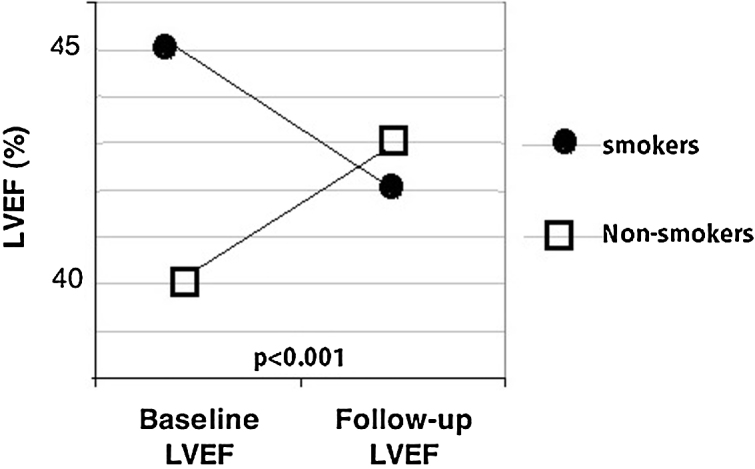
Assessment of smoking status in STEMI patients predicts worsening of left ventricular systolic function.
Table 2.
The comparison of the basal clinical and echocardiographic parameters taken at second days of hospitalization in both group.
| Anterior STEMI patients undergoing p-PCI | Smokers (n: 37) | Non-smokers (n: 37) | p value |
|---|---|---|---|
| Heart rate (beat per minute, mean ± SD) | 82.19 ± 9.67 | 78.78 ± 8.62 | 0.114 |
| Systolic blood pressure (mmHg, median, IQR) | 130 (20) | 130 (35) | 0.318 |
| Diastolic blood pressure (mmHg, median, IQR) | 80 (20) | 80 (15) | 0.431 |
| Echocardiographic findings | |||
| LVEF (%) (mean ± SD) | 44.95 ± 7.93 | 40.32 ± 7.28 | 0.011 |
| LVEDD (cm) (median, IQR) | 4.8 (0.1) | 4.9 (0.5) 23.08 ± 4.55 | 0.007 |
| SPAP (mmHg) (mean ± SD) | 25.08 ± 4.91 | 0.073 | |
LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; SPAP, systolic pulmonary artery pressure; SD, standard deviation; IQR, interquartile range.
Table 3.
Comparison of the echocardiographic LVEF taken at second day of hospitalization and at post-discharge third month control.
| Anterior STEMI patients undergoing p-PCI | At second days hospitalization | At post-discharge third months | p value |
|---|---|---|---|
|
Smokers;n = 37 Echocardiographic findings | |||
| LVEF (%) (mean ± SD) | 44.95 ± 7.93 | 42.24 ± 10.84 | 0.009 |
| LVDD (cm) (median, IQR) | 4.80 (0.2) | 5.00 (0.5) | 0.043 |
| Heart rate (beat per minute, mean ± SD) | 82.19 ± 9.67 | 78.81 ± 10.94 | 0.077 |
| Systolic blood pressure (mmHg, median, IQR) | 130 (25) | 125 (20) | 0.010 |
| Diastolic blood pressure (mmHg, median, IQR) | 80(20) | 75 (9) | 0.001 |
| SPAP (mmHg) at (mean ± SD) | 25.08 ± 4.91 | 26.00 ± 4.97 | 0.074 |
| Mean change in LVEF (%) at post-discharge 3 months | −2.70 ± 5.95 | ||
|
Non-smokers;n = 37 Echocardiographic findings | |||
| LVEF (%) (mean ± SD) | 40.32 ± 7.28 | 43.30 ± 12.52 | 0.039 |
| LVEDD (cm) (median, IQR) | 4.90 (0.5) | 5.10 (0.7) | <0.001 |
| Heart rate (beat per minute, mean ± SD) | 78.78 ± 8.62 | 72.16 ± 10.87 | 0.001 |
| Systolic blood pressure (mmHg, median, IQR) | 130 (35) | 125 (20) | <0.001 |
| Diastolic blood pressure (mmHg, median, IQR) | 80(15) | 76 (11) | <0.001 |
| SPAP (mmHg) (mean ± SD) | 23.08 ± 4.55 | 23.49 ± 5.11 | 0.333 |
| Mean change in LVEF (%) at post-discharge 3 months | +2.97 ± 8.45 | ||
STEMI, ST elevation myocardial infarction; p-PCI, primary percutaneous coronary intervention; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; SPAP, systolic pulmonary artery pressure.
Smoking quitting rate at 3 months post-discharge was % 84. The reduction in LVEF was more prominent in those who continued smoking compared to quitting smoking (mean decrease in LVEF: −2.00 ± 5.39% in quitting smoking vs. −7.33 ± 7.20% continued smoking; p = 0.042).
In univariate analysis, diabetes mellitus, peak troponin I, current smoking, the lower TIMI flow grade after p-PCI, pain-to-door time and door-to-balloon times were predictors of adverse left ventricular remodelling. After multivariate logistic regression analysis, smoking at admission, lower TIMI flow grade after p-PCI, the pain-to-door time and door-to-balloon time remained independent predictors of deterioration in LVEF after STEMI (Table 4).
Table 4.
Univariate and multivariate predictors of adverse left ventricular remodelling.
| Variable | Univariate |
Multivariate |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| p | OR | 95% CI | p | OR | 95% CI | ||||
| Age | 0.339 | 1.7 | – | ||||||
| Male gender | 0.466 | 1.9 | – | 0.000E | |||||
| Hypertension | 0.639 | 1.8 | – | 0.000E | |||||
| Diabetes mellitus | 0.041 | 3.06 | 1.05–8.96 | 0.127 | 2400 | – | |||
| Dyslipidemia | 0.940 | ||||||||
| Premature family history of CAD | 0.170 | ||||||||
| Body mass index | 0.332 | ||||||||
| Peak troponin I | 0.034 | 1.03 | 1.00–1.06 | 0.901 | |||||
| LVEF at admission | 0.137 | ||||||||
| Current smoking at admission | 0.028 | 2.92 | 1.12–7.58 | 0.008 | 5.91 | 1.60–21.81 | |||
| TIMI 0–2 flow grade after p-PCI | 0.001 | 7.50 | 2.17–25.91 | 0.038 | 4.97 | 1.09–22.57 | |||
| Killip classification | 0.122 | ||||||||
| Pain-to-door time | 0.028 | 1.21 | 1.02–1.43 | 0.011 | 1.34 | 1.07–1.69 | |||
| Door-to-balloon time | 0.001 | 1.15 | 1.05–1.25 | 0.031 | 1.01 | 1.03–1.24 | |||
CAD, coronary artery disease; LVEF, left ventricular ejection fraction; p-PCI, primary percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
4. Discussion
The results of our study suggest that the ‘smoker's paradox’ is a pseudo-paradoxs in STEMI patients undergoing p-PCI. Although the LVEF during the early phase (in-hospital second days) was higher in smokers compared to non-smokers; it worsened in smokers at follow-up, whereas it improved in non-smokers. Therefore, we speculated that true or persistent ‘smoker's paradox’ does not appear to be relevant among STEMI patients undergoing p-PCI.
Multiple studies in the past have shown that smokers, who underwent PCI seem to have paradoxically better outcomes than non-smokers.5, 8, 14, 18, 19 There has been some speculation regarding mechanisms involved in the paradoxical effects of active CS following p-PCI.10, 14, 19 Firstly, the PREMIAR (the protection of distal embolization in high-risk patients with acute STEMI) investigators reported that CS in STEMI patients undergoing p-PCI was associated with better myocardial reperfusion than non-smokers,20 and postulated that this may be the mechanism behind the smoker's paradox and its beneficial effect in the short-term clinical outcome. The other possible mechanisms might be a ‘pre-conditioning’-like effect of nicotine, an active component of the CS in early phase, but the CS might have a deleterious effect on cardiac or ventricular remodelling in the late phase.21 Experimental and clinical studies have shown the direct toxic effects of CS on the myocardium, independent of vascular effects.22, 23, 24 Tobacco smoke contains more than 4000 chemical compounds that might have an effect on human coronary collaterals.25 In addition to smoking-induced chronic hypoxia and endothelial dysfunction, nicotine was shown to be a potent physiological and/or pathological angiogenic agent effective through an endogenous nicotinic cholinergic pathway in endothelial cells and in part by activation of endothelial–monocyte interactions involved in arteriogenesis.25 Despite this stimulating effect on collateralization, other clinical studies revealed that smoking was negatively and independently correlated with reduced coronary collateralization26, 27 particularly in diabetic patients.26 Although diabetes was a significant predictor of adverse left ventricular remodelling in our univariate model, it was not significant in our multivariate model, which could be the result of loss of cardio-protection in diabetics with smoking. Although diabetes is well known CAD risk factor, some endo/exogenous protective mechanisms including ischemic preconditioning might be more effective in some diabetic patients.28, 29, 30 However, the concomitant presence of diabetes and current smoking as a deadly duet may impair the collateral development and prevent endogenous cardio-protective mechanisms.26, 31, 32, 33 Thirdly, acute coronary artery obstructions in individuals, who smoke are likely greater thrombogenic and less atherogenic than those of non-smokers.8 Therefore, the smokers have been shown to have a higher proportion of single vessel disease at the diagnosis of STEMI than non-smokers, and this paradox can be explained in part by their less atherosclerotic plaque burden in smokers.8 Moreover, smokers have a higher risk of sudden cardiac death before hospital admission compared with non-smokers,34 and the apparent decrease in case fatality in smokers after an acute cardiac event is restricted to admitted patients.35 Thus, the ‘smoker's paradox’ may be largely explained by a greater case fatality before admission to hospital in smokers.10 Therefore, the apparent smoker's paradox seen in early phase of hospitalization should be interpreted with caution. Moreover, the vast majority of these studies did not include appropriate adjustment for confounding factors and were based on secondary analyses of data derived in randomized trials, which had enrolled highly selected populations. Of the patients suffering from STEMI, smokers are more than 10 years younger than non-smokers requiring coronary revascularization at a much younger age, which may be a major confounding factor causing ‘smoker's paradox.36, 37 After adjusting for differences in age, most studies did not reveal any protective effect of smoking on cardiovascular outcomes following PCI.12, 37, 38
This study has some limitations. Because of small size of study population, we could find no evidence to support these findings with clinical endpoint. Second, there may have been other factors interlinked with smoking behavior that we did not record. For example, smoking cessation could be accompanied with other life style changes such as a diet. Another explanation could be selection bias since many smokers tend to die of fatal MIs before they had any chance to undergo PCI.
Studies demonstrate that many hospitals do not consistently offer tobacco use interventions to their patients.36 This vexing problem is currently being addressed by the Joint Commission for Smoking Cessation.39 Whereas persistent smokers in the presence of established coronary artery disease have an increased risk of re-infarction and sudden cardiac death, even after coronary revascularization with PCI or bypass grafting,40 many CS-mediated prothrombotic changes are quickly reversible upon CS cessation.41 This message needs to be disseminated to patients, primary and secondary care physicians, and the general population. Public health efforts should urgently promote our understanding of current CS-induced cardiovascular pathology to encourage individuals to reduce their exposure to CS. Therefore, it is important to inquire about smoking status at each clinical encounter, and appropriate advice should be offered to help patients to stop CS. We believe that the “smoker's paradox” is in fact a pseudo-paradox in STEMI patient undergoing p-PCI; however, further studies with larger numbers may be warranted.
Funding
This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
The authors have none to declare.
References
- 1.Zhang Y.J., Iqbal J., van Klaveren D. Smoking is associated with adverse clinical outcomes in patients undergoing revascularization with PCI or CABG: the SYNTAX trial at 5-year follow-up. JACC. 2015;65:1107–1115. doi: 10.1016/j.jacc.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg I., Jonas M., Tenenbaum A. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med. 2003;163:2301–2305. doi: 10.1001/archinte.163.19.2301. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: an update. JACC. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 4.Zahger D., Cercek B., Cannon C.P. How do smokers differ from nonsmokers in their response to thrombolysis? (the TIMI-4 trial) Am J Cardiol. 1995;75:232–236. doi: 10.1016/0002-9149(95)80026-o. [DOI] [PubMed] [Google Scholar]
- 5.Barbash G.I., Reiner J., White H.D. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: mechanism of the “smoker's paradox” from the GUSTO-I trial, with angiographic insights. Global Utilization of Streptokinase and Tissue-Plasminogen Activator for Occluded Coronary Arteries. JACC. 1995;26:1222–1229. doi: 10.1016/0735-1097(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 6.Hasdai D., Garratt K.N., Grill D.E., Lerman A., Holmes D.R., Jr. Effect of smoking status on the long-term outcome after successful percutaneous coronary revascularization. NEJM. 1997;336:755–761. doi: 10.1056/NEJM199703133361103. [DOI] [PubMed] [Google Scholar]
- 7.Kelly T.L., Gilpin E., Ahnve S., Henning H., Ross J., Jr. Smoking status at the time of acute myocardial infarction and subsequent prognosis. Am Heart J. 1985;110:535–541. doi: 10.1016/0002-8703(85)90071-7. [DOI] [PubMed] [Google Scholar]
- 8.Grines C.L., Topol E.J., O’Neill W.W. Effect of cigarette smoking on outcome after thrombolytic therapy for myocardial infarction. Circulation. 1995;91:298–303. doi: 10.1161/01.cir.91.2.298. [DOI] [PubMed] [Google Scholar]
- 9.Reinstadler S.J., Eitel C., Fuernau G. Association of smoking with myocardial injury and clinical outcome in patients undergoing mechanical reperfusion for ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2016 doi: 10.1093/ehjci/jew030. [DOI] [PubMed] [Google Scholar]
- 10.Aune E., Roislien J., Mathisen M., Thelle D.S., Otterstad J.E. The “smoker's paradox” in patients with acute coronary syndrome: a systematic review. BMC Med. 2011;9:97. doi: 10.1186/1741-7015-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca G., Parodi G., Sciagra R. Smoking and infarct size among STEMI patients undergoing primary angioplasty. Atherosclerosis. 2014;233:145–148. doi: 10.1016/j.atherosclerosis.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Weisz G., Cox D.A., Garcia E. Impact of smoking status on outcomes of primary coronary intervention for acute myocardial infarction – the smoker's paradox revisited. Am Heart J. 2005;150:358–364. doi: 10.1016/j.ahj.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Andrikopoulos G.K., Richter D.J., Dilaveris P.E. In-hospital mortality of habitual cigarette smokers after acute myocardial infarction; the “smoker's paradox” in a countrywide study. Eur Heart J. 2001;22:776–784. doi: 10.1053/euhj.2000.2315. [DOI] [PubMed] [Google Scholar]
- 14.Symons R., Masci P.G., Francone M. Impact of active smoking on myocardial infarction severity in reperfused ST-segment elevation myocardial infarction patients: the smoker's paradox revisited. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehv738. [DOI] [PubMed] [Google Scholar]
- 15.Li Y.H., Lin G.M., Lai C.P., Lin C.L., Wang J.H. The “smoker's paradox” in Asian versus non-Asian patients with percutaneous coronary intervention longer than 6 months follow-up: a collaborative meta-analysis with the ET-CHD registry. Int J Cardiol. 2013;168:4544–4548. doi: 10.1016/j.ijcard.2013.06.093. [DOI] [PubMed] [Google Scholar]
- 16.Brooks G.C., Lee B.K., Rao R. Predicting persistent left ventricular dysfunction following myocardial infarction: the PREDICTS study. JACC. 2016;67:1186–1196. doi: 10.1016/j.jacc.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang R.M., Bierig M., Devereux R.B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. JASE. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Katayama T., Iwasaki Y., Sakoda N., Yoshioka M. The etiology of ‘smoker's paradox’ in acute myocardial infarction with special emphasis on the association with inflammation. Int Heart J. 2008;49:13–24. doi: 10.1536/ihj.49.13. [DOI] [PubMed] [Google Scholar]
- 19.Cohen D.J., Doucet M., Cutlip D.E., Ho K.K., Popma J.J., Kuntz R.E. Impact of smoking on clinical and angiographic restenosis after percutaneous coronary intervention: another smoker's paradox? Circulation. 2001;104:773–778. doi: 10.1161/hc3201.094225. [DOI] [PubMed] [Google Scholar]
- 20.Albertal M., Cura F., Escudero A.G. Mechanism involved in the paradoxical effects of active smoking following primary angioplasty: a subanalysis of the protection of distal embolization in high-risk patients with acute myocardial infarction trial. J Cardiovasc Med. 2008;9:810–812. doi: 10.2459/JCM.0b013e3282f73519. [DOI] [PubMed] [Google Scholar]
- 21.Minicucci M.F., Azevedo P.S., Polegato B.F., Paiva S.A., Zornoff L.A. Cardiac remodeling induced by smoking: concepts, relevance, and potential mechanisms. Inflamm Allergy Drug Targets. 2012;11:442–447. doi: 10.2174/187152812803589958. [DOI] [PubMed] [Google Scholar]
- 22.Santos P.P., Oliveira F., Ferreira V.C. The role of lipotoxicity in smoke cardiomyopathy. PLOS ONE. 2014;9:e113739. doi: 10.1371/journal.pone.0113739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gokulakrisnan A., Jayachandran Dare B., Thirunavukkarasu C. Attenuation of the cardiac inflammatory changes and lipid anomalies by (−)-epigallocatechin-gallate in cigarette smoke-exposed rats. Mol Cell Biochem. 2011;354:1–10. doi: 10.1007/s11010-011-0785-6. [DOI] [PubMed] [Google Scholar]
- 24.Gu L., Pandey V., Geenen D.L., Chowdhury S.A., Piano M.R. Cigarette smoke-induced left ventricular remodelling is associated with activation of mitogen-activated protein kinases. Eur J Heart Fail. 2008;10:1057–1064. doi: 10.1016/j.ejheart.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koerselman J., de Jaegere P.P., Verhaar M.C., Grobbee D.E., van der Graaf Y., Group S.S. Coronary collateral circulation: the effects of smoking and alcohol. Atherosclerosis. 2007;191:191–198. doi: 10.1016/j.atherosclerosis.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Ozeke O., Gungor M., Topaloglu S., Aras D., Ozer C. Chronic total artery occlusions in noninfarct-related coronary arteries. Int J Angiol. 2014;23:17–22. doi: 10.1055/s-0033-1356648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billinger M., Fleisch M., Eberli F.R., Garachemani A., Meier B., Seiler C. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? JACC. 1999;33:1027–1035. doi: 10.1016/s0735-1097(98)00674-3. [DOI] [PubMed] [Google Scholar]
- 28.Altunkaynak H.O., Ozcelikay A.T. Cardioprotective effect of postconditioning against ischemia-reperfusion injury is lost in heart of 8-week diabetic rat. Gen Physiol Biophys. 2016;35:63–69. doi: 10.4149/gpb_2015032. [DOI] [PubMed] [Google Scholar]
- 29.Rezende P.C., Rahmi R.M., Uchida A.H. Type 2 diabetes mellitus and myocardial ischemic preconditioning in symptomatic coronary artery disease patients. Cardiovasc Diabetol. 2015;14:66. doi: 10.1186/s12933-015-0228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balakumar P., Sharma N.K. Healing the diabetic heart: does myocardial preconditioning work? Cell Signal. 2012;24:53–59. doi: 10.1016/j.cellsig.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Haire-Joshu D., Glasgow R.E., Tibbs T.L., American Diabetes Association Smoking and diabetes. Diabetes Care. 2003;26(suppl 1):S89–S90. doi: 10.2337/diacare.26.2007.s89. [DOI] [PubMed] [Google Scholar]
- 32.Lejay A., Fang F., John R. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. 2016;91:11–22. doi: 10.1016/j.yjmcc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinandy P., Hausenloy D.J., Heusch G., Baxter G.F., Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 34.Elosua R., Vega G., Rohlfs I. Smoking and myocardial infarction case-fatality: hospital and population approach. Eur J Cardiovasc Prev Rehabil. 2007;14:561–567. doi: 10.1097/HJR.0b013e32804955b3. [DOI] [PubMed] [Google Scholar]
- 35.Sonke G.S., Stewart A.W., Beaglehole R., Jackson R., White H.D. Comparison of case fatality in smokers and non-smokers after acute cardiac event. BMJ. 1997;315:992–993. doi: 10.1136/bmj.315.7114.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakowski T., Siudak Z., Dziewierz A., Dubiel J.S., Dudek D. Impact of smoking status on outcome in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Thromb Thrombolysis. 2012;34:397–403. doi: 10.1007/s11239-012-0764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamedali B., Shroff A. Impact of smoking status on cardiovascular outcomes following percutaneous coronary intervention. Clin Cardiol. 2013;36:372–377. doi: 10.1002/clc.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakabayashi K., Romaguera R., Laynez-Carnicero A. Impact of smoking on acute phase outcomes of myocardial infarction. Coron Artery Dis. 2011;22:217–222. doi: 10.1097/MCA.0b013e3283441d28. [DOI] [PubMed] [Google Scholar]
- 39.Fiore M.C., Goplerud E., Schroeder S.A. The Joint Commission's new tobacco-cessation measures – will hospitals do the right thing? NEJM. 2012;366:1172–1174. doi: 10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metz L., Waters D.D. Implications of cigarette smoking for the management of patients with acute coronary syndromes. Prog Cardiovasc Dis. 2003;46:1–9. doi: 10.1016/s0033-0620(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 41.Csordas A., Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]


