Summary
Background
Human papillomavirus (HPV) vaccination programmes were first implemented in several countries worldwide in 2007. We did a systematic review and meta-analysis to assess the population-level consequences and herd effects after female HPV vaccination programmes, to verify whether or not the high efficacy reported in randomised controlled clinical trials are materialising in real-world situations.
Methods
We searched the Medline and Embase databases (between Jan 1, 2007 and Feb 28, 2014) and conference abstracts for time-trend studies that analysed changes, between the pre-vaccination and post-vaccination periods, in the incidence or prevalence of at least one HPV-related endpoint: HPV infection, anogenital warts, and high-grade cervical lesions. We used random-effects models to derive pooled relative risk (RR) estimates. We stratified all analyses by age and sex. We did subgroup analyses by comparing studies according to vaccine type, vaccination coverage, and years since implementation of the vaccination programme. We assessed heterogeneity across studies using I2 and χ2 statistics and we did trends analysis to examine the dose–response association between HPV vaccination coverage and each study effect measure.
Findings
We identified 20 eligible studies, which were all undertaken in nine high-income countries and represent more than 140 million person-years of follow-up. In countries with female vaccination coverage of at least 50%, HPV type 16 and 18 infections decreased significantly between the pre-vaccination and post-vaccination periods by 68% (RR 0·32, 95% CI 0·19–0·52) and anogenital warts decreased significantly by 61% (0·39, 0·22–0·71) in girls 13–19 years of age. Significant reductions were also recorded in HPV types 31, 33, and 45 in this age group of girls (RR 0·72, 95% CI 0·54–0·96), which suggests cross-protection. Additionally, significant reductions in anogenital warts were also reported in boys younger than 20 years of age (0·66 [95% CI 0·47–0·91]) and in women 20–39 years of age (0·68 [95% CI 0·51–0·89]), which suggests herd effects. In countries with female vaccination coverage lower than 50%, significant reductions in HPV types 16 and 18 infection (RR 0·50, 95% CI 0·34–0·74]) and in anogenital warts (0·86 [95% CI 0·79–0·94]) occurred in girls younger than 20 years of age, with no indication of cross-protection or herd effects.
Interpretation
Our results are promising for the long-term population-level effects of HPV vaccination programmes. However, continued monitoring is essential to identify any signals of potential waning efficacy or type-replacement.
Funding
The Canadian Institutes of Health Research.
Introduction
Since 2007, 52 out of 195 countries worldwide have implemented human papillomavirus (HPV) vaccination programmes, including 41% of high-income countries and 15% of low-income and middle-income countries.1–4 The population-level effect of HPV vaccination programmes is expected to vary substantially between these countries, depending on the vaccine used, implementation strategies, and vaccination coverage achieved. Two HPV vaccines are currently available worldwide: the bivalent vaccine, which targets HPV types 16 and 18 (which are associated with 70–80% of cervical cancers globally5), and the quadrivalent vaccine, which also targets HPV types 6 and 11 (associated with 85–95% of cases of anogenital warts6). Most high-income countries are using the quadrivalent vaccine, whereas a mixed picture exists for low-income and middle-income countries.2,7 Although all HPV vaccination programmes target pre-adolescent girls (and might also include catch-up programmes for older girls and women), a few countries, such as the USA and Australia, have recently begun to include boys.8,9 Finally, in high-income countries, vaccination coverage in the younger cohorts of girls ranges from nearly 90% to less than 50%, mostly depending on whether the countries have school-based or non-school-based vaccination programmes.10
Large international randomised controlled clinical trials have shown both HPV vaccines to be safe and well tolerated, highly efficacious against vaccine-type persistent HPV infection and precancerous cervical lesions in women (vaccine efficacy 93–100%),11,12 and to provide some degree of cross-protection against three non-vaccine types (HPV types 31, 33, and 45),12–14 which are associated with 10–15% of cervical cancers worldwide.15 Existing evidence from clinical trials also suggests that cross-protective vaccine efficacy estimates against infections and lesions associated with HPV types 31, 33, and 45 are higher for the bivalent vaccine than for the quadrivalent vaccine.16 Following clinical trials, mathematical models have been used to predict the long-term population-level effectiveness and cost-effectiveness of vaccination programmes delivered in different settings. Modelling studies have consistently predicted that the overall burden of HPV-related diseases in women will decrease substantially in the next few decades through vaccination, and that vaccination of girls against HPV is highly cost effective in most countries.17–19 Despite consistency in model predictions of the direct effects of HPV vaccination in vaccinated girls, uncertainty remains about the potential population-level effects of cross-protection and herd protection (eg, the indirect consequences of vaccinating girls on HPV in unvaccinated boys, men, and adult women), and the vaccination coverage necessary to achieve substantial herd effects.20–24 This information is crucial to help guide vaccine choices and inform decisions about vaccination of boys and men.
Now that more than 7 years have elapsed since the implementation of the first HPV vaccination programmes in 2007 (appendix pp 2–4), it is timely to verify whether or not the promising results from clinical trials and model projections are materialising at the population level. An increasing number of post-vaccination surveillance studies have recently been published using several intermediate endpoints (eg, HPV infection, anogenital warts, and precancerous cervical lesions). The aim of this systematic review and meta-analysis is to summarise existing evidence about the population-level effect of HPV vaccination, as measured in time-trend studies in girls and young women targeted for vaccination, and in boys, men, and older women. We focused on three HPV-related endpoints: HPV infection, anogenital warts, and high-grade cervical lesions.
Methods
Search strategy and selection criteria
We systematically reviewed the global literature and report it in accordance with the PRISMA guidelines.25 Studies were eligible for inclusion if they fulfilled the following criteria: they provided data about at least one endpoint of HPV infection, anogenital warts, histopathologically confirmed high-grade cervical lesions (cervical intraepithelial neoplasia [CIN] 2 or worse); if they assessed the population-level effect by comparing the frequency (prevalence or incidence) of the endpoint between the pre-vaccination and post-vaccination periods (ie, time-trend studies); and if data from the pre-vaccination and post-vaccination periods were collected from the same population sources with use of the same recruitment methods.
We excluded studies with the following characteristics because they did not measure population-level effect: if HPV vaccination was administered as part of an individual-based randomised trial, or HPV vaccination effect was assessed by comparing the frequency of the endpoint between vaccinated and unvaccinated people during the post-vaccination period.
Our search strategy had three stages. First, we searched the Medline and Embase databases between Jan 1, 2007, and Feb 28, 2014, with a combination of the following Medical Subject Heading (MeSH) terms, title, or abstract words, with no restriction on the language of publication: (“papillomavirus vaccine”, “papillomavirus vaccination”, “HPV vaccine”, or “HPV vaccination”) and (“program evaluation”, “population surveillance”, “sentinel surveillance”, “incidence”, or “prevalence”), and (“papillomavirus infection”, “condylomata acuminata”, “anogenital warts”, “cervical intraepithelial neoplasia”, “cervical dysplasia”, “uterine cervical neoplasm”, or “HPV related diseases”). We identified eligible studies by reviewing titles and abstracts, and we also searched the reference lists of eligible articles. Second, we reviewed the abstracts of recent major conferences on HPV (the European Research Organisation on Genital Infection and Neoplasia [EUROGIN] Congress 2013 and the International Papillomavirus Conference 2012) to identify additional unpublished studies. Third, MD and MB contacted the authors of conference abstracts to obtain unpublished data. MD and EB independently assessed the eligibility of all studies. Additionally, DM independently assessed the eligibility of studies of HPV infection. If more than one publication from the same data source and research team was available, we kept the publication that presented the most recent data.
Data extraction and quality assessment
Our primary outcomes were the relative risks (RR) comparing the pre-vaccination and post-vaccination periods for: the prevalence of HPV infection for four HPV type subgroups (high-oncogenic risk vaccine types [HPV16 and HPV18], three types with the greatest evidence of cross-protective efficacy [HPV31, HPV33, and HPV45],16 the five potentially cross-protective types [HPV31, HPV33, HPV45, HPV52, and HPV58],16 and all high-oncogenic risk non-vaccine types [all high-risk HPV types except for HPV16 and HPV18]); the frequency (prevalence or incidence) of anogenital wart diagnosis; and the frequency (prevalence or incidence) of high-grade cervical lesions. Two authors (MD and EB) independently extracted the study characteristics and outcomes using a standardised form. MD and MB contacted authors to request supplementary extractions to standardise data stratifications between studies for comparison and pooling (eg, same age and HPV type groupings). We also collected information about the vaccination programme characteristics and vaccination coverage of the country or region of each study (appendix pp 2–4). For the HPV prevalence studies, we collected age-specific vaccination coverage directly from each study, since vaccination status was available for all study participants. Finally, the authors of each article validated the data from their study.
Before contacting the study investigators, MD, AM, PLM, and MB assessed whether or not the studies had sufficient methodological quality to be included in the meta-analysis. The quality of the studies (potential for bias and confounding, and external validity) was assessed independently from the investigators of the original studies. Potential for bias and confounding within studies were assessed by review of the participant selection or recruitment procedures, endpoint definitions, algorithms used to identify cases, and potential confounders considered in the statistical analyses (appendix pp 5–9).
Data analysis
Because mostly girls (<20 years of age) were vaccinated in the study populations, we decided a priori to stratify all our analyses by sex and age. Furthermore, because only the quadrivalent vaccine includes HPV types 6 and 11 (which are responsible for roughly 90% of cases of anogenital warts6), we decided a priori to stratify our analyses for anogenital warts by the type of vaccine.
To ensure comparability of the study results included in the meta-analysis, we first defined pre-vaccination and post-vaccination periods for all studies (appendix pp 10–11). Second, for comparability, we used prevalence or incidence rate ratios as the measure of effect for all HPV-related endpoints. For HPV infection, most studies presented RR (crude and/or adjusted prevalence ratios) and 95% CIs. When available, we included adjusted RR in the meta-analysis. When only crude HPV prevalence over time was available, we calculated prevalence ratios by dividing the post-vaccination and pre-vaccination prevalence and estimated the 95% CI (CI approximation for prevalence ratios26). For anogenital warts and precancerous lesions, all studies presented annual frequency (prevalence or incidence) over time. We estimated pre-vaccination frequency by aggregating the data for up to 3 years before vaccination, and calculated RR by dividing each post-vaccination year by the pre-vaccination estimate.
We derived summary estimates of the effect of HPV vaccination for each endpoint by using random-effects models on the log scale.27,28 We did a subgroup analysis to identify potential sources of heterogeneity by comparing the summary estimates obtained from subsets of studies or groups within studies grouped by: vaccine type (bivalent or quadrivalent), vaccination coverage (low <50% or high ≥50%—we used study-specific coverage estimates for HPV infection, and country/region-level coverage for the other outcomes), age (<20, 20–24, 25–29, or 30–39 years), years since the vaccination programme was implemented (1, 2, 3, or 4 years), source of study data (population based, health provider/insurance based, or clinic based), and by whether or not the impact measure was adjusted (yes or no). We examined heterogeneity across studies using I2 and χ2 statistics.28 I2 values less than 50% represent low heterogeneity, between 50–75% substantial heterogeneity, and more than 75% high heterogeneity.29 The p value associated with the χ2 statistic represents the statistical significance of heterogeneity. Finally, we analysed the dose–response association between HPV vaccination coverage (independent variable) and the log-RR of each study (dependent variable) by fitting a linear regression, weighted by the inverse variances of the log-RR.30 We used Review Manager 5.2 and SAS 9.4 for all analyses.
Role of the funding source
The funders had no role in the study design, data collection, analysis and interpretation, or writing of the report. MB had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
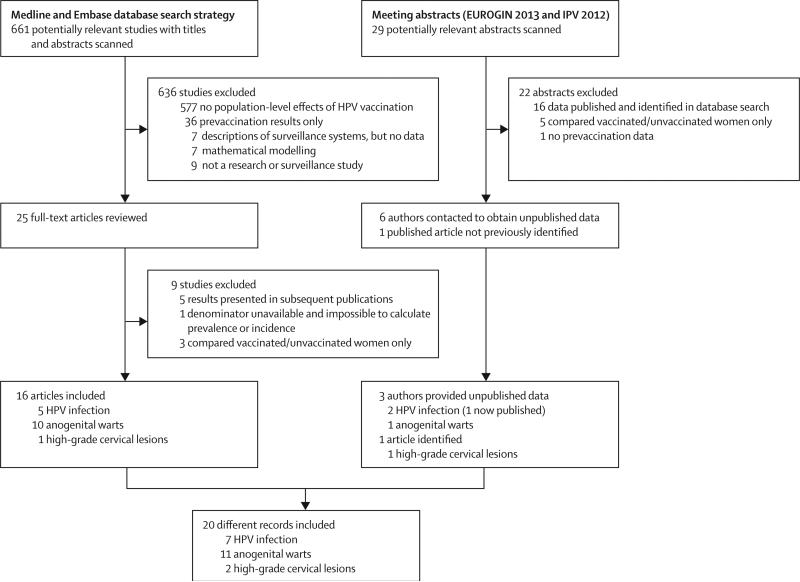
In our searches we identified 661 articles and 29 conference abstracts, of which 20 records met the inclusion criteria (seven on HPV infection,31–37 11 on anogenital warts,38–48 and two on high-grade cervical lesions;49,50 figure 1, table). The studies were done in nine high-income countries (the USA, Australia, England, Scotland, New Zealand, Sweden, Denmark, Canada, and Germany) and assessed the population-level consequences of vaccination in 16 600 women for HPV infection, more than 125 million person-years of follow-up for anogenital warts, and 15 million female-years of follow-up for high-grade cervical lesions (table). The vaccine used, vaccination strategy, delivery, and vaccination coverage varied substantially (table and appendix pp 2–4). All studies had sufficient methodological quality to be included in the meta-analysis (appendix pp 5–9). However, because two studies analysed the entire Danish population during identical time periods,43,48 we only included Baandrup and colleagues’ study43 in our main analysis (the choice of study had no effect on our results; appendix pp 12–14).
Figure 1.
Study selection
EUROGIN=EUropean Research Organisation on Genital Infection and Neoplasia. IPV=International Papillomavirus Conference. HPV=human papillomavirus.
Table.
Characteristics of the studies included in the systematic review and meta-analysis
| Vaccine used | Data source* | Study population | Population used in meta- analysis |
Data collection dates† |
Sample size used in meta- analysis‡ |
Case definition | Effect measure in publication |
Effect measure recalculated§ |
|
|---|---|---|---|---|---|---|---|---|---|
| HPV infection | |||||||||
| Cummings et al, 2012 (USA)31 | Quadrivalent | Clinic based | Girls aged 14–17 years attending one of three urban primary care clinics in Indianapolis (IN, USA) | Girls aged 14–17 years | Pre-vaccine: 1999–2005; post-vaccine: 2010 | Pre-vaccine: n=150; post-vaccine: n=75 | HPV+ Roche Linear Array (Roche Diagnostics, Indianapolis, IN, USA; 37 types) | OR of HPV prevalence (crude) | RR of HPV prevalence (crude) |
| Kahn et al, 2012 (USA)32 | Quadrivalent | Clinic based | Girls and women aged 13–26 years attending one hospital-based adolescent clinic and one community health centre in Cincinnati (OH, USA) | Girls and women aged 13–24 years who had had sexual contact | Pre-vaccine: 2006–07; post-vaccine: 2009–10 | Pre-vaccine: n=336; post-vaccine: n=383 | HPV+ Roche Linear Array (Roche Molecular Systems, Alameda, CA, USA; 37 types) | HPV prevalence difference (adjusted) | RR of HPV prevalence (adjusted) |
| Tabrizi et al, 2012 (Australia)33 | Quadrivalent | Clinic based | Women aged 18–24 years attending one of six family planning clinics in Sydney, Melbourne, and Perth (Australia) | Women aged 18–24 years | Pre-vaccine: 2005–07; post-vaccine: 2010–11 | Pre-vaccine: n=202; post-vaccine: n=1058 | HPV+ Roche Linear Array (Roche Molecular Systems; 13 types) | OR of HPV prevalence (adjusted) | RR of HPV prevalence (adjusted) |
| Markowitz et al, 2013 (USA)34 | Quadrivalent | Population based: NHANES study participants | Nationally representative sample of US girls and women aged 14–59 years | Girls and women aged 14–24 years | Pre-vaccine: 2003–06; post-vaccine: 2007–10 | Pre-vaccine: n=1795; post-vaccine: n=1185 | HPV+ Roche Linear Array (Roche Diagnostics; 37 types) | RR of HPV prevalence (crude) | RR of HPV prevalence (crude) |
| Mesher et al, 2013 (England, UK)35 | Bivalent | Clinic based | Girls and women aged 16–24 years undergoing chlamydia screening in community sexual health services, general practice, youth clinics in 7 regions around England | Girls and women aged 16–24 years | Pre-vaccine: 2008; post-vaccine: 2010–12 | Pre-vaccine: n=2354; post-vaccine: n=4178 | 2008: Hybrid Capture 2 (Qiagen) and Roche Linear Array (Roche Molecular Systems); 2010–12: HPV+ In-house multiplex PCR and Luminex-based genotyping test; 18 types)¶ | OR of HPV prevalence (adjusted) | RR of HPV prevalence (adjusted) |
| Sonnenberg et al, 2013 (England, Scotland, and Wales, UK)36 | Bivalent | Population based: NATSAL study participants | Nationally representative sample of men and women aged 16–74 years in Britain | Women aged 18–24 years | Pre-vaccine: 1999–2001; post-vaccine: 2010–12 | Pre-vaccine: n=328; post-vaccine: n=795 | HPV+ In-house Luminex-based genotyping assay (18 types)¶ in urine samples | OR of HPV prevalence (age-adjusted) | RR of HPV prevalence (crude) |
| Kavanagh et al, 2014 (Scotland, UK)37 | Bivalent | Population based: Scottish Cervical Screening Call & Recall System | Women aged 20–21 years participating in routine cervical cancer screening in Scotland | Women aged 20–21 years | Pre-vaccine: 2009–10; post-vaccine: 2011–12 | Pre-vaccine: n=2704; post-vaccine: n=1975 | HPV+ Multimetrix HPV assay (Diamex, Heidelberg, Germany; 18 types) | HPV prevalence over time (no effect measure) | RR of HPV prevalence (crude) |
| Anogenital warts | |||||||||
| Oliphant and Perkins, 2011 (New Zealand)38 | Quadrivalent | Clinic based | New clients of one sexual health service in Auckland aged 10 years and older | Female and male clients aged 15–39 years | 2007–10. Pre-vaccine: 2007–08; post-vaccine: 2009–10 | Person-years pre-vaccine: 17 517; person-years post-vaccine: 15 508 | Clinical diagnosis | Annual proportion of new clients diagnosed with anogenital warts | RR of anogenital warts proportion (crude) |
| Bauer et al, 2012 (USA)39 | Quadrivalent | Health provider/insurance based: clinical encounters claims data of a health programme | Clients of the California Family Planning, Access, Care and Treatment (PACT) programme aged 10 years and older (87% are female clients) | Female and male clients aged 15–39 years. Programme serves low-income individuals | 2007–10. Pre-vaccine: 2007; post-vaccine: 2008–10 | Person-years pre-vaccine: 1 750 980; person-years post-vaccine: 5 555 420 | ICD-9 codes 07 840, 07 811 OR prescription of imiquimod or podophyllotoxin | Annual proportion of PACT clients diagnosed with anogenital warts | RR of anogenital warts proportion (crude) |
| Kliewer et al, 2012 (Canada)40 | Quadrivalent | Population based: medical claims and hospital discharge database | Entire population of Manitoba (Canada) | People (both sexes) aged 15–39 years | 1985–2009. Pre-vaccine: 2006–08; post-vaccine: 2009 | Person-years pre-vaccine: 737 366; person-years post-vaccine: 250 984 | Treatments (one of 14 tariff codes for anogenital wart treatments) OR admission to hospital for anogenital warts with ICD-9 code 078.11 OR 0781, 078.10, 07 819 and related procedure OR ICD-10 A63.0 OR B07 and related procedure | Annual incidence rate of diagnosed anogenital warts in the population | RR of anogenital warts incidence (crude) |
| Leval et al, 2012 (Sweden)41 | Quadrivalent | Population-based: Statistics Sweden, National Patient Register, and Prescribed Drug Register | Entire population of Sweden aged 10 years and older | People (both sexes) aged 15–39 years | 2006–10. Pre-vaccine: 2006; post-vaccine: 2007–10 | Person-years pre-vaccine: 2 942 525; person-years post-vaccine: 12 043 886 | ICD-10 code A63.0 OR prescription of imiquimod or podophyllotoxin | Annual incidence rate of diagnosed anogenital warts in the population | RR of anogenital warts incidence (crude) |
| Ali et al, 2013 (Australia)42 | Quadrivalent | Clinic based | New clients of eight sexual health centres across Australia aged 12 years and older (Australian born) | Australian-born people (both sexes) aged 15–39 years | 2004–11. Pre-vaccine: 2005–07; post-vaccine: 2008–12 | Person-years pre-vaccine: 24 147; person-years post-vaccine: 37 237 | Clinical diagnosis | Annual proportion of new clients with diagnosed anogenital warts | RR of anogenital warts proportion (crude) |
| Baandrup 2013 (Denmark)43 | Quadrivalent | Population based: Statistics Denmark, National Patient Registry | Entire population of Denmark aged 10 years and older | People (both sexes) aged 15–39 years | 2006–11. Pre-vaccine: 2007–09; post-vaccine: 2010–11 | Person-years pre-vaccine: 5 140 633; person-years post-vaccine: 2 598 265 | ICD-10 code A63.0 | Annual incidence rate of diagnosed anogenital warts in the population | RR of anogenital warts incidence (crude) |
| Howell-Jones et al, 2013 (England)44 | Bivalent | Population based: genitourinary medicine (GUM) clinics | Entire population of England aged 15–24 years | People (both sexes) aged 15–24 years | 2002–11. Pre-vaccine: 2006–08; post-vaccine: 2009–11 | Person-years pre-vaccine: 6 790 231; person-years post-vaccine: 20 610 282 | Clinical diagnosis | Annual incidence rate of diagnosed anogenital warts in the population | RR of anogenital warts incidence (crude) |
| Flagg et al, 2013 (USA)45 | Quadrivalent | Health provider/insurance based: Truven Health Analytics Market Scan Commercial Claims and Encounters Database | Enrollees in roughly 100 health private insurance plans across the USA aged 10–39 years | People (both sexes) aged 15–39 years: insured employees, early retirees, and their dependents | 2003–10. Pre-vaccine: 2004–06; post-vaccine: 2007–10 | Person-years pre-vaccine: 11 864 207; person-years post-vaccine: 36 000 783 | 1) ICD-9 codes 078.41, OR 2) ICD-9 code 078.1, 078.10, or 078.19 AND therapeutic procedure or diagnosis of benign anogenital neoplasm, OR 3) At least one prescription for anogenital wart treatment AND therapeutic procedure or diagnosis of benign anogenital neoplasm | Annual proportion of insured individuals with diagnosed anogenital warts | RR of anogenital warts proportion (crude) |
| Mikolajczyk et al, 2013 (Germany)46 | Quadrivalent | Health provider/insurance based: German Pharmaco-epidemiological Research Database | Enrollees in one large health insurance company across Germany aged 10–79 years | People (both sexes) aged 15–39 years | 2005–08. Pre-vaccine: 2005–07; post-vaccine: 2008 | Person-years pre-vaccine: 4 439 256; person-years post-vaccine: 1 621 308 | ICD-10 code A63.0 | Annual incidence rate of diagnosed anogenital warts in insured individuals | RR of anogenital warts incidence (crude) |
| Nsouli-Maktabi et al, 2013 (USA)47 | Quadrivalent | Health provider/insurance based: Defense Medical Surveillance System | Members of the US Armed Forces (both sexes) across the USA aged 17 years and older | Members of the forces (both sexes) any time between 2000 and 2012, aged 17–39 years | 2000–12. Pre-vaccine: 2004–06; post-vaccine: 2007–11 | Person-years pre-vaccine: 3 569 823; person-years post-vaccine: 4 736 303 | ICD-9 code 078.1 | Annual incidence rate of diagnosed anogenital warts in US force members | RR of anogenital warts incidence (crude) |
| Sando et al, 2013 (Denmark)48 | Quadrivalent | Population based: Statistics Denmark, National Patient Registry, and Medical Products Statistics Registry | Entire population of Denmark aged 15–34 years | People (both sexes) aged 15–34 years | 2001–11. Pre-vaccine: 2007–09; post-vaccine: 2010–11 | Person-years pre-vaccine: 1 326 573; person-years post-vaccine: 2 687 020 | ICD-10 code A63.0, OR prescription of podophyllotoxin | Annual proportion of the population with diagnosed anogenital warts | RR of anogenital warts proportion (crude) |
| High-grade precancerous cervical lesions | |||||||||
| Brotherton et al, 2011 (Australia)49 (and Australian Institute of Health and Welfare, 2013)∥ | Quadrivalent | Health provider/insurance based: cervical cancer screening programme registry | Girls and women younger than 69 years of age participating in the National Cervical Screening Program | Girls and women aged 15–39 years | 2004–11. Pre-vaccine: 2005–07; post-vaccine: 2008–11 | Person-years pre-vaccine: 6 028 918; person-years post-vaccine: 7 814 102 | Histopathologically confirmed CIN2+ | Annual incidence of high-grade cervical lesions in screened girls and women | RR of high-grade lesion incidence (crude) |
| Niccolai et al, 2013 (USA)50 | Quadrivalent | Health provider/insurance based: statewide surveillance (all 34 pathology laboratories report CIN2+/adenocarcinoma in situ) | Women aged 21–39 years from Connecticut screened for cervical cancer | Women aged 21–39 years | 2008–11. Pre-vaccine: 2008; post-vaccine: 2009–11 | Person-years pre-vaccine: 411 624; person-years post-vaccine: 823 248 | Histopathologically confirmed CIN2+ | Annual incidence of high-grade lesions in women aged 21–39 years in Connecticut | RR of high-grade lesion incidence (crude) |
OR=odds ratio. HPV=human papillomavirus. RR=relative risk (post-vaccination prevalence or incidence/pre-vaccination prevalence or incidence). CIN=cervical intraepithelial neoplasia.
Data sources are regarded as population based when the study population includes the entire population of a given country or region; health provider or insurance based when the study population consists of a subgroup of the total population participating in a specific health programme or insurance plan; and clinic based when the study population comprises a finite number of clinics or hospital's clients.
For studies of HPV infection, the pre-vaccination and post-vaccination periods were already established in the original publications (except for Kavanagh et al37). For studies of anogenital warts and cervical lesions, the pre-vaccination and post-vaccination periods were established for the purpose of this systematic review as described in appendix pp 10–11.
The sample size is restricted to the age groups used in the review. For studies of HPV infection, the pre-vaccination and post-vaccination sample sizes were already established in the original studies. For studies of anogenital warts and cervical lesions, the pre-vaccination sample size corresponds to the cumulative number of person-years up to 3 years pre-vaccination, including the year of the introduction of HPV vaccination. The post-vaccination sample size corresponds to the cumulative number of person-years from 1–4 years after the introduction of vaccination, depending on the data available in each study.
For HPV infection, the investigators recalculated the RR of prevalence using the original data from their specific studies. For anogenital warts and precancerous lesions, we estimated pre-vaccination frequency by aggregating the data for up to three years before vaccination, and calculated RR by dividing each post-vaccination year by the pre-vaccination estimate.
13 high-risk HPV types were presented in the original publications, whereas the 18 high-risk HPV types available were used for the purposes of this meta-analysis.
Data from this study are restricted to the Victorian registry data. Supplementary data from the Australian Institute of Health and Welfare 2013 report51 were provided by Brotherton (JML Brotherton, National HPV Vaccination Program Register, Victorian Cytology Service, East Melbourne, Melbourne, VIC, Australia, personal communication). Since the report covers all regions of Australia, we used it as our main data source for the review.
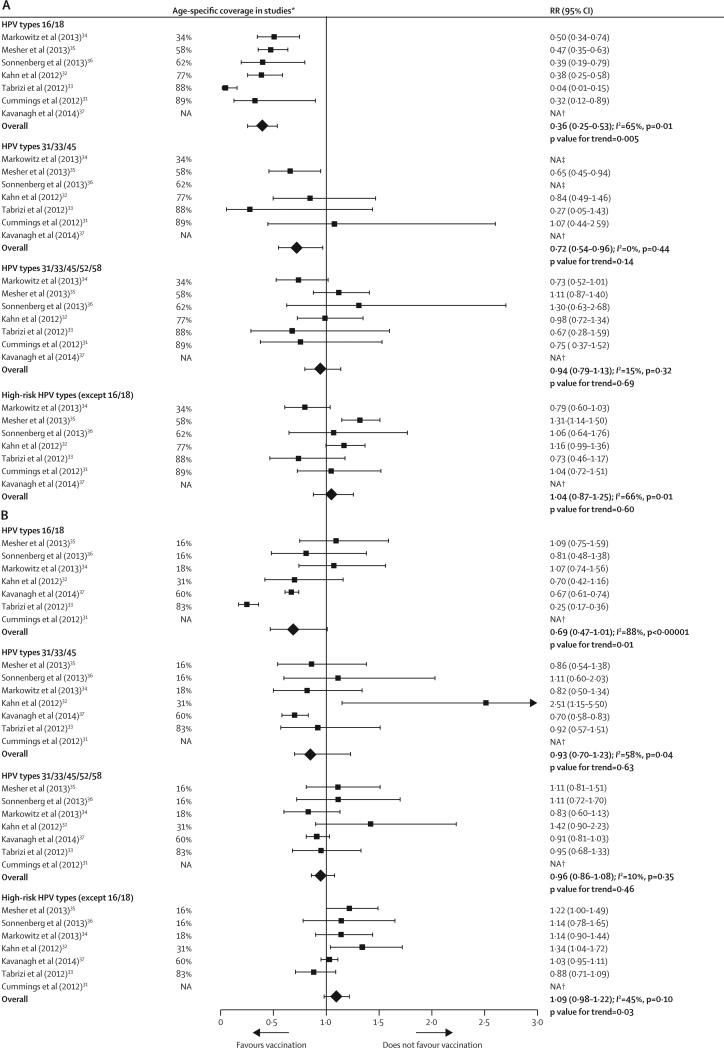
In girls 13–19 years of age, the overall prevalence of HPV types 16 and 18 decreased significantly by 64% in the post-vaccination period (RR 0·36 [95% CI 0·25–0·53]) compared with the pre-vaccination period (figure 2A), with a significant dose–response association with vaccination coverage (p=0·005). The overall prevalence of HPV types 31, 33, and 45 also decreased significantly post-vaccination by 28% (RR 0·72 [95% CI 0·54–0·96]), but the reductions were not associated with vaccination coverage. The overall prevalence of HPV types 31, 33, 45, 52, and 58, and non-vaccine high-risk types (ie, all high-risk HPV types except HPV16 and HPV18) did not change significantly between the pre-vaccination and post-vaccination periods (figure 2A).
Figure 2.
Changes in the prevalence of HPV infections between the prevaccination and postvaccination periods in (A) girls aged 13–19 years and (B) women aged 20–24 years, ranked by age-specific vaccination coverage (≥1 dose) reported in studies
RR=relative risk. HPV=human papillomavirus. NA=not available. p values for trends were obtained by fitting a linear regression between the log RR and the age-specific coverage of each study, weighted by the inverse variances of the log RR. The minimum age of participants varied between studies (see table 1). *Age-specific proportion of female participants, included in the analysis of each study, who received at least one dose of the HPV vaccine. †Data not available for girls aged 13–19 years in Kavanagh et al, and for women aged 20–24 years in Cummings et al. ‡Data not provided because they were judged potentially unreliable according to National Health and Nutrition Examination Survey analytic guidelines:52 prevalence estimates had a relative standard error of >30% and the sample size was below that recommended for analyses of complex survey data, by design effect and specified proportion. The only other data excluded were for HPV types 31/33/45 from NATSAL: unweighted prevaccination prevalence: 3/85; unweighted postvaccination prevalence: 16/215; weighted prevalence ratio 3·50 (95% CI 0·97–12·67)
In women 20–24 years of age, the overall prevalence of HPV types 16 and 18 decreased by 31% (RR 0·69 [95% CI 0·47–1·01]) in the post-vaccination period (figure 2B). Although the overall reduction in HPV16 and HPV18 infection was not significant, it showed a dose–response association with vaccination coverage (p=0·01). No significant decreases in prevalence or dose–response associations with vaccination coverage were recorded for HPV types 31, 33, and 45, or for HPV types 31, 33, 45, 52, and 58. Finally, a small—but non-significant—increase in non-vaccine high-risk HPV types occurred (RR 1·09, 95% CI 0·98–1·22), which was negatively associated with increasing vaccination coverage (p=0·03).
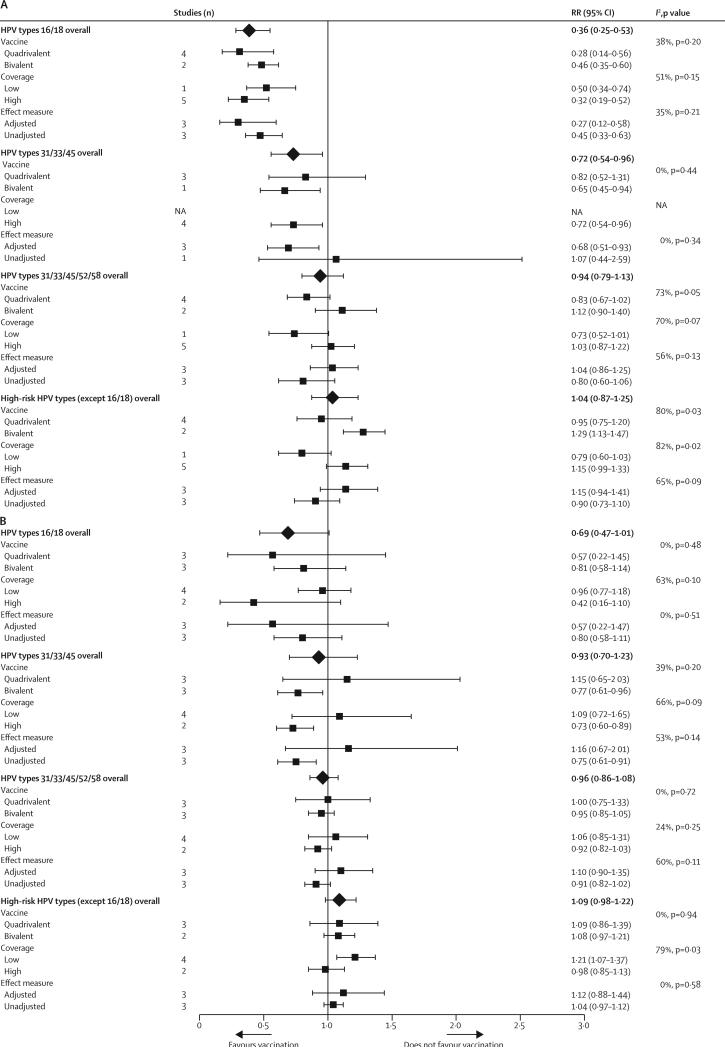
In addition to vaccination coverage, the use of adjusted or crude RRs emerged as a substantial source of heterogeneity between studies (I2 ranged between 50% and 75% for many endpoints; figure 3). Notably, the point estimates of adjusted RRs were lower than were the crude RRs for HPV subgroups with substantial post-vaccination reductions (ie, HPV types 16 and 18 in 20–24-year-old women; figure 3B, and HPV types 16 and 18 and types 31, 33, and 45 in 13–19 year-old girls; figure 3A), but were higher than the crude RRs for the other endpoints (figure 3).
Figure 3.
Subgroup analyses of the changes in the prevalence of HPV infections between the prevaccination and postvaccination periods in (A) girls aged 13–19 years and (B) women aged 20–24 years
RR=relative risk. HPV=human papillomavirus. NA=not available.
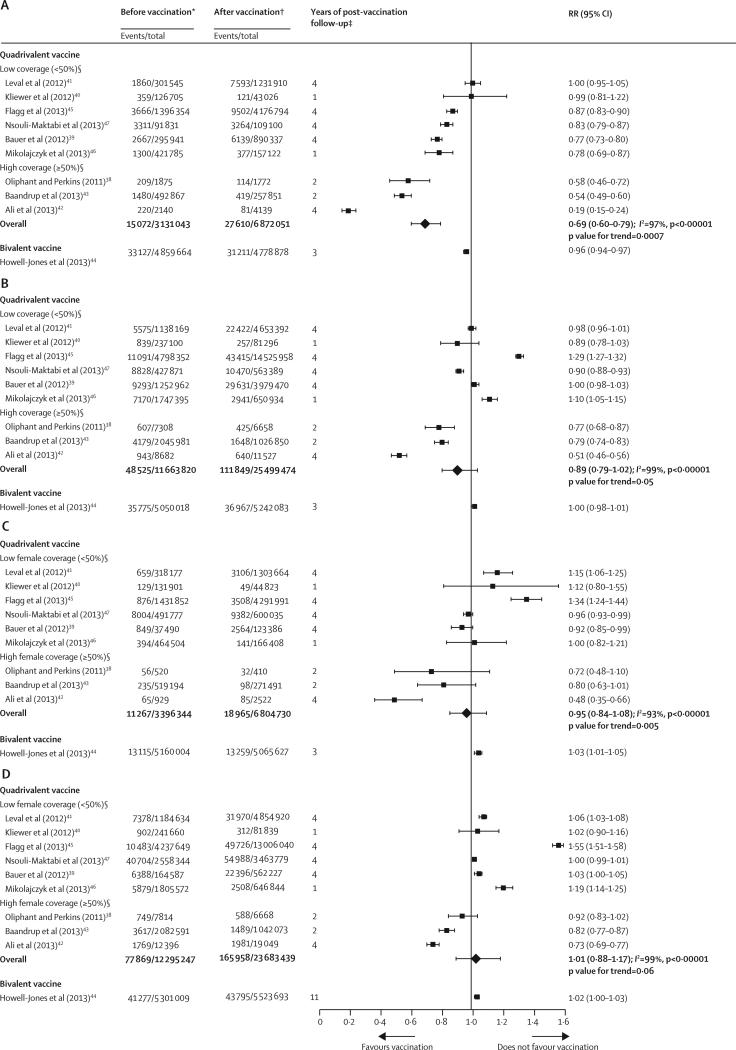
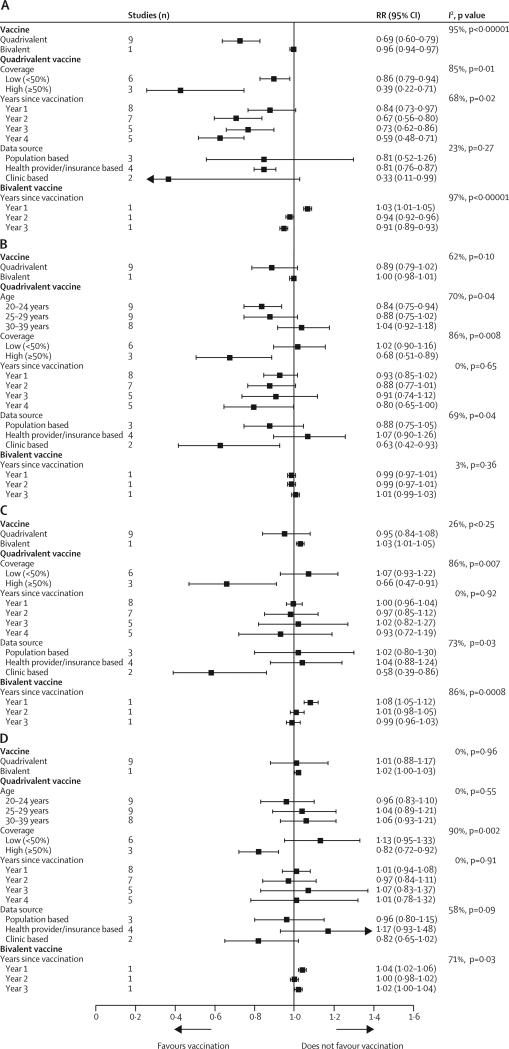
In girls aged 15–19 years in countries using the quadrivalent vaccine, anogenital warts decreased significantly by 31% (RR 0·69 [95% CI 0·60–0·79]) in the post-vaccination period. A notable dose–response association was recorded between anogenital wart reduction and increase in population-level female vaccination coverage (p=0·0007; figure 4A). In the same group, anogenital warts were reduced more substantially (by 61%) in studies with high vaccination coverage than in those with low vaccination coverage (14% reduction; figure 5A). In addition to vaccination coverage, years since the start of vaccination emerged as a significant source of heterogeneity (I2=68%, p=0·02; figure 5A).
Figure 4.
Changes in anogenital wart diagnosis between the pre-vaccination and post-vaccination periods in (A) girls aged 15–19 years, (B) women aged 20–39 years, (C) boys aged 15–19 years, and (D) men aged 20–39 years, ranked by the national or setting-specific female vaccination coverage
RR=relative risk. p values for trends were obtained by fitting a linear regression between the log RR and the rank of vaccination coverage of each study, weighted by the inverse variances of the log RR. *Before vaccination: cumulative number of cases and person-years up to 3 years pre-vaccination, including the year of the introduction of the human papillomavirus (HPV) vaccine. †After vaccination: cumulative number of cases and person-years 1–4 years after the introduction of vaccination, depending on data available in each study. ‡Years of post-vaccination follow-up: number of years after the introduction of HPV vaccination considered in the meta-analysis (see appendix pp 10–11 for more details). §Studies were ranked qualitatively by the national or setting-specific vaccination coverage, for which we considered the number of cohorts vaccinated and vaccination coverage achieved in each cohort. However, we could not estimate the overall vaccination coverage for each study (see appendix pp 2–4 for details about the programme description, number of cohorts vaccinated, and three-dose vaccination coverage for each study).
Figure 5.
Subgroup analyses of the changes in anogenital wart diagnosis between the pre-vaccination and post-vaccination periods in (A) girls aged 15–19 years, (B) women aged 20–39 years, (C) boys aged 15–19 years, and (D) men aged 20–39 years
Data are for years with female-only vaccination programmes. RR=relative risk.
In countries that used the quadrivalent vaccine, nonsignificant decreases in anogenital warts were recorded post-vaccination in women 20–39 years of age (11%, RR 0·89 [95% CI 0·79–1·02]) and in boys 15–19 years of age (5%, 0·95 [0·84–1·08]; figure 4B, 4C). Again, these reductions showed a significant dose–response association with increased population-level female vaccination coverage (p=0·05 for older women and p=0·005 for young men). Subgroup analyses showed that female vaccination coverage was a main source of heterogeneity (figure 5B, 5C). In countries with high female vaccination coverage, anogenital warts were reduced significantly by 32% in women aged 20–39 years (RR 0·68 [95% CI 0·51–0·89]) and by 34% in boys aged 15–19 years (0·66 [0·47–0·91]). No changes in anogenital warts were recorded in men aged 20–39 years in countries using the quadrivalent vaccine (figure 4D).
The only study that assessed population-level changes in anogenital warts following vaccination with the bivalent vaccine44 reported a small but significant decrease in these warts in girls aged 15–19 years (figure 4A). Conversely, a small but significant increase in anogenital warts was recorded in boys aged 15–19 years (figure 4C), and no significant effect was noted in older people of either sex (figure 4B, 4D).
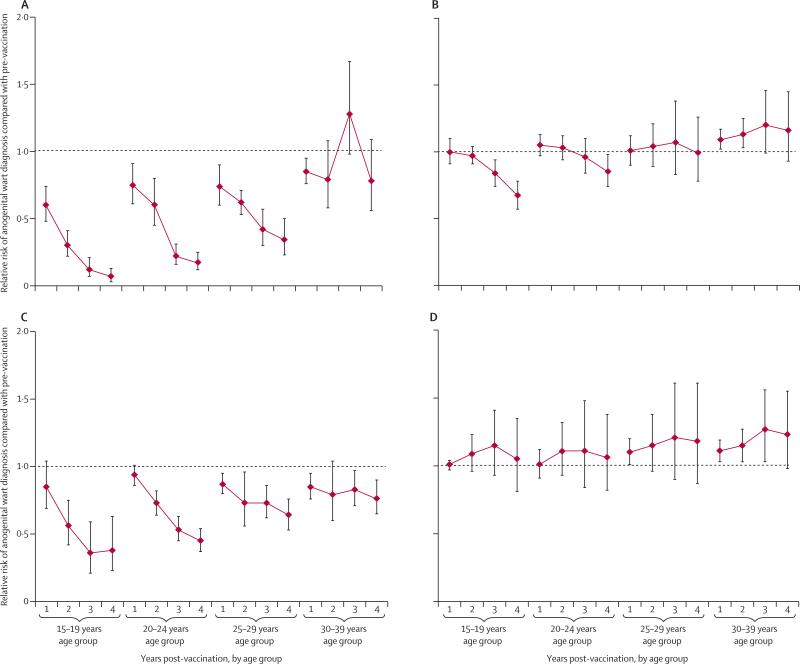
Figure 6 shows the changes over time in anogenital warts in studies of the quadrivalent vaccine, with the main sources of heterogeneity taken into consideration. Figure 6A clearly shows a rapid and significant decrease in anogenital warts over time in girls and women younger than 30 years of age in studies with high vaccination coverage. However, in studies with low vaccination coverage (figure 6B), the decline was recorded only in girls younger than 20 years of age, and became significant only in the third year after vaccination implementation. A rapid and significant decline in anogenital warts over time also occurred in boys and men younger than 30 years of age in studies with high female vaccination coverage (figure 6C). However, in studies with low female vaccination coverage, there was a general pattern of anogenital warts increasing over time, particularly in older men (figure 6D).
Figure 6.
Changes in anogenital wart diagnosis during the first 4 years after the introduction of human papillomavirus vaccination with the quadrivalent vaccine
Results are stratified by age and female vaccination coverage: (A) Girls and women, with high female vaccination coverage (≥50%); (B) girls and women, with low female vaccination coverage (<50%); (C) boys and men, with high female vaccination coverage (≥50%); (D) boys and men, with low female vaccination coverage (<50%). For high coverage, the results from the following studies were combined depending on the years of follow-up available: years 1 and 2: Oliphant and Perkins (2011),38 Baandrup et al (2013),43 and Ali et al (2013);42 years 3 and 4: Ali et al (2013).42 For low coverage, the results from the following studies were combined depending on the years of follow-up available: year 1: Leval et al (2013),41 Kliewer et al (2012),40 Flagg et al (2013),45 Nsouli-Maktabi et al (2013),47 and Mikolajczyk et al (2013);46 years 2, 3, and 4: Leval et al (2013),41 Flagg et al (2013),45 Nsouli-Maktabi et al (2013),47 and Bauer et al (2013).39 See appendix pp 2–4 for information about vaccination coverage in each study.
A significant decrease in high-grade precancerous cervical lesions was recorded in the only study49 that reported these data for girls aged 15–19 years (RR 0·69, 95% CI 0·66–0·73), but no significant change was recorded in the two studies reporting data in women aged 20 years and older (appendix p 15).
Discussion
This systematic review and meta-analysis, representing more than 140 million person-years of follow-up data from nine high-income countries, reports significant population-level decreases in HPV-related outcomes up to 4 years after the implementation of HPV vaccination programmes. In countries with high vaccination coverage, HPV16 and HPV18 infection, and anogenital warts decreased by more than 60% in girls younger than 20 years of age, starting after the first year of the vaccination programmes. Furthermore, in these countries, our results suggest evidence of vaccine cross-protection and herd effects, with significant reductions in HPV31, HPV33, and HPV45 infection in girls younger than 20 years of age, and in anogenital warts in men and older women. In countries with low vaccination coverage, significant reductions were recorded for HPV16 and HPV18 infection and anogenital warts in girls younger than 20 years of age, but no significant reductions were noted for HPV31, HPV33, and HPV45 in this group, or HPV-related outcomes in boys, men, and older women (ie, no indication of cross-protection or herd effects). Our findings provide strong evidence that HPV vaccination is highly effective and can provide cross-protection outside trial settings, and reinforce the need for early vaccination and high vaccination coverage to maximise population-level effectiveness and herd effects.
Although this meta-analysis is based on time-trend ecological studies, and therefore causality cannot be concluded, several factors strongly suggest that the reported reductions in population-level HPV-related outcomes can be attributed to HPV vaccination. These factors are: the magnitude of the effect; the dose–response association between vaccination coverage and effect; and consistency, both between the studies included in the review (despite the different methods and settings) and with results from clinical trials and mathematical modelling. First, reductions in HPV types 16 and 18, anogenital warts, and high-grade cervical lesions were large and statistically significant in the target age groups for vaccination (girls <20 years of age). Second, we found a statistically significant positive association between increases in vaccination coverage and reduction in HPV types 16 and 18 infection in girls younger than 20 years of age and anogenital warts in both women and men. Furthermore, reductions in anogenital warts increased over time since vaccination (as the number of vaccinated cohorts increased), especially in youngest age groups with highest vaccination coverage. Third, the results showed consistency between countries with similar levels of vaccination coverage. Furthermore, in the studies in which the vaccine status was available, vaccinated women had significantly lower HPV-related outcomes than did unvaccinated women in the post-vaccination era.32–34,37,42,53–56 Our results are also consistent with data from clinical trials that showed a high vaccine-type efficacy,11,12 and suggested some degree of cross-protection against HPV types 31, 33, and 45, but not against types 52 or 58.16 However, the higher bivalent cross-protective efficacy reported in a recent meta-analysis of clinical trial data16 was not shown in our population-level meta-analysis. Finally, the large herd effects reported with high vaccination coverage are consistent with predictions from dynamic models.20–24
The studies included in the meta-analysis possess the strengths and weaknesses inherent in ecological studies. They provide a wealth of timely information about the effects of HPV vaccination using large study populations, but are especially vulnerable to information bias and confounding (appendix pp 5–9). However, the three most important potential sources of bias and confounding in these studies are likely to underestimate the effect of vaccination. First, because of increased awareness of anogenital warts from licensing of the HPV vaccines and the launch of the vaccination programmes, potential exists for confounding related to possible increases in health-seeking behaviours and information bias from increased diagnosis of anogenital warts over time.
Second, most studies had insufficient or no information to adequately control for sexual activity, which might have been increasing over time.43,57,58 These limitations might explain the slight increase in the prevalence of non-vaccine high-risk HPV types and anogenital wart consultations in the post-vaccination period within groups with low or no vaccination coverage (eg, women older than 20 years, and men).
Third, information bias might be present as a consequence of masking by HPV type 16 and 18, especially in the pre-vaccine period.59 That is, by preventing HPV16 and HPV18 infection, vaccination could remove the potential masking effect of these types, producing increased detection of non-vaccine types. Conversely, the main potential source of overestimation of vaccination effects is present in clinic-based studies that measure the proportion of consultations attributable to anogenital warts in sexual health clinics (appendix pp 7–8).38,42 Indeed, changes in the clientele of the clinics between the pre-vaccination and post-vaccination periods could overestimate the vaccination effect on anogenital warts if consultations due to other causes (eg, chlamydia consultations42) became more frequent. Clinic-based studies represent two-thirds of the studies assessing the population-level effect of vaccination on anogenital warts in countries with high vaccination coverage, and could partly explain slight reductions in anogenital warts in adult men.
Fourth, the external validity of the studies was generally good (appendix pp 5–9). However, because most studies were undertaken on individuals consulting the health system, the results for the effect of HPV vaccination might not be completely generalisable to groups with lower levels of health-seeking behaviour, especially in countries in which the HPV vaccine is delivered in health-care clinics. Finally, in view of the indirect nature of our inferences, our analysis might not have had adequate sensitivity to detect small post-vaccination effects (eg, type-replacement, or herd effects and cross-protection when vaccination coverage is low).
Our results should be interpreted cautiously because they represent only the short-term population-level effects of HPV vaccination programmes. First, the cohorts of vaccinated girls have not yet reached the ages with highest incidence rates of HPV infection, anogenital warts, and cervical lesions (ie, between 20 and 35 years of age). Therefore, the direct and herd effects are expected to continue to increase over time as overall population-level vaccination coverage increases.
Second, the existing evidence is insufficient to draw conclusions about the existence of net type-replacement (eg, no significant increase in the prevalence of high-risk non-vaccine HPV types in groups with the highest vaccination coverage), which could be because no type-replacement is occurring, or partly a consequence of the short follow-up time or dilution of type-specific changes by grouping HPV types.
Third, the time horizon was too short to examine waning of vaccine efficacy. However, randomised controlled trials have shown no signs of waning vaccine efficacy after 9·5 years of follow-up.60
Fourth, in view of the long lag time between infection and cancer, no direct evidence of the effect of vaccination on HPV-related cancers is currently available. However, since HPV infection is the cause—and high-grade precancerous cervical lesions the precursors—of cervical cancer, these intermediate outcomes have been judged acceptable proxies for efficacy against cervical cancer by regulatory bodies worldwide.61–64 Nevertheless, one should be careful in using reductions in precancerous cervical lesions from screening databases as proxies for cervical cancer because they might represent changes in screening recommendations and participation, and they are not HPV type-specific. Additionally, surveillance studies based on cervical screening registries could overestimate the population-level effect of HPV vaccination, if vaccine uptake is higher in women who undergo screening.65–68
Finally, as previously shown, trends in HPV types 6 or 11-related disease (eg, anogenital warts) are a poor proxy of change in HPV types 16 and 18 and related diseases (eg, cervical cancer).69 This is because HPV6 and HPV11 will be easier to eliminate and control through vaccination than HPV16 and HPV18 because of their shorter durations of infectiousness and lower transmissibility.
Our overall findings are likely generalisable to high-income countries, since most of the heterogeneity between countries disappeared once results were stratified by vaccination coverage and age, and given similarities in sexual behaviour,58 HPV type distribution,70,71 age profile of HPV prevalence,72 and cervical cancer incidence between high income countries.73 However, precise estimates of population-level effect will vary between countries according to their programmatic specificities, such as the characteristics of catch-up campaigns.
Our results should be extrapolated to low-income and middle-income countries with caution because all studies in the meta-analysis were from high-income countries and substantial differences exist between these countries and low-income and middle-income countries in sexual behaviour,58 HPV epidemiology,72,73 and potential cofactors of HPV infection and disease, such as high HIV prevalence.74 However, no evidence exists to suggest that vaccine efficacy would be lower in low-income and middle-income countries, especially because the vaccine has been shown safe and immunogenic in women with HIV infection.75 On the other hand, herd effects could differ in low-income and middle-income countries with very different population-level sexual behaviour (eg, increased mixing between older men and younger women, and more concurrency in partnerships). Even in the unlikely scenario that there would be no herd effects in such countries, a recent global modelling study (PRIME)19 has shown that HPV vaccination would be highly cost effective, in view of the very high cervical cancer incidence and mortality in these countries,
This first meta-analysis of the population-level effect of HPV vaccination programmes shows compelling evidence of a strong and statistically significant dose–response association between HPV vaccination coverage and reductions in HPV16 and HPV18 infection and anogenital warts in cohorts of girls and women targeted for vaccination. Additionally, our study provides the first evidence of a dose–response association between female vaccination coverage and reduction of anogenital warts in older women and men.
Our results have important policy implications. The sharpest declines in HPV-related outcomes in both male and female participants were recorded in countries with school-based vaccine delivery (eg, the UK, Australia, and New Zealand), which suggests that this strategy helps faster rollout and higher vaccination coverage than non-school-based vaccine programmes. Our study also shows population-level data supporting clinical trial evidence of HPV vaccine cross-protection against HPV types 31, 33, and 45, although no dose–response with vaccination coverage was recorded.
In conclusion, the results of this study are very promising for the long-term population-level effect of HPV vaccination programmes on cervical cancer and other HPV-related diseases. However, we must continue to monitor and evaluate HPV vaccination programmes to confirm these results, and we need to remain vigilant for evidence of potential waning efficacy, type-replacement, or lower vaccination coverage in groups at greater risk of HPV-related cancers.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This work was supported by the Canada Research Chairs programme (support for MB) and an operating grant from the Canadian Institutes of Health Research (grant no. MOP-119427). The authors would also like to thank Caty Blanchette for doing statistical analysis, Michael Malloy for supplementary analysis of the Australian data, Rebecca Howell-Jones who did the original analyses of decreases in genital warts in England, and Kavita Panwar who undertook the human papillomavirus testing for the report by Mesher and colleagues.
Footnotes
The first three authors and senior author are listed in order of contribution. All other authors are listed in alphabetical order
Contributors
MD, MB, and M-CB conceived the study. MD and EB did the literature search and did the analysis. MB, M-CB, AM, PL-M, and JB participated in the analysis. MD and MB codrafted the first version of the report. DM independently assessed eligibility of studies on human papillomavirus infection. All other authors (HA, LB, HB, SB, JMLB, TC, BD, CKF, EWF, AMJ, JAK, KK, SKK, EVK, LM, DM, LN, JO, KGP, KS, PS, SNT, and CT) provided data, after having done supplementary analysis for the purposes of this meta-analysis. All authors interpreted the results and critically revised the report for scientific content. All authors approved the final version of the report.
Declaration of interests
In the past 3 years, MB reports unrestricted grants from Merck (for herpes zoster research—none are ongoing). MD has done consulting work for GlaxoSmithKline (herpes zoster vaccine). HA has received a grant from bioCSL for the Australian genital warts surveillance network. SB, DM, and KS have received grants from GlaxoSmithKline for human papillomavirus testing of some samples (study number EPI-HPV-109903). JMLB has received grants from bioCSL and Merck. BD has received grants from bioCSL and speaker fees from Merck and Sanofi Pasteur MSD. CKF owns shares in CSL Biotherapies who have licensed the Gardasil vaccine to Merck, and has received travel reimbursement and speaker fees from Merck. AMJ has been a Governor of the Wellcome Trust since 2011. JAK has received a grant from Merck. SKK has received grants from Merck and is a member of the Scientific Advisory Board of Merck and an expert lecturer for Sanofi Pasteur MSD. EVK has received grants from Merck and GlaxoSmithKline and personal fees from Merck. LN has received consulting fees from Merck. SNT has received grants from bioCSL. EB, M-CB, LB, HB, JB, TC, EWF, KK, PL-M, LM, AM, JO, KGP, PS, and CT declare no competing interests.
References
- 1.WHO . Countries with HPV vaccine in the national immunization programme and planned introductions. World Health Organization/IVB Database; Jan, 2014. [Sept 15, 2014]. http://www.who.int/immunization/diseases/hpv/decision_implementation/en/ [Google Scholar]
- 2.Cervical Cancer Action (CCA) Progress in cervical cancer prevention. [Sept 15, 2014];The CCA report card. 2012 Dec; http://www.cervicalcanceraction.org/pubs/CCA_reportcard_low-res.pdf.
- 3.The World Bank [Sept 15, 2014];Countries and economies. 2006 http://data.worldbank.org/country.
- 4.Ladner J, Besson MH, Rodrigues M, Audureau E, Saba J. Performance of 21 HPV vaccination programs implemented in low and middle-income countries, 2009–2013. BMC Public Health 2014. 14:670. doi: 10.1186/1471-2458-14-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 6.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction-the first five years. Vaccine. 2012;30(suppl 5):F139–48. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Recommendations on the use of quadrivalent human papillomavirus vaccine in males-Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–08. [PubMed] [Google Scholar]
- 9.Georgousakis M, Jayasinghe S, Brotherton J, Gilroy N, Chiu C, Macartney K. Population-wide vaccination against human papillomavirus in adolescent boys: Australia as a case study. Lancet Infect Dis. 2012;12:627–34. doi: 10.1016/S1473-3099(12)70031-2. [DOI] [PubMed] [Google Scholar]
- 10.Paul P, Fabio A. Literature review of HPV vaccine delivery strategies: considerations for school- and non-school based immunization program. Vaccine. 2014;32:320–26. doi: 10.1016/j.vaccine.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 12.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler CM, Castellsague X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler CM, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16–26 years. J Infect Dis. 2009;199:936–44. doi: 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 15.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 16.Malagon T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–89. doi: 10.1016/S1473-3099(12)70187-1. [DOI] [PubMed] [Google Scholar]
- 17.Brisson M, Van de Velde N, Boily MC. Economic evaluation of human papillomavirus vaccination in developed countries. Public Health Genomics. 2009;12:343–51. doi: 10.1159/000214924. [DOI] [PubMed] [Google Scholar]
- 18.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31:3786–804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 19.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health. 2014;2:e406–14. doi: 10.1016/S2214-109X(14)70237-2. [DOI] [PubMed] [Google Scholar]
- 20.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 21.Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis. 2011;204:372–76. doi: 10.1093/infdis/jir285. [DOI] [PubMed] [Google Scholar]
- 22.Choi YH, Jit M, Gay N, Cox A, Garnett GP, Edmunds WJ. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine. 2010;28:4091–102. doi: 10.1016/j.vaccine.2009.09.125. [DOI] [PubMed] [Google Scholar]
- 23.Baussano I, Dillner J, Lazzarato F, Ronco G, Franceschi S. Upscaling human papillomavirus vaccination in high-income countries: impact assessment based on transmission model. Infect Agent Cancer. 2014;9:4. doi: 10.1186/1750-9378-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogaards JA, Kretzschmar M, Xiridou M, Meijer CJ, Berkhof J, Wallinga J. Sex-specific immunization for sexually transmitted infections such as human papillomavirus: insights from mathematical models. PLoS Med. 2011;8:e1001147. doi: 10.1371/journal.pmed.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner B. Fundamentals of biostatistics. 4th edn. Wadsworth Publishing Company; Belmont: 1995. [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Deeks JJ, Higgins PT. [May 28, 2014];Statistical algorithms in Review Manager 5. 2010 http://www.cochrane.org/sites/default/files/uploads/handbook/Statistical_Methods_in_RevMan5-1.pdf.
- 29.The Cochrane Collaboration Higgins JPT, Green S, editors. [May 28, 2014];Cochrane handbook for systematic reviews of interventions, version 5.1.0. 2011 www.cochrane-handbook.org.
- 30.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–28. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Cummings T, Zimet GD, Brown D, et al. Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine. 2012;30:5496–99. doi: 10.1016/j.vaccine.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130:e249–56. doi: 10.1542/peds.2011-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabrizi SN, Brotherton JML, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–51. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 35.Mesher D, Soldan K, Howell-Jones R, et al. Reduction in HPV 16/18 prevalence in sexually active young women following the introduction of HPV immunisation in England. Vaccine. 2013;32:26–32. doi: 10.1016/j.vaccine.2013.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenberg P, Clifton S, Beddows S, et al. Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382:1795–806. doi: 10.1016/S0140-6736(13)61947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavanagh K, Pollock KG, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110:2804–11. doi: 10.1038/bjc.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliphant J, Perkins N. Impact of the human papillomavirus (HPV) vaccine on genital wart diagnoses at Auckland Sexual Health Services. N Z Med J. 2011;124:51–58. [PubMed] [Google Scholar]
- 39.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007-2010. Am J Public Health. 2012;102:833–85. doi: 10.2105/AJPH.2011.300465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kliewer E, Mahmud SM, Demers AA, Lambert P, Musto G. Quadrivalent HPV vaccination and the incidence of anogenital warts in Manitoba, Canada.. 28th International Papillomavirus Conference; San Juan, Puerto Rico. Nov 30–Dec 6, 2012; Abstract E07-663. [Google Scholar]
- 41.Leval A, Herweijer E, Arnheim-Dahlstrom L, et al. Incidence of genital warts in sweden before and after quadrivalent human papillomavirus vaccine availability. J Infect Dis. 2012;206:860–66. doi: 10.1093/infdis/jis405. [DOI] [PubMed] [Google Scholar]
- 42.Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032. doi: 10.1136/bmj.f2032. [DOI] [PubMed] [Google Scholar]
- 43.Baandrup L, Blomberg M, Dehlendorff C, Sand C, Andersen KK, Kjaer SK. Significant decrease in the incidence of genital warts in young Danish women after implementation of a national human papillomavirus vaccination program. Sex Transm Dis. 2013;40:130–35. doi: 10.1097/OLQ.0b013e31827bd66b. [DOI] [PubMed] [Google Scholar]
- 44.Howell-Jones R, Soldan K, Wetten S, et al. Declining genital warts in young women in England associated with HPV 16/18 vaccination: an ecological study. J Infect Dis. 2013;208:1397–403. doi: 10.1093/infdis/jit361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health 2013. 103:1428–35. doi: 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikolajczyk RT, Kraut AA, Horn J, Schulze-Rath R, Garbe E. Changes in incidence of anogenital warts diagnoses after the introduction of human papillomavirus vaccination in Germany–an ecologic study. Sex Transm Dis. 2013;40:28–31. doi: 10.1097/OLQ.0b013e3182756efd. [DOI] [PubMed] [Google Scholar]
- 47.Nsouli-Maktabi H, Ludwig SL, Yerubandi UD, Gaydos JC. Incidence of genital warts among U.S. service members before and after the introduction of the quadrivalent human papillomavirus vaccine. MSMR. 2013;20:17–20. [PubMed] [Google Scholar]
- 48.Sando N, Kofoed K, Zachariae C, Fouchard J. A reduced national incidence of anogenital warts in young Danish Men and women after introduction of a national quadrivalent human papillomavirus vaccination programme for young women—an ecological study. Acta Derm Venereol. 2014;94:288–92. doi: 10.2340/00015555-1721. [DOI] [PubMed] [Google Scholar]
- 49.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 50.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiol Biomarkers Prev. 2013;22:1446–50. doi: 10.1158/1055-9965.EPI-13-0272. [DOI] [PubMed] [Google Scholar]
- 51.Australian Institute of Health and Welfare Cervical screening in Australia 2010–2011. [June 4, 2014];National Cervical Screening Program. 2013 AIHW Cancer series no. 76, http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129543399.
- 52.US Department of Health and Human Services, Centers for Disease Control and Prevention National Health And Nutrition Examination Survey: Analytic Guidelines, 1999–2010. [July 17, 2014];Data evaluation and methods research. 2013 Sep; Series 2, number 161, http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf.
- 53.Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ. 2014;348:g1458. doi: 10.1136/bmj.g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leval A, Herweijer E, Ploner A, et al. Quadrivalent human papillomavirus vaccine effectiveness: a Swedish national cohort study. J Natl Cancer Inst. 2013;105:469–74. doi: 10.1093/jnci/djt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blomberg M, Dehlendorff C, Munk C, Kjaer SK. Strongly decreased risk of genital warts after vaccination against human papillomavirus: nationwide follow-up of vaccinated and unvaccinated girls in Denmark. Clin Infect Dis. 2013;57:929–34. doi: 10.1093/cid/cit436. [DOI] [PubMed] [Google Scholar]
- 56.Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227. doi: 10.1186/1741-7015-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382:1781–94. doi: 10.1016/S0140-6736(13)62035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet. 2006;368:1706–28. doi: 10.1016/S0140-6736(06)69479-8. [DOI] [PubMed] [Google Scholar]
- 59.Mori S, Nakao S, Kukimoto I, Kusumoto-Matsuo R, Kondo K, Kanda T. Biased amplification of human papillomavirus DNA in specimens containing multiple human papillomavirus types by PCR with consensus primers. Cancer Sci. 2011;102:1223–27. doi: 10.1111/j.1349-7006.2011.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10:2147–62. doi: 10.4161/hv.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagliusi SR, Teresa Aguado M. Efficacy and other milestones for human papillomavirus vaccine introduction. Vaccine. 2004;23:569–78. doi: 10.1016/j.vaccine.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 62.National Advisory Committee on Immunization (NACI) Statement on human papillomavirus vaccine. [June 15, 2014];Canada Communicable Disease Report. 2007 Feb 15;33 http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/07vol33/acs-02/index-eng.php. [Google Scholar]
- 63.US Food and Drug Administration [June 15, 2014];Clinical review—human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant—Gardasil. 2006 http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM111287.pdf.
- 64.European Centre for Disease Prevention and Control [June 15, 2014];Guidance for the introduction of HPV vaccines in EU countries. 2008 http://www.ecdc.europa.eu/en/Documents/4940_0801_HPV_guidance.pdf.
- 65.Steens A, Wielders CC, Bogaards JA, Boshuizen HC, de Greeff SC, de Melker HE. Association between human papillomavirus vaccine uptake and cervical cancer screening in the Netherlands: implications for future impact on prevention. Int J Cancer. 2013;132:932–43. doi: 10.1002/ijc.27671. [DOI] [PubMed] [Google Scholar]
- 66.Kliewer EV, Mahmud SM, Demers AA, Lambert P. Human papillomavirus vaccination and Pap testing profile in Manitoba, Canada. Vaccine. 2013;32:33–38. doi: 10.1016/j.vaccine.2013.10.082. [DOI] [PubMed] [Google Scholar]
- 67.Spencer AM, Roberts SA, Brabin L, Patnick J, Verma A. Sociodemographic factors predicting mother's cervical screening and daughter's HPV vaccination uptake. J Epidemiol Community Health. 2014;68:571–77. doi: 10.1136/jech-2013-202629. [DOI] [PubMed] [Google Scholar]
- 68.Jemal A, Simard EP, Dorell C, et al. Annual report to the nation on the status of cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013. 105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brisson M, Van de Velde N, Boily MC. Different population-level vaccination effectiveness for HPV types 16, 18, 6 and 11. Sex Transm Infect. 2011;87:41–43. doi: 10.1136/sti.2010.044412. [DOI] [PubMed] [Google Scholar]
- 70.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–59. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 71.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–05. doi: 10.1038/sj.bjc.6601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 73.International Agency for Research on Cancer Globocan 2008. [May 14, 2012];Cervical cancer incidence and mortality worldwide. 2008 http://globocan.iarc.fr/factsheets/cancers/cervix.asp.
- 74.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of HPV-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26:1645–52. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: a partially-blind randomised placebo-controlled study. Vaccine. 2013;31:5745–53. doi: 10.1016/j.vaccine.2013.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.