Abstract
In contrast to simian immunodeficiency viruses (SIVs), which induce immunodeficiency over a 1- to 3-year period, highly pathogenic simian-human immunodeficiency viruses (SHIVs) cause a complete, irreversible, and systemic depletion of CD4+ T lymphocytes in rhesus monkeys within weeks of infection. By using small-molecule competitors specific for CCR5 and CXCR4 in ex vivo assays, we found that highly pathogenic SHIVDH12R exclusively uses CXCR4 for infection of rhesus peripheral blood mononuclear cells, whereas SIVmac239 and SIVsmE543 use CCR5 for entry into the same cells. During the period of peak virus production in SHIVDH12R- or SHIV89.6P-infected rhesus monkeys, massive elimination of CXCR4+ naïve CD4+ T cells occurred. In contrast, circulating CCR5+ memory CD4+ T cells were selectively depleted in rapidly progressing SIV-infected monkeys. At the time of their death, two SIV rapid progressors had experienced a nearly complete loss of the memory CD4+ T cell subset from the blood and mesenteric lymph nodes. Thus, pathogenic SHIVs and SIVs target different subsets of CD4+ T cells in vivo, with the pattern of CD4+ T lymphocyte depletion being inextricably linked to chemokine receptor use. In the context of developing an effective prophylactic vaccine, which must potently control virus replication during the primary infection, regimens that suppress SHIVs might not protect monkeys against SIV or humans against HIV-1.
Highly pathogenic simian-human immunodeficiency viruses (SHIVs) cause a rapid, systemic, and complete loss of CD4+ T lymphocytes within weeks of inoculation of rhesus monkeys, high and sustained levels (>107 copies per ml of plasma) of viral RNA, and death from immunodeficiency in 12–30 weeks (1–3). This rapid and irreversible CD4+ T cell-depleting phenotype is markedly different from that observed for either simian immunodeficiency virus (SIV) or HIV-1, both of which generally induce more gradual and moderate losses of CD4+ T cells, and much slower development of clinical disease in macaques and humans, respectively. Surprisingly, SHIVs have proven to be more controllable by the same vaccine regimens that are ineffective against SIV (4–7). Vaccine strategies that readily and durably suppress plasma SHIV RNA levels and prevent the associated global loss of CD4+ T lymphocytes only transiently control SIV viral load, and SIV-challenged macaques invariably succumb to AIDS after similar immunizations.
The distinctive features of in vivo SHIV and SIV infections enumerated above suggest that the underlying mechanism(s) of disease induction by each virus may be different. Side-by-side comparisons of CD4+ T cell subsets targeted by SHIVs and SIV during infections of rhesus monkeys were therefore carried out. In the case of SIV, four (of the 10 initially inoculated) rapidly progressing animals were studied because they exhibit virological parameters and clinical symptoms that are similar to those reported for macaques infected with SHIVs (8). Ex vivo experiments, employing competitors specific for CCR5 and CXCR4, revealed that SIVs exclusively used CCR5 to enter and spread through cultured rhesus monkey peripheral blood mononuclear cells (PBMCs), whereas SHIVDH12R used CXCR4, but not CCR5, during productive infections of the same cells. In inoculated rhesus monkeys, massive depletion of naïve CD4+ T lymphocytes was observed during the period of peak SHIV production (weeks 1 to 3). In contrast, memory CD4+ T cells were selectively eliminated from the blood and lymph nodes in rapidly progressing SIV-infected animals. The expression of CXCR4 on virtually all naïve and a significant fraction of memory CD4+ T lymphocytes ultimately makes both subsets the target of highly pathogenic SHIVs, and explains the rapid, irreversible, and complete depletion of CD4+ T cells in SHIV, but not SIV infections of macaques.
Materials and Methods
Virus and Animals. The origin and preparation of the tissue culture-derived SHIVDH12R and SHIVDH12R-CL-7 stock have been described (1, 9). SHIV89.6P was kindly provided by N. Letvin (Harvard University, Boston; ref. 3). An infectious molecular clone of SIVsmE543 was generated and prepared as described (10). Rhesus macaques (Macaca mulatta) were maintained in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals (11) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Phlebotomies and virus inoculations [500–100,000 tissue culture 50% infectious dose (TCID50) of SHIVDH12R or SHIVDH12R-CL-7 i.v., 1:500 dilution of SHIV89.6P i.v, or 2,000 TCID50 of SIVsmE543 i.v.] were performed as described (12).
Effect of Coreceptor-Specific Inhibitors on SIV and SHIV Replication. AMD3100 (13) was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. AD101 (14) was provided by B. Baroudy (Schering–Plough Research Institute, Bloomfield, NJ). Chemokine coreceptor usage in PBMC was determined as described (9, 15).
Quantitation of Plasma Viral RNA Levels. Plasma viral RNA levels were determined by real-time PCR (ABI Prism 7700 sequence detection system, Applied Biosystems) using reverse-transcribed viral RNA as templates as described (12).
Lymphocyte Immunophenotyping and Data Analysis. EDTA-treated blood samples and mesenteric lymph node suspensions were stained with combinations of the following fluorochrome-conjugated mAbs: CD3 [FITC or phycoerythrin (PE)], CD4 [peridinin chlorophyll protein-Cy5.5 or allophycocyanin (APC)], CD28 (FITC or PE), CD95 (APC), CD45RA (FITC or PE), CD11a (PE or APC), CCR7 (PE or PE-Cy7), CXCR4 (PE or APC), CCR5 (PE or APC), and isotype-matched controls (mouse IgG1, mouse IgG2, and rat IgG2). All antibodies were obtained from BD Biosciences Pharmingen (San Diego) and analyzed by four-color flow cytometry (FACSCalibur; BD Biosciences Immunocytometry Systems). For CD4+ T lymphocyte subset frequencies, a CD4+ small-lymphocyte gate was used for further phenotypying (16, 17). Data analysis was performed by using cellquest pro (BD Biosciences) and flowjo (TreeStar, San Carlos, CA). The statistical analysis was performed by using statview software (Abacus Concepts, Berkeley, CA). The significance of differences between paired groups was analyzed with the Mann–Whitney U test.
Results
SIV and SHIVs Use Different Coreceptors to Establish Infections of Cultured Rhesus Monkey PBMC. Small-molecule coreceptor-targeted inhibitors, specific for CCR5 or CXCR4, were used to assess coreceptor utilization by SIVs and SHIVs for macaque PBMCs. As shown in Fig. 1, SIVsmE543, SIVmac239, and SIVmac316 spreading infections were readily suppressed by the CCR5-specific AD101 (14), but not by the CXCR4 inhibitor, AMD3100 (13). The opposite result was observed with a molecularly cloned derivative of the highly pathogenic SHIVDH12R, designated SHIVDH12R-CL-7 (18): infection of monkey PBMC was completely blocked by the CXCR4-targeting AMD 3100, but not by the CCR5 inhibitor AD101. These results indicate that the three SIV strains used CCR5 and the highly pathogenic SHIVDH12R-CL-7 used CXCR4 to enter and spread in cultured rhesus macaque PBMC. Similar findings have been reported for uncloned SHIVDH12R and SHIV89.6PD (9, 15).
Fig. 1.
Coreceptor use by SIVs (A–C) and SHIVDH12R-CL-7 (D). SIVsmE543–3, SIVmac239, SIVmac316, and SHIVDH12R-CL-7 were spinoculated onto rhesus PBMCs in the presence of the indicated amounts of chemokine receptor inhibitors. The reverse transcriptase activity released into the medium on day 5 after infection was determined in the absence (dashed line) or presence of inhibitor.
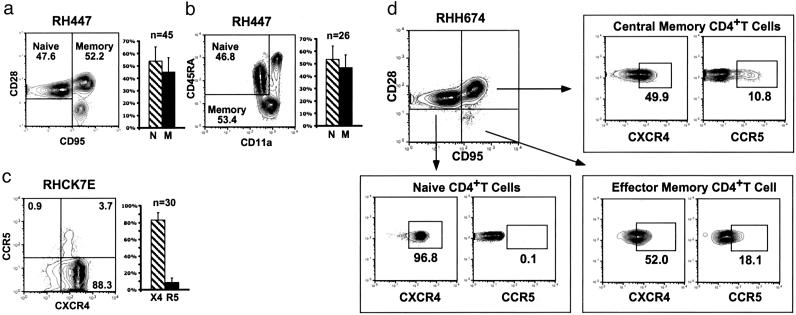
Flow Cytometric Analysis of Macaque naïve and Memory CD4+ T Lymphocytes. To ascertain whether the coreceptor utilization results obtained with cultured PBMCs were applicable to SHIV and SIV infections of rhesus monkeys, fluorescence-activated cell sorter (FACS) analysis was performed to measure the frequencies of CD4+ T lymphocyte subsets in uninfected and virus-infected animals. Two combinations of mAbs were used for the detection of naïve and memory macaque CD4+ T cells. In the first (Fig. 2a), PBMCs from a representative uninfected rhesus monkey were gated on CD4+ lymphocytes expressing CD28 and CD95 as reported (17). Under these conditions, naïve T cells were identified by their CD95lowCD28high phenotype, whereas central and effector memory CD4+ T cells, as defined for rhesus monkey lymphocytes (17), were CD95highCD28high and CD95highCD28low, respectively. By using these criteria for analysis, the circulating CD4+ T lymphocytes from 45 uninfected monkeys, ranging in age from 2 to 9 years, consisted of slightly more naïve (mean = 53.9%) than memory (mean = 45.0%) cells. Within the CD4+ memor y subset, the fraction of CD95highCD28low effector memory cells varied greatly (0.6–33%) among different macaques compared with the central memory cells, which comprised 24–56% of the total CD4+ T cells in the animals analyzed. When the combination of CD45RA and CD11a expression was used to measure naïve and memory CD4+ T cells in macaques, as reported for analyses of monkey T cell subsets (16), similar values were observed (Fig. 2b). Under these conditions, naïve CD4+ T cells were CD45RA+CD11adim, and two populations of memory cells were detected: CD45RA– and CD45RA+CD11ahigh.
Fig. 2.
Expression of memory/naïve markers and CCR5/CXCR4 on uninfected rhesus monkey CD4+ T lymphocytes. (a–c) Cells from a representative uninfected macaque were initially gated by flow cytometry on CD4 and were then analyzed for CD28/CD95 (a), CD45RA/CD11a (b), or CCR5/CXCR4 (c) expression. The percentage of total cells within each sector is indicated. The bar graphs depict the fraction of: (i) CD4+ naïve and memory cells in 45 (a) and 26 (b) uninfected macaques or (ii) CD4+CXCR4+ and CD4+CCR5+ lymphocytes in 30 uninfected animals (c). (d) Chemokine expression on CD4+ naïve and memory cells was determined by initially gating on CD95lowCD28high, CD95highCD28low, or CD95highCD28high cells and then by analyzing for CXCR4 or CCR5. The boxes show the gates used to quantitate CXCR4 and CCR5 cells based on the staining with isotype matched control mAbs.
In addition to the CD4 molecule, primate lentiviruses also use chemokine receptors (primarily CCR5 and CXCR4) to enter their target cells. As shown in Fig. 2c for both a representative monkey and a total of 30 uninfected animals, >80% of circulating CD4+ T lymphocytes were CXCR4-positive (mean = 83%), and <10% (mean = 8.6%) expressed CCR5. Chemokine receptor expression was also measured on naïve and memory subsets of monkey CD4+ T cells. After initially gating on CD95lowCD28high-naïve lymphocytes, individual samples were stained for CXCR4 or CCR5. Virtually all of the naïve cells were positive for CXCR4 and negative for CCR5 in the representative animal shown (Fig. 2d). When extended to a total of 11 uninfected macaques, the majority (mean = 95.7%) of naïve CD4+ T lymphocytes were CXCR4+. A similar analysis of CD95highCD28high central memory CD4+ T cells revealed that a mean of 63.2% expressed CXCR4, and 17.4% were CCR5+.
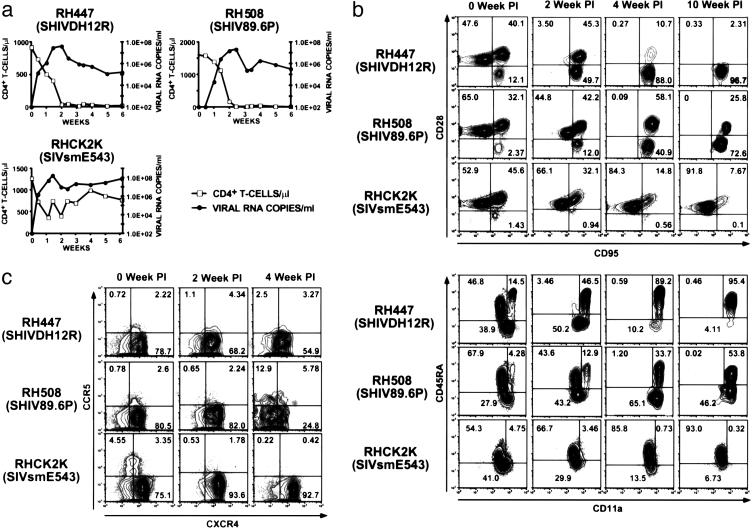
SIVs and Highly Pathogenic SHIVs Target Different CD4+ T Lymphocyte Subsets During Infections of Rhesus Monkeys. The plasma virus loads and levels of circulating CD4+ T lymphocytes during the first 6 weeks of infection for individual animals inoculated intravenously with SHIVDH12R, SHIV89.6P, or SIVsmE543 are shown in Fig. 3a. All three animals generated peak levels of plasma viremia between days 10 and 21 after inoculation and sustained high virus loads thereafter. As expected, the two monkeys (RH447 and RH508) inoculated with the pathogenic SHIVs both experienced a rapid, precipitous, and complete loss of CD4+ T cells, whereas the SIV-infected macaque (RHCK2K) had fluctuating but relatively normal levels of CD4+ T lymphocytes during the acute phase of infection.
Fig. 3.
SHIVs preferentially target the depletion of CD4+ CXCR4+ naïve cells, whereas SIV induces the loss of CD4+ CCR5+ memory cells during the first 10 weeks of infection. (a) SHIVDH12R, SHIV89.6P, and SIVsmE543 all replicate to high levels in rhesus monkeys, but only the SHIVs induce a rapid and complete loss of CD4+ T lymphocytes. (b and c) EDTA-treated samples of blood, collected at the indicated times from SIV- or SHIV-infected animals, were gated on CD4+ T cells and analyzed for the indicated surface markers, which distinguish naïve and memory (b) or CCR5+ and CXCR4+ (c) cells. The percentage of total cells within each sector is indicated.
To ascertain whether distinct CD4+ T cell subsets were targeted by pathogenic SHIVs and SIVs, flow cytometric analyses were performed on PBMC samples collected during the first 10 weeks after virus inoculation, by using the two combinations of mAbs that distinguish memory and naïve lymphocytes. As shown in Fig. 3b, SHIVDH12R and SHIV89.6P both induced the rapid depletion of naïve CD4+ T cells within the first 4 weeks after infection. The two SHIVs also caused a marked loss of central memory CD4+ T lymphocytes so that by week 10, the effector memory cells became the predominant remaining CD4+ T cell subset in the representative animals shown. In contrast, the SIVsmE543-inoculated monkey experienced a fairly rapid loss of circulating memory CD4+ T cells (both central and effector subsets). At week 10, >90% of the circulating CD4+ T lymphocytes in animal RHCK2K were naïve cells.
Two of the rapidly progressing SIVsmE543-infected macaques (RHCK2K and RHCK2F) were killed on weeks 15 and 31 after inoculation, respectively, because of their deteriorating clinical condition. FACS analyses of CD4+ T cells from the blood and mesenteric lymph node suspensions, employing the two combinations (CD95/CD28 or CD45RA/CD11a) of mAbs, revealed that >95% of the samples consisted of naïve cells (Fig. 4). Virtually all of the CD95lowCD28high and CD45RA+CD11adim CD4+ T lymphocytes from both body compartments were also CCR7+ (data not shown), further validating their naïve cell status. Thus, at the time of death, both of the rapidly progressing SIVsmE543-infected monkeys had experienced a nearly complete loss of memory CD4+ T cells from the blood and lymphoid tissues. A similar analysis could not be performed on specimens collected at the necropsy of immunodeficient SHIV-infected animals because of the extremely low levels of CD4+ T lymphocytes in the blood (<5 cells per microliter) and lymphoid tissues (19).
Fig. 4.
At the time of death, two SIV-infected monkeys had lost virtually all of their CD4+ memory T cells from the blood and lymph nodes. EDTA-treated blood samples or mesenteric lymph node suspensions, collected before death from macaques RHCK2K and RHCK2F, were gated on CD4+ T cells and stained for the indicated surface markers that distinguish naïve and memory cells. The percentages are indicated for each CD4+ T cell subset.
FACS analyses were also conducted on CXCR4- and CCR5-expressing CD4+ T cells collected from the blood of SHIV- and SIV-infected monkeys. Not unexpectedly, CXCR4+ CD4+ T lymphocytes were selectively eliminated in the two representative SHIV-infected animals and CCR5+ CD4+ T cells were depleted in the SIV-infected rapid progressor (Fig. 3c).
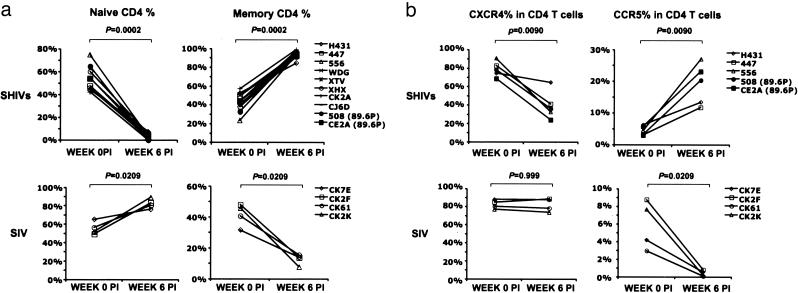
The flow cytometric analyses of CD4+ T lymphocytes from 10 SHIV (8 SHIVDH12R and 2 SHIV89.6P) and 4 rapidly progressing SIVsmE543-infected monkeys at weeks 0 and 6 are summarized in Fig. 5. The data show that the highly pathogenic SHIVs preferentially target the depletion of CXCR4+ naïve cells, whereas SIV induces the loss of CCR5+ memory CD4+ T lymphocytes from the blood of infected macaques.
Fig. 5.
Changes in the levels of naïve (a), memory (a), CXCR4+ (b), and CCR5+ (b) CD4+ T cells in rhesus monkeys inoculated with SHIVs and SIVs at week 0 and week 6 after infection. The significance of differences between each paired group was analyzed by using the Mann–Whitney U test.
Discussion
We have shown that highly pathogenic SHIVs and SIVs target different subsets of CD4+ T cells in vivo, explaining the distinctive laboratory findings and clinical courses induced by each virus in infected rhesus monkeys. Ex vivo, SHIVs exclusively used CXCR4 to enter and spread through cultured macaque PBMCs. In infected monkeys, SHIVs cause a nearly complete depletion of naïve CD4+ T lymphocytes within 4 weeks of inoculation and the elimination of virtually all of the central memory cells by week 10. In contrast, SIVmac239 and SIVsmE543 both used CCR5, but not CXCR4, to infect cultured rhesus monkey PBMCs. CCR5 is exclusively expressed on memory CD4+ T lymphocytes and at significantly higher levels on cells that reside in nonlymphoid tissues (e.g., in the gastrointestinal tract and lung; refs. 20 and 21). As a consequence, the SIV-infected monkeys exhibiting rapid disease progression in this study experienced the selective loss of circulating CCR5+ CD4+ memory T cells during the first 6 weeks of infection. In two SIV-infected animals killed at weeks 15 and 31 with severe clinical disease, >95% of the CD4+ T lymphocytes in the blood and lymph nodes were naïve T cells. Thus, the pattern of CD4+ T lymphocyte depletion induced by SHIVs and SIVs is inextricably linked to the chemokine coreceptor used by each virus. One could therefore argue that in the context of an effective prophylactic vaccine, which must potently control virus replication during the initial weeks of the primary infection, regimens that suppress SHIVs might not be effective against SIV in monkeys and HIV-1 in humans. If X4 SHIVs are to be used to evaluate antibody-based vaccine strategies, it would be more appropriate to use sterilizing protection, rather than preventing the complete loss of CD4+ T lymphocytes, as the endpoint for vaccine efficacy.
The specific targeting and rapid elimination of CXCR4-positive CD4+ T cells in the blood in macaques inoculated with pathogenic SHIVs is consistent with reports describing the enhanced cytopathicity and more vigorous replicative properties of syncytium-inducing (primarily X4) HIV-1 variants compared with nonsyncytium inducing (exclusively R5) strains in vitro (22, 23). Similar results have been obtained by using the ex vivo human tonsil histoculture system in which X4 HIV-1 variants were found to be more cytopathic than R5 viruses (24–26). Although comparable results have also been reported for X4 HIV-1 isolates tested in the SCID-hu-Thy-Liv mouse model (27), the presence of large pools of immature T lymphocytes/thymocytes in that system makes it less applicable to primate lentivirus infections, in monkeys and humans, that target CD4+ T cells that have survived thymic selection.
It has been recognized for >10 years that the emergence of X4 variants in HIV-1-infected individuals is associated with accelerated CD4+ T cell depletion and more rapid progression to AIDS (22, 28). The CXCR4 coreceptor, used by late-appearing X4 viruses, is primarily expressed on cells with a resting naïve phenotype. CXCR4+ cells also comprise the major population of CD4+ T lymphocytes in the circulation and lymphoid tissues (Fig. 2c and ref. 24). The CCR5 chemokine coreceptor, on the other hand, is expressed on a much smaller pool of activated memory CD4+ T cells; some memory T lymphocytes express both CCR5 and CXCR4 (Fig. 2d and ref. 24). Thus, a report showing that carriers of X4 HIV-1 variants have 30- to 70-fold higher frequencies of virus-infected naïve cells than individuals who harbor only R5 virus (29) is consistent with the coreceptor utilization properties of each viral strain. The accelerated and specific decline of naïve CD4+ T lymphocytes after the emergence of X4 variants is a further reflection of coreceptor-mediated targeting and depletion of this abundant T cell subset (30).
Multiple studies have shown that resting and/or naïve PBMC in culture are highly refractory to HIV-1 infection, whereas activated memory cells are quite susceptible (31–35). The block to HIV-1 replication in resting PBMCs is not presently understood, although the small pool sizes of ribonucleotides, deoxyribonucleotide triphosphates, and ATP in quiescent cells may play a role (36). Nonetheless, HIV-1 has been recovered from CD45RA+/CD62L+ naïve CD4+ T lymphocytes of infected individuals (37), and this same T cell subset becomes preferentially infected and depleted in human tonsil explant cultures by X4 virus strains (38). It has also been recently reported that although resting CD45RO+ CD57– CD4+ memory cells are the predominant virus-producing cell in HIV-1-infected persons, naïve CD4+ T lymphocytes are also virus positive at a 10-fold lower frequency in the same individuals (39). At present, we have no definitive information as to whether naïve CD4+ T cells in SHIV-infected rhesus monkeys are the sole source of the prodigious viremia observed at week 2 after inoculation (Fig. 3). Nevertheless, in preliminary experiments, we were unable to demonstrate any increase in activation/proliferation markers such as CD25, CD69, or Ki-67 on CD4+ T cells in the blood or lymph nodes, before or during the peak viremia associated with the SHIV infection (Y.N., unpublished work), suggesting that they are the source of prodigious amounts of progeny virions generated.
The induction of immunodeficiency by pathogenic SHIVs requires the elimination of virtually all CD4+ T lymphocytes. Although CXCR4 is expressed on both naïve and memory CD4+ T lymphocyte subsets, its higher surface expression on the former (Fig. 2d) may explain why the loss of memory cells lags behind that of naïve lymphocytes when SHIV-induced depletions are complete (Fig. 3b). This depletion of naïve CD4+ T lymphocytes may also markedly impact the pool of virus-specific memory CD4+ T cells, resulting in an ineffective antiviral immune response. In contrast, after the inoculation of nonpathogenic SHIVs or when the SHIV-induced loss of CD4+ T cells is only partial or transient (12), the resulting infections are rarely associated with clinical symptoms; such SHIV-infected animals usually remain disease-free for several years (18). In monkeys experiencing partial depletions of CD4+ T cells, the loss primarily affects the naïve cell subset (Y.N., unpublished work). The preservation of CD4+ memory T lymphocytes in these SHIV-infected macaques is very likely responsible for their long-term asymptomatic clinical course. Similarly, the relative sparing of CD4+ memory T cells during SHIV versus SIV infections may explain why comparable vaccine regimens appear to be more effective against SHIV challenges.
Compared with SHIVs, SIV infections of Asian macaques usually proceed at a much slower pace with the development of clinical disease, occurring 1–2 years after virus inoculation (40, 41). During acute SIV infections, marked depletions of CD4+ T cells from the gut-associated lymphoid tissues, not blood and lymph nodes, have been reported (42, 43). This loss specifically affects activated memory cells. In the 25–30% of SIV-infected rhesus monkeys, termed rapid progressors, which develop persistent antigenemia, high levels of plasma viremia, no SIV-specific humoral responses, and immunodeficiency within 3–4 months after inoculation, only minor declines in circulating CD4+ T lymphocytes have been reported in animals experiencing severe immunologic deficits (8). This result was also observed in the cohort of the four SIV-infected macaques in the present study. In these rapid progressors, SIV initially targeted CD4+ central memory cells, which express high levels of CCR5, and subsequently infected CD4+ effector memory lymphocytes, which express lower levels of the chemokine receptor (Fig. 2d). This targeting resulted in marked depletions of both memory subsets in the blood during the initial weeks of the acute infection (Figs. 3b and 5). In contrast, conventional SIV progressors only experience the partial loss of circulating CD4+ central memory cells during the first 10 weeks of infection and have minimal to no changes affecting their effector memory subsets, very likely reflecting the differential expression of CCR5 (Y.N., unpublished work).
Taken together, the SIV/macaque system would appear to be a better model for HIV-1-induced immunodeficiency than would the SHIV system. First, R5, but not X4-using HIV-1 isolates, are commonly recovered during the asymptomatic phase of infection (44, 45). Like SIV, such R5 HIV-1 strains preferentially infect CD4+ memory T lymphocytes and induce clinical disease over extended periods of time without requiring the total elimination of CD4+ T cells. These facts notwithstanding, there are features of HIV-1 infections never observed with SIV, such as the emergence of X4 variants in late-stage individuals with severe disease. Whether the latter HIVs spontaneously arise de novo from R5 predecessors or are archival remnants of X4 strains that were readily controlled/out-competed after the primary infection, these viruses share multiple properties with the highly pathogenic SHIVs in macaques, and their appearance heralds a rapidly deteriorating clinical course in individuals with an underlying dysfunctional immune system.
Acknowledgments
We thank Lowrey Rhodes, Joel Beren, Russ Byrum, Liz Scanlon, Frances Banks, and Wes Thornton for their diligence and assistance in the care and maintenance of our animals; Christopher Erb and Ronald Plishka for determining viral RNA levels; John P. Moore (Weill Medical College of Cornell University, New York) for providing AMD3100; Bahige Baroudy and Jayaram Tagat (Schering–Plough Research Institute) for providing AD101; Ron Willey for critical comments during the preparation of this paper; and Mario Roederer and Joseph Mattapallil (Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health) for helpful suggestions concerning FACS analysis.
Abbreviations: SIV, simian immunodeficiency virus; SHIV, simian-human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; FACS, fluorescence-activated cell sorter; PE, phycoerythrin; APC, allophycocyanin.
References
- 1.Igarashi, T., Endo, Y., Englund, G., Sadjadpour, R., Matano, T., Buckler, C., Buckler-White, A., Plishka, R., Theodore, T., Shibata, R. & Martin, M. (1999) Proc. Natl. Acad. Sci. USA 96, 14049–14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joag, S. V., Li, Z., Foresman, L., Stephens, E. B., Zhao, L.-J., Adany, I., Pinson, D. M., McClure, H. M. & Narayan, O. (1996) J. Virol. 70, 3189–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimann, K. A., Li, J. T., Veazey, R., Halloran, M., Park, I.-W., Karlsson, G. B., Sodroski, J. & Letvin, N. L. (1996) J. Virol. 70, 6922–6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., Villinger, F., Altman, J. D., Lydy, S. L., O'Neil, S. P., Staprans, S. I., Montefiori, D. C., Xu, Y., Herndon, J. G., Wyatt, L. S., et al. (2001) Science 292, 69–74. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., Santra, S., Schmitz, J. E., Kuroda, M. J., Fu, T.-M., Wagner, W., Bilska, M., Craiu, A., Zheng, X. X., Krivulka, G. R., et al. (2000) Science 290, 486–492. [DOI] [PubMed] [Google Scholar]
- 6.Horton, H., Vogel, T. U., Carter, D. K., Vielhuber, K., Fuller, D. H., Shipley, T., Fuller, J. T., Kunstman, K. J., Sutter, G., Montefiori, D. C., et al. (2002) J. Virol. 76, 7187–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ourmanov, I., Brown, C. R., Moss, B., Carroll, M., Wyatt, L., Pletneva, L., Goldstein, S., Venzon, D. & Hirsch, V. M. (2000) J. Virol. 74, 2740–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, V. M., Santra, S., Goldstein, S., Plishka, R., Buckler-White, A., Seth, A., Ourmanov, I., Brown, C. R., Engle, R., Montefiori, D., et al. (2004) J. Virol. 78, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igarashi, T., Donau, O. K., Imamichi, H., Dumaurier, M.-J., Sadjadpour, R., Plishka, R. J., Buckler-White, A., Buckler, C., Suffredini, A. F., Lane, H. C., et al. (2003) J. Virol. 77, 13042–13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch, V., Adger-Johnson, D., Campbell, B., Goldstein, S., Brown, C., Elkins, W. R. & Montefiori, D. C. (1997) J. Virol. 71, 1608–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl. Inst. Health, Bethesda), DHHS Publ. No. (NIH) 85-23.
- 12.Endo, Y., Igarashi, T., Nishimura, Y., Buckler, C., Buckler-White, A., Plishka, R., Dimitrov, D. S. & Martin, M. A. (2000) J. Virol. 74, 6935–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donzella, G. A., Schols, D., Lin, S. W., Este, J. A., Nagashima, K. A., Maddon, P. J., Allaway, G. P., Sakmar, T. P., Henson, G., De Clercq, E. & Moore, J. P. (1998) Nat. Med. 4, 72–77. [DOI] [PubMed] [Google Scholar]
- 14.Trkola, A., Kuhmann, S. E., Strizki, J. M., Maxwell, E., Ketas, T., Morgan, T., Pugach, P., Xu, S., Wojcik, L., Tagat, J., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang, Y.-J., Lou, B., Lal, R. B., Gettie, A., Marx, P. A. & Moore, J. P. (2000) J. Virol. 74, 6893–6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattapallil, J. J., Letvin, N. L. & Roederer, M. (2004) AIDS 18, 13–23. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher, C. J., Hagen, S. I., Walker, J. M., Lum, R., Mitchell, B. L., Maino, V. C., Axthelm, M. K. & Picker, L. J. (2002) J. Immunol. 168, 29–43. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi, T., Endo, Y., Nishimura, Y., Buckler, C., Sadjadpour, R., Donau, O. K., Dumaurier, M.-J., Plishka, R. J., Buckler-White, A. & Martin, M. A. (2003) J. Virol. 77, 10829–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igarashi, T., Brown, C. R., Byrum, R. A., Nishimura, Y., Endo, Y., Plishka, R. J., Buckler, C., Buckler-White, A., Miller, G., Hirsch, V. M. & Martin, M. A. (2002) J. Virol. 76, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douek, D. C., Picker, L. J. & Koup, R. A. (2003) Annu. Rev. Immunol. 21, 265–304. [DOI] [PubMed] [Google Scholar]
- 21.Veazey, R. S., Mansfield, K. G., Tham, I. C., Carville, A. C., Shvetz, D. E., Forand, A. E. & Lackner, A. A. (2000) J. Virol. 74, 11001–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor, R. I., Mohri, H., Cao, Y. & Ho, D. D. (1993) J. Virol. 67, 1772–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouchier, R. A. M., Meyaard, L., Brouwer, M., Hovenkamp, E. & Schuitemaker, H. (1996) Virology 219, 87–95. [DOI] [PubMed] [Google Scholar]
- 24.Grivel, J.-C. & Margolis, L. B. (1999) Nat. Med. 5, 344–346. [DOI] [PubMed] [Google Scholar]
- 25.Grivel, J.-C., Penn, M. L., Eckstein, D. A., Schramm, B., Speck, R. F., Abbey, N. W., Herndier, B., Margolis, L. & Goldsmith, M. A. (2000) J. Virol. 74, 5347–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penn, M. L., Grivel, J.-C., Schramm, B., Goldsmith, M. A. & Margolis, L. (1999) Proc. Natl. Acad. Sci. USA 96, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkowitz, R. D., Alexander, S., Bare, C., Linquist-Stepps, V., Bogan, M., Moreno, M. E., Gibson, L., Wieder, E. D., Kosek, J., Stoddart, C. A. & McCune, J. M. (1998) J. Virol. 72, 10108–10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koot, M., Keet, I. P. M., Vos, A. H. V., de Goede, R. E. Y., Roos, M. T. L., Coutinho, R. A., Miedema, F., Schellekens, P. T. A. & Tersmette, M. (1993) Ann. Intern. Med. 118, 681–688. [DOI] [PubMed] [Google Scholar]
- 29.Blaak, H., van't Wout, A. B., Brouwer, M., Hooibrink, B., Hovenkamp, E. & Schuitemaker, H. (2000) Proc. Natl. Acad. Sci. USA 97, 1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazenberg, M. D., Otto, S. A., Hamann, D., Roos, M. T., Schuitemaker, H., de Boer, R. J. & Miedema, F. (2003) AIDS 17, 1419–1424. [DOI] [PubMed] [Google Scholar]
- 31.Chou, C.-S., Ramilo, O. & Vitetta, E. S. (1997) Proc. Natl. Acad. Sci. USA 94, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDougal, J. S., Mawle, A., Cort, S. P., Nicholson, J. K. A., Cross, G. D., Scheppler-Campbell, J. A., Hicks, D. & Sligh, J. (1985) J. Immunol. 135, 3151–3162. [PubMed] [Google Scholar]
- 33.Roederer, M., Raju, P. A., Mitra, D. K., Herzenberg, L. A. & Herzenberg, L. A. (1997) J. Clin. Invest. 99, 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnittman, S. M., Lane, H. C., Greenhouse, J., Justement, J. S., Baseler, M. & Fauci, A. S. (1990) Proc. Natl. Acad. Sci. USA 87, 6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spina, C. A., Prince, H. E. & Richman, D. D. (1997) J. Clin. Invest. 99, 1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien, W. A., Namazi, A., Kalhor, H., Mao, S. H., Zack, J. A. & Chen, I. S. (1994) J. Virol. 68, 1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski, M. A., Chun, T.-W., Justement, S. J., Motola, I., Spinelli, M. A., Adelsberger, J., Ehler, L. A., Mizell, S. B., Hallahan, C. W. & Fauci, A. S. (1999) J. Virol. 73, 6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckstein, D. A., Penn, M. L., Korin, Y. D., Scripture-Adams, D. D., Zack, J. A., Kreisberg, J. F., Roederer, M., Sherman, M. P., Chin, P. S. & Goldsmith, M. A. (2001) Immunity 15, 671–682. [DOI] [PubMed] [Google Scholar]
- 39.Brenchley, J. M., Hill, B. J., Ambrozak, D. R., Price, D. A., Guenaga, F. J., Casazza, J. P., Kuruppu, J., Yazdani, J., Migueles, S. A., Connors, M., et al. (2004) J. Virol. 78, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kestler, H., Kodama, T., Ringler, D., Marthas, M., Pedersen, N., Lackner, A., Regier, D., Sehgal, P., Daniel, M., King, N., et al. (1990) Science 248, 1109–1112. [DOI] [PubMed] [Google Scholar]
- 41.Orandle, M. S., Williams, K. C., MacLean, A. G., Westmoreland, S. V. & Lackner, A. A. (2001) J. Virol. 75, 4448–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veazey, R. S., DeMaria, M., Chalifoux, L. V., Shvetz, D. E., Pauley, D. R., Knight, H. L., Rosenzweig, M., Johnson, R. P., Desrosiers, R. C. & Lackner, A. A. (1998) Science 280, 427–431. [DOI] [PubMed] [Google Scholar]
- 43.Smit-McBride, Z., Mattapallil, J. J., McChesney, M., Ferrick, D. & Dandekar, S. (1998) J. Virol. 72, 6646–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connor, R. I., Sheridan, K. E., Ceradini, D., Choe, S. & Landau, N. R. (1997) J. Exp. Med. 185, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuitemaker, H., Koot, M., Kootstra, N. A., Dercksen, M. W., de Goede, R. E., van Steenwijk, R. P., Lange, J. M., Schattenkerk, J. K., Miedema, F. & Tersmette, M. (1992) J. Virol. 66, 1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]