Abstract
As root symbionts of cycad trees, cyanobacteria of the genus Nostoc produce β-methylamino-l-alanine (BMAA), a neurotoxic nonprotein amino acid. The biomagnification of BMAA through the Guam ecosystem fits a classic triangle of increasing concentrations of toxic compounds up the food chain. However, because BMAA is polar and nonlipophilic, a mechanism for its biomagnification through increasing trophic levels has been unclear. We report that BMAA occurs not only as a free amino acid in the Guam ecosystem but also can be released from a bound form by acid hydrolysis. After first removing free amino acids from tissue samples of various trophic levels (cyanobacteria, root symbioses, cycad seeds, cycad flour, flying foxes eaten by the Chamorro people, and brain tissues of Chamorros who died from amyotrophic lateral sclerosis/Parkinsonism dementia complex), we then hydrolyzed the remaining fraction and found BMAA concentrations increased 10- to 240-fold. This bound form of BMAA may function as an endogenous neurotoxic reservoir, accumulating and being transported between trophic levels and subsequently being released during digestion and protein metabolism. Within brain tissues, the endogenous neurotoxic reservoir can slowly release free BMAA, thereby causing incipient and recurrent neurological damage over years or even decades, which may explain the observed long latency period for neurological disease onset among the Chamorro people. The presence of BMAA in brain tissues from Canadian patients who died of Alzheimer's disease suggests that exposure to cyanobacterial neurotoxins occurs outside of Guam.
Keywords: biomagnification, cyanobacteria, cycad, symbiosis, ALS/PDC
We recently reported (1) that β-methylamino-l-alanine (BMAA), a neurotoxic, nonprotein amino acid identified from the seeds of the indigenous Guam cycad Cycas micronesica Hill by Vega and Bell (2), is biomagnified in the Guam ecosystem, in which it is associated with amyotrophic lateral sclerosis/Parkinsonism dementia complex (ALS/PDC), a devastating neurological disease of the indigenous Chamorro people. Because Chamorros eat tortillas made from cycad seed flour, BMAA was proposed by Spencer et al. (3) as the cause of the high incidence of ALS/PDC among the Chamorro people (4). This hypothesis was rejected by Duncan et al. (5), who found only low concentrations of BMAA in washed cycad flour.
We previously reported that BMAA is produced by cyanobacteria of the genus Nostoc, which are root symbionts of cycads (1). BMAA is then biomagnified in both the cycad seeds and the flying foxes that forage on them. Consumption of flying foxes by the Chamorro people has been implicated as a delivery mechanism of relatively high doses of BMAA in the Chamorro diet (6, 7). Support for this hypothesis has come from the findings of high levels of BMAA in Guam flying foxes (8) and the discovery of BMAA within brain tissues of Chamorro patients who have died of ALS/PDC in Guam (1).
One of the more puzzling aspects of ALS/PDC in Guam is the long latency period of the disease. Years and even decades after Chamorro people leave Guam, the incidence rate of ALS/PDC among Chamorro expatriates is still four times the background incidence of ALS worldwide (9). This latency period led Spencer et al. to suggest that BMAA might function as a “slow toxin” (10). The Spencer et al. proposal was disputed by Duncan et al. (11), who argued that “the concept of a slow neurotoxin is without precedence, and it seems unlikely that a toxic dietary component...plays a causative role in the development of motor neuron and related neurodegenerative diseases.” There is no known environmental neurotoxin that produces both significantly delayed onset of symptoms and a progressive neurological disease from a single exposure (12).
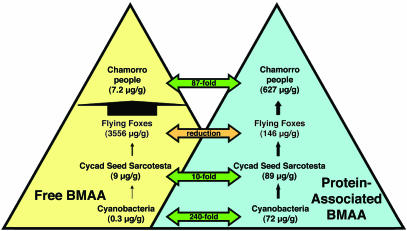
Given our previous findings of BMAA in the brain tissues of patients with ALS/PDC (1), it seemed that a reservoir of BMAA must exist within the body to generate repetitive exposures sufficient to cause neurological disease. However, the chemical nature of BMAA would make the molecule at first seem to be a poor candidate for bioaccumulation within the ecosystem. Unlike other biomagnified compounds including dichlorodiphenyltrichloroethane and polychlorinated biphenyls, BMAA is not lipophilic and thus would likely not accumulate in fatty tissues. Because BMAA accumulates through increasing trophic levels in the Guam ecosystem (Fig. 1), an alternative mechanism is required to explain BMAA biomagnification. Because amino acids are components of proteins and BMAA has been artificially incorporated in synthetic peptides (13), we decided to investigate the possibility of BMAA occurring in a bound form in different trophic levels of the Guam ecosystem.
Fig. 1.
Biomagnification of free and protein-bound BMAA in the Guam ecosystem.
Materials and Methods
Cyanobacteria were isolated from infected coralloid roots of C. micronesica Hill grown from three living accessioned specimens in the National Tropical Botanical Garden in Lawai, Kauai, Hawaii, and grown in repeated generations as axenic cultures for analysis. The elimination of external soil-borne bacteria was ensured by surface sterilization of cycad root tissues in a solution of 70% (vol/vol) ethanol for 3 min followed by a 30-min immersion in 1.6% (vol/vol) of a 5.4% stock solution of sodium hypochlorite with two drops of surfactant and three sequential washes with sterile deionized water before culture onto standard BG-11 medium, pH 7.1, solidified with gellam gum (Sigma). Cultures were maintained in a controlled-environment room with a 16-h photoperiod at a light intensity of 35–45 μmol/m2 per s and temperatures of 25–30°C for a period of 4 months with monthly subculture and sampling for analysis of BMAA. Histological characterizations were used to verify cyanobacterial culture identity and purity. To assess the effects of amino acids on cyanobacterial growth, glutamate or glutamine (0, 125, or 250 μmol/liter) were supplemented to the BG-11 medium after filter sterilization. Cyanobacterial growth was increased 2-fold by supplementation with either amino acid.
Whole tissues of three replicate samples of leaf, root, seed, and reproductive organs of C. micronesica were harvested from a living accessioned specimen at the National Tropical Botanical Garden. Samples of five processed cycad flours prepared on Guam from the sliced gametophytes of the seeds of C. micronesica in the traditional manner by Chamorros and collected in 1987–1988 by neurologist J. C. Steele (Guam Memorial Hospital, Tamuning, Guam) were analyzed. Comparable flour samples have been analyzed by other workers (11, 14–18).
Samples of flying foxes from Guam (Pteropus mariannus mariannus) were collected by H. C. Reynolds in 1951 and preserved as dried specimens at the Museum of Vertebrate Zoology (University of California, Berkeley). Samples analyzed in the current study were hair and skin excised from the wing membranes and body skin.
Superior frontal gyrus tissues of 6 Chamorro patients clinically diagnosed with ALS/PDC, 2 Chamorro individuals who did not die of neurological illness, and a comparison group of 15 Canadians (2 patients with Alzheimer's disease and 13 patients who did not die of neurological illness) were obtained from P. L. McGeer (Kinsmen Laboratory of Neurological Research, University of British Columbia, Vancouver, BC, Canada). In addition, samples of frontal cortex, temporal cortex, parahippocampal gyrus, caudate, and cerebellum from seven patients with Alzheimer's disease and one patient who did not die of a neurological illness were obtained from the same source. All human tissues were blinded, and the blind was not broken until after the tests were run. The time interval between death and autopsy varied from 4 h to 5 days (19). Specimens were fixed in paraformaldehyde before storage in a 15% buffered sucrose maintenance solution. Extensive characterization of the familial relationships, clinical histories, and histochemical characteristics of the patients has been published (19).
Samples were homogenized twice in 0.1 M trichloroacetic acid and centrifuged at 15,800 × g for 3 min to precipitate proteins and extract free amino acids. The precipitates then were hydrolyzed at 110°C in constant-boiling 6 M HCl for 24 h. Particulate matter was removed from a 500-μl aliquot by ultrafiltration (Ultrafree-MC, Millipore) at 15,800 × g, and the resulting extract was freeze-dried. The residue was resuspended in 20 mM HCl and applied to a Sep-Pac C18 cartridge equilibrated with sequential washes of 100% methanol, 50% (vol/vol) methanol, and a gradient of borate buffer/acetonitrile (0.5 M borate/0–60% CH3CN) at 20% increments. BMAA was derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate following standardized protocols (8).
BMAA was separated from the protein amino acids by reverse-phase elution (Waters Nova-Pak C18 column, 300 × 3.9 mm) with 140 mM sodium acetate, 5.6 mM triethylamine, pH 5.2 (mobile phase A), and 60% acetonitrile in water (mobile phase B) at 37°C. The standard amino acid elution gradient was modified for BMAA separation (0.0 min = 100% A, 2.0 min = 90% A curve 11, 5.0 min = 86% A curve 11, 10.0 min = 86% A curve 6, 18.0 min = 73% A curve 6, 30.0 min = 60% A curve 10, 35.0 min = 40% A curve 6, 39.0 min = 10% A curve 6, 40.0 min = 100% B, 45 min = 100% A). BMAA concentration was determined by detection of the fluorescent tag (Waters 2487 Dual-l fluorescence detector) with excitation at 250 nm and emission at 395 nm.
Identification and quantification of BMAA was verified by liquid chromatography/MS analysis with an Agilent liquid chromatography mass spectrometer including a variable-wavelength diode-array detector, single-quadrupole MS with an atmospheric pressure ionization source, electrospray ionization, and a Waters Symmetry column heated at 30°C. BMAA was eluted over a linear gradient of CH3CN (10–40%) in water for 10 min at 0.4 ml/min. Nitrogen gas was purified and supplied to the electrospray ionization interface with a nebulizing pressure of 35 psi (1 psi = 6.89 kPa) and a drying gas of 11.5 liters/min at a temperature of 350°C. The diode-array detector detected analytes at 254 nm (bandwidth 4 nm) with a full spectral scan from 190 to 600 nm and 0.5-nm resolution within a semimicroflow cell. MS signals were in positive mode, scanning between 100- and 600-Da range at a 50-V fragmentor voltage, at a gain of 1.0 V. BMAA was identified by using the extracted ion chromatogram in which the molecular ion peak was confirmed.
Results
We found BMAA, a nonprotein amino acid, as a bound component of bacterial, plant, animal, and human tissues in the Guam ecosystem. Axenic cultures of cyanobacteria contained 72 μg/g BMAA in the bound form, 240 times the level (0.3 μg/g BMAA) previously quantified as free amino acid (1). In C. micronesica, bound BMAA was quantified in cyanobacteria-infected roots (2 μg/g). BMAA in a bound form was found as well in leaf tissue (738 μg/g), the outer seed layer (48 μg/g), the seed sarcotesta (89 μg/g), and female gametophyte (81 μg/g). The results of the analysis of five cycad flours prepared from female gametophytes of cycad seeds in Guam between 1987 and 1988 show that significant amounts of BMAA were retained as a bound form within the prepared flours (Table 1). Previous research results with the same flours showed a huge variation in free BMAA concentration but did not quantify the neurotoxin present in the bound form.
Table 1. Comparison of BMAA concentrations detected in flours prepared from the gametophyte of cycad seeds on the island of Guam (1987–2000).
| Free BMAA (μg/g) from literature accounts*
|
||||
|---|---|---|---|---|
| Flour sample | Protein-associated BMAA, μg/g | Ref. 8 | Ref. 16 | Refs. 5 and 15 |
| Yigo | 48.97 (±7.63) to 39.79 (±2.26) | ND | 0.27 (±0.09) to 9.53 (±1.72) | ND to 11.8 |
| Agat | 92.68 (±16.33) | 8 | 1.02 (±0.51) | 3.9 |
| Merizo | 91.55 (±8.97) to 168.90 (±32.16) | 3 | 18.39 (±0.54) | ND to 146 |
ND, not detected.
Ref. 17 cites free-BMAA levels of 0.00008 to 0.003 for various villages in Guam
In flying foxes, BMAA was found in a bound form in hair (146 μg/g) and wing membrane (2 μg/g) in 52-year-old museum specimens. This discovery indicates processes for ingestion, digestion, and reincorporation of BMAA in mammals.
BMAA in a bound form was found in the superior frontal gyrus tissues of all six patients with ALS/PDC from Guam, both of the Canadian patients with Alzheimer's disease, and one of the two Chamorros who had not been diagnosed with neurodegenerative disease (20). No BMAA was detected in the 13 Canadian individuals from the comparison group who died of causes unrelated to neurodegeneration (1). We found that BMAA can occur in brain tissues other than the frontal gyrus. BMAA was variously distributed at high levels in the frontal cortex, temporal cortex, parahippocampal gyrus, or caudate of six of the seven patients with Alzheimer's disease, but we did not detect BMAA in homologous tissues from the comparison patient who died of causes unrelated to neurodegeneration (Table 2). In general, there was an ≈60- to 130-fold greater quantity of BMAA in the bound form than was recovered from the free amino acid pool.
Table 2. Bound BMAA (μg/g) in brain tissues from patients with Alzheimer's disease (AD).
| Patient | Disease | Frontal cortex | Temporal cortex | Parahippocampal gyrus | Caudate | Cerebellum |
|---|---|---|---|---|---|---|
| 074 | Control | — | — | — | — | — |
| 489 | AD | 25.9 | — | — | — | — |
| 568 | AD | 41.2 | 33.7 | — | — | — |
| 574 | AD | — | 53.1 | — | — | — |
| 575 | AD | — | — | — | — | — |
| 576 | AD | — | 170.8 | 235.6 | 185.5 | — |
| 577 | AD | — | — | 34.2 | — | — |
| 582 | AD | 45.7 | — | — | 29.6 | — |
—, not detected.
Discussion
Our findings demonstrate that bound BMAA in addition to free BMAA can bioaccumulate from cyanobacterial symbionts in the root tissues of C. micronesica throughout the food chain, from which it is consumed by Chamorro people in their traditional diets (Fig. 1). For example, it seems that significant levels of bound BMAA are associated with the protein fraction of cycad flours, a fraction that was not analyzed by previous investigators (Table 1). Model animals fed the same cycad flours developed the early symptoms of neurodegenerative disease consistent with many of the aspects of ALS/PDC in humans (18, 21). The possible association of BMAA with protein provides one explanation for the neurotoxic effects attributed to the flour even though the quantified levels of free BMAA in the flour was low (17, 18).
Additional sources of neurotoxin in the Chamorro diet include flying foxes (6, 7). Unlike the Chamorro people, who eat flour prepared from cycad gametophytes (81 μg/g), flying foxes primarily consume the seed sarcotesta (89 μg/g). BMAA was detected in the protein fraction from hair and skin of 50-year-old flying fox specimens, providing an indication of the stability of the BMAA–protein complex. Because all these samples have been stored for more than five decades, however, it is possible that the original ratio of bound BMAA to free BMAA has been altered by protein degradation.
As BMAA passes through the Guam ecosystem, cyanobacterially produced neurotoxin is biomagnified, as measured by both free and bound forms, until it reaches the top trophic level in the Guam ecosystem: the indigenous Chamorro people. BMAA, both as a free amino acid and likely as a protein-bound amino acid, occurs in the brain tissues of Chamorro patients who died from ALS/PDC (Fig. 1). Similar ecological biomagnification of BMAA, or its ingestion from cyanobacterially contaminated water in other parts of the world, is suggested by our finding of bound BMAA in brain tissues of Canadian patients with Alzheimer's disease.
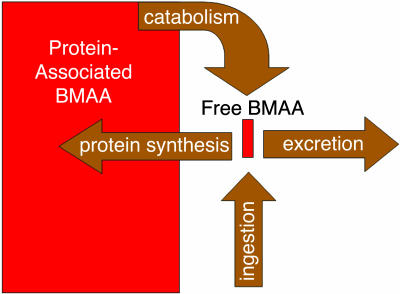
We suggest that BMAA is bound to protein and functions as an endogenous neurotoxic reservoir (Fig. 2) that slowly releases neurotoxin directly into the cerebral tissues through protein metabolism. On the basis of previous studies, the endogenous neurotoxic reservoir delivery of neurotoxin inside of the brain can have at least five different neuropathological impacts. First, the incorporation of nonprotein amino acids may alter tertiary folding of neuroproteins, altering their biological activity. Second, the potential for protein-associated BMAA to form dimers that covalently bind metal ions (22) can result in a protein punctuated with reactive nonprotein amino acid complexes that alter ionic balance in neuronal cells, generate free radicals, or even catalyze deleterious chemical processes. Third, capture and release of metal ions such as those of Zn, Cu, Ca, and Al by BMAA complexes can interfere with the proper function of N-methyl-d-aspartate (NMDA) (23) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (24) receptors. Fourth, BMAA incorporation may truncate proteins before completed synthesis or collapse proteins after release from the ribosome. Such truncation of protein synthesis is characteristic of many of the tauopathies. Fifth, BMAA can be released slowly in free form through protein metabolism in the brain, serving as an agonist at AMPA, NMDA, and other neuroreceptor sites (25). This latter activity can effectively translate a single or episodic ingestion of BMAA into a highly prolonged, constant low-level exposure of BMAA within brain tissues, resulting in neuronal death through excitotoxicity. Etiologically, such prolonged low-level exposure may not produce acute disease, such as has been observed in animals dosed with high levels of BMAA, but instead might result in both the latency and progressive nature typified by ALS/PDC among the Chamorro people.
Fig. 2.
The endogenous neurotoxic reservoir.
Protein-associated BMAA in the endogenous neurotoxic reservoir may therefore be the slow toxin that Spencer et al. hypothesized, wherein disease manifestation occurs after a latency period measured in decades (10). The manifestation of disease arising from the endogenous neurotoxic reservoir depends on the rate of amino acid flux between free and protein-associated BMAA and therefore may well be responsive to individual variations, diet, and nutritional status. As well, variations arising from genetic vulnerabilities, age, endocrine function, or idiopathic differences might occur.
Because BMAA-induced progressive neurodegenerative disease has not yet been demonstrated in an animal model, Koch's postulates have not been fulfilled. It is possible also that other unknown neurotoxins may be biomagnified in tandem with BMAA and in fact may be the causative toxins, with BMAA merely serving as a biomarker for neurodegeneration. Pending development of an appropriate animal model, further corroboration of the deleterious role of BMAA could be provided by in vitro studies of the effects of BMAA in neuronal cultures as well as theoretical models of neurotoxin-induced ALS/PDC among the Chamorro people. Studies of BMAA production of other species of cyanobacteria as well as analyses of other possible routes of biomagnification (26) would help in the assessment of possible human exposure to the neurotoxin outside of Guam.
Acknowledgments
We thank the Acacia Foundation and B. and J. Lane for laboratory equipment and the Amyotrophic Lateral Sclerosis Association and the Castle Foundation for funding.
Abbreviations: BMAA, β-methylamino-l-alanine; ALS, amyotrophic lateral sclerosis; PDC, Parkinsonism dementia complex.
References
- 1.Cox, P. A., Banack, S. A. & Murch, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 13380-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vega, A. & Bell, E. A. (1967) Phytochemistry 6, 759-762. [Google Scholar]
- 3.Spencer, P. S., Nunn, P. B., Hugon, J., Ludolph, A. C., Ross, S. M. & Roy, D. N. (1987) Science 237, 517-522. [DOI] [PubMed] [Google Scholar]
- 4.Whiting, M. G. (1963) Econ. Bot. 17, 271-302. [Google Scholar]
- 5.Duncan, M. W., Steele, J. C., Kopin, I. J. & Markey, S. P. (1990) Neurology 40, 767-772. [DOI] [PubMed] [Google Scholar]
- 6.Cox, P. A. & Sacks, O. W. (2002) Neurology 58, 956-959. [DOI] [PubMed] [Google Scholar]
- 7.Monson, C. S., Banack, S. A. & Cox, P. A. (2003) Conserv. Biol. 17, 678-686. [Google Scholar]
- 8.Banack, S. A. & Cox, P. A. (2003) Neurology 61, 387-389. [DOI] [PubMed] [Google Scholar]
- 9.Torres, J., Iriarte, L. L. G. & Kurland, L. T. (1957) Calif. Med. 86, 385-386. [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer, P. S., Kisby, G. E. & Ludolph, A. C. (1991) Neurology 41, Suppl. 2, 41-68. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, M. W., Kopin, I. J., Garruto, R. M., Lavine, L. & Markey, S. P. (1988) Lancet 2, 631-632. [DOI] [PubMed] [Google Scholar]
- 12.Spencer, P. S. & Schaumburg, H. H. (2000) Experimental and Clinical Neurotoxicology (Oxford Univ. Press, New York), 2nd Ed.
- 13.Seebach, D., Studer, A., Pfammatter, E. & Widmer, H. (1994) Helv. Chim. Acta 77, 2035-2050. [Google Scholar]
- 14.Duncan, M. W., Marini, A. M., Watters, R., Kopin, I. J. & Markey S. P. (1992) J. Neurosci. 12, 1523-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan, M. W. (1991) Adv. Neurol. 56, 301-310. [PubMed] [Google Scholar]
- 16.Kisby, G. E., Ellison, M. & Spencer, P. S. (1992) Neurology 42, 1336-1340. [DOI] [PubMed] [Google Scholar]
- 17.Khabazian, I., Bains, J. S., Williams, D. E., Cheung, J., Wilson, J. M. B., Pasqualotto, B. A., Pelech, S. L., Andersen, R. J., Wang, Y.-T., Liu, L., et al. (2002) J. Neurochem. 82, 516-528. [DOI] [PubMed] [Google Scholar]
- 18.Wilson, J. M. B., Khabazian, I., Wong, M. C., Seyedalikhani, A., Bains, J. S., Pasqualotto, B. A., Williams, D. E., Andersen, R. J., Simpson, R. J., Smith, R., et al. (2002) Neuromol. Med. 1, 207-221. [DOI] [PubMed] [Google Scholar]
- 19.McGeer, P. L., Schwab, C., McGeer, E. G., Haddock, R. L. & Steele, J. C. (1997) Neurology 49, 400-409. [DOI] [PubMed] [Google Scholar]
- 20.Murch, S. J., Cox, P. A., Banack, S. A., Steele, J. C. & Sacks, O. W. (2004) Acta Neurol. Scand., in press. [DOI] [PubMed]
- 21.Wilson, J. M. B., Khabazian, I., Pow, D. V., Craig, U.-K. & Shaw, C. A. (2003) Neuromolecular Med. 3, 105-117. [DOI] [PubMed] [Google Scholar]
- 22.Hursthouse, M. B., Motevalli, M., O'Brien, P. & Nunn, P. B. (1990) J. Chem. Soc. Dalton Trans., 1985-1987.
- 23.Nunn, P. B., O'Brien, P., Pettit, L. D. & Pyburn, S. I. (1989) J. Inorg. Biochem. 37, 175-183. [DOI] [PubMed] [Google Scholar]
- 24.Weiss, J. H. & Sensi, S. L. (2000) Trends Neurosci. 23, 365-371. [DOI] [PubMed] [Google Scholar]
- 25.Carriedo, S. G., Yin, Z. H. & Weiss, J. H. (1996) J. Neurosci. 16, 4069-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Codd, G. A., Bell, S. G., Kaya, K., Ward, C. J., Beattie, K. A. & Metcalf, J. S. (1999) Eur. J. Phycol. 34, 405-415. [Google Scholar]