Abstract
Purpose
Crohn’s disease is a type of inflammatory bowel disease affecting estimated 4 million people worldwide. Therapy stratification of Crohn’s disease (CD) is mainly based on the inflammatory activity being assessed by endoscopic biopsy and clinical criteria. Cross-sectional imaging allows for the assessment of structural characteristics of the entire gastrointestinal tract including small bowel loops and may provide potential non-invasive image-based biomarkers for the inflammatory activity of CD. The aim of this study was to explore the predictive value of Computed Tomography-based morphologic patterns for inflammatory activity in CD.
Material and methods
42 patients diagnosed with CD were included in a retrospective study (13 male, 29 female, median age 32 years). Abdominal CT imaging was carried out on symptomatic patients at a single institution 0–10 days prior to endoscopic biopsy or surgery using a protocol optimized for the characterization of structural bowel alterations. Image data were initially reviewed independently by three radiologists and discrepancies were settled in consensus with a focus on mesenteric fat stranding and combing, mesenteric adenopathy, mesenteric abscess, intraperitoneal free fluid, fistula, skip lesions, highest wall thickness and the localization of the affected bowel. The extent of inflammatory activity in the bowel wall was determined subsequently by histological analysis.
Results
All intestinal and extraintestinal CT findings except the mesenteric comb sign showed a tendency towards higher extent or prevalence in patients with high histological inflammatory activity score, especially median bowel wall thickness (6.0 mm vs. 3.5 mm), mesenteric abscesses (32% vs. 0%) and mesenteric adenopathy (94% vs. 45%). Spearman rank order correlation coefficient indicated a significant correlation of bowel wall thickness (r = 0.40, p < 0.05), mesenteric adenopathy (r = 0.54, p < 0.05), mesenteric abscess (r = 0.33, p < 0.05) and mesenteric fat stranding (r = 0.33, p < 0.05) with the histological inflammatory activity score.
Conclusion
CT-based biomarkers including wall thickness, mesenteric fat stranding, mesenteric lymphadenopathy and mesenteric abscess positively correlated with the histological inflammatory activity score and therefore provided additional information for therapy stratification in symptomatic patients with CD, particularly as most of these biomarkers are hidden from endoscopy.
Keywords: CT Crohn Small bowel Computed Tomography, Histopathology
1. Introduction
Crohn’s Disease (CD) is regarded as an unpredictable, relapsing, transmural inflammatory disease potentially affecting any part of the gastrointestinal tract (GI) [1]. It usually involves the large intestine and the distal small bowel causing inflammation, ulcerations, bleeding and ultimately fibrosis and scarring of the GI tract wall [2].
Proper diagnosis of CD is challenging and requires multiple tests. Diagnosis is based on medical history, physical examination, laboratory tests, endoscopy including biopsy and imaging studies. The Montreal classification [3] with age at diagnosis, location, behavior and the CD Activity Index (CDAI) [4] are widely used for disease assessment and therapy stratification. However, these scores are unreliable in differentiating remission and active CD [5]. Currently, endoscopy and biopsy are the gold standard for assessing the inflammatory activity of CD. Nevertheless, diagnosis in the small bowel disease is difficult to achieve, because common endoscopy is limited to the colon and distal small bowel. Furthermore, endoscopic examination including biopsy is not always immediately available and can be stressful for the patient. In contrast, Computed Tomography (CT) imaging is widely available and provides a non-invasive method for the evaluation of the entire GI tract including extraintestinal manifestations and complications of CD, which are not visible by endoscopy. CT and MRI are the imaging modalities of choice to depict the localization and CD severity and provide information on disease activity. Imaging of the morphological characteristics of CD include assessment of mucosal alterations, transmural involvement and extraintestinal manifestations. In this context, CT may provide image-based biomarkers for the inflammatory activity of CD, which could further contribute to effective therapy stratification. Here, we explore the potential value of CT morphologic pattern as predictor of inflammatory activity in CD.
2. Material and methods
2.1. Patient characteristics
We researched retrospectively the hospital database at a single institution for a period of 5 years. Inclusion criteria were a CT scan performed 0–10 days prior to endoscopic biopsy or surgery and a histopathologically proven diagnosis of CD. Patients with other imaging modalities or with a CT scan but without histopathological correlation were excluded from the study. We included 42 patients diagnosed with CD in our retrospective study (13 male, 29 female, median age 32 years), who underwent abdominal Multidetector Computed Tomography (MDCT) imaging 0–10 days prior to biopsy or surgery. MDCT datasets were acquired at a single institution.
The study entailed patients with acute symptoms and a broad spectrum of inflammatory activity, which was determined by histological analysis of bowel wall tissue specimens. Absence of inflammatory activity (score = 0) was found in 2/42 patients, mild inflammatory activity (score = 1) in 5/42 patients, moderate inflammatory activity (score = 2) in 4/42 patients and high inflammatory activity (score = 3) in 31/42 patients (Table 1). Patients were predominantly female. Median age was 32 years (Table 1). The study was approved by the local Ethics Committee. The work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Informed consent was waived.
Table 1.
Summary of patient characteristics.
| Histological inflammatory activity score |
|||
|---|---|---|---|
| 0–2 | 3 | Overall | |
| n | 11 | 31 | 42 |
| Male (n)/Female (n) | 2/9 | 11/20 | 13/29 |
| Age (y) | 26 (20–36) | 38 (23–65) | 32 (20–65) |
Data are median and range in brackets.
2.2. Histological analysis
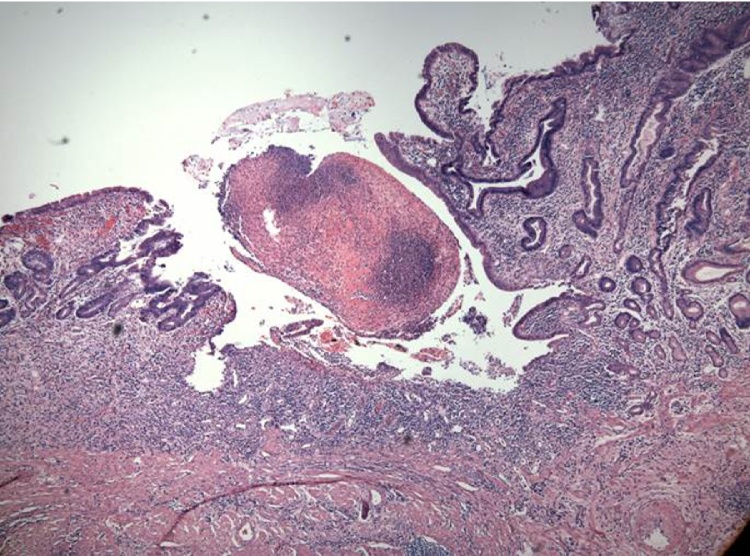
The histological evaluation of the inflammatory activity in the bowel wall served as a reference standard. After macroscopic examination, endoscopic and surgical bowel specimens underwent fixation with formalin and standard hematoxylin-eosin safran stain and microscopic analysis. The extent of inflammatory activity was reported by a pathologist with experience in inflammatory bowel disease who was blinded for clinical, biological and radiological data. The standardized evaluation process was based on a semiquantitative visual 4-point scale of 0 = absence of inflammation, 1 = mild inflammation (neutrophil infiltrate limited to the mucosa), 2 = moderate inflammation (neutrophil infiltrate limited to mucosa and submucosa), and 3 = high inflammation (transmural neutrophil infiltrate affecting the muscularis propria or fistula/abscess of the subserosa) (Fig. 3).
Fig. 3.
40 year old women, typical active inflammatory reaction of the gastrointestinal mucosa characterized by neutrophilic invasion of mucosal glands.
2.3. Computed tomography imaging
CT imaging was performed using a 16 row MDCT unit (GE 16 Light Speed CT scanner, General Electric, Milwaukee, WI, USA) using an imaging protocol dedicated to the assessment of structural alterations of the GI tract. Distension of small bowel loops was obtained by administration of approximately 1.0 L oral contrast medium (3% Gastrografin solution, meglumine diatrizoate, Bristol-Myers Squibb) over 60 min. Intravenous contrast of 100 mL iopromide (Ultravist 300, 300 mg iodine/mL; Bayer HealthCare Pharmaceuticals Inc, Wayne, NJ) was injected at a rate of 3.0 mL/sec and bolus-tracking with a region of interest in the descending aorta at the level of the first lumbar vertebra was applied to generate enteric phase CT data. Image acquisition started at 35 s after the trigger (trigger threshold level 100HU [Hounsfield Unit]) during breathhold at a mean inspiratory level. Axial and coronal CT images (slice thickness 5.0 mm, reconstruction interval 3.0 mm) were reconstructed and transferred to a picture archiving and communication (PACS) system for image interpretation.
2.4. MDCT image evaluation
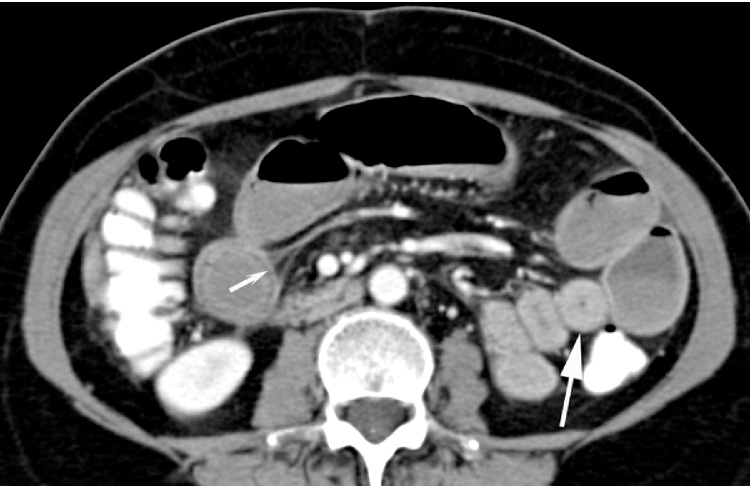
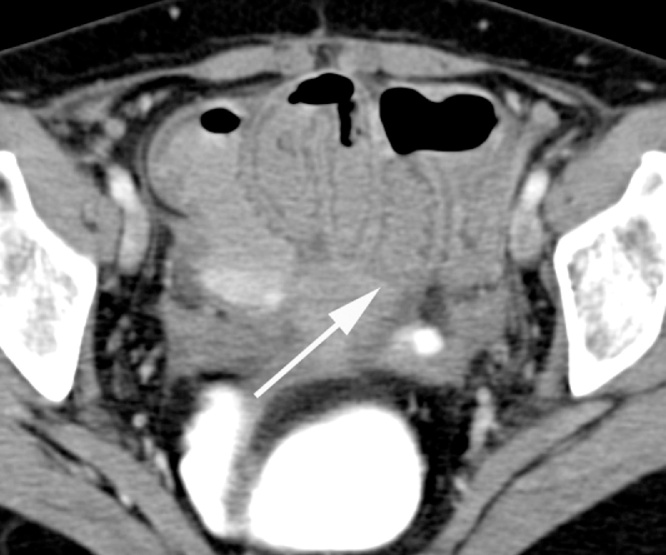
Image data were independently reviewed by three experienced radiologists who were blinded to clinical or histopathological data. Discrepancies were settled in consensus. The presence or absence of potential CT findings related to CD including mesenteric fat stranding, comb sign (Fig. 1), mesenteric adenopathy, mesenteric abscess, free intraperitoneal fluid, fistula, skip lesions, wall thickness (Fig. 2) and localization of the affected bowel segment were reported. The maximum wall thickness of inflamed bowel was quantified by manual measurement on axial or coronal image reconstructions, depending on the most appropriate image orientation. Image quality was evaluated visually with a semi-quantitative 5-point scale (0 = poor, not diagnostic, 1 = reduced but diagnostic, 2 = moderate, 3 = good, 4 = excellent) .
Fig. 1.
33 year old man, axial contrast-enhanced MDCT image of small bowel wall thickening (big arrow) and comb sign (small arrow) involving the distal ileum.
Fig. 2.
40 year old women, axial contrast-enhanced MDCT image of bowel wall thickening and mucosal enhancement involving long segment of distal ileum (big arrow).
2.5. Statistical analysis
Basic descriptive data were expressed as medians with interquartile range (Q1–Q3). Calculations were performed with SPSS 22 (IBM, Armonk, New York, USA). Spearman’s rank correlation coefficient was calculated for potential CT-biomarkers and histological inflammatory activity score. By convention, r between 0.0–0.2 was regarded as negligible, 0.2–0.4 as weak, 0.4–0.7 moderate, 0.7–0.9 strong, and 0.9–1.0 very strong correlation [6]. Multiple testing was compensated for by the Bonferroni-Holm method [7].
3. Results
All patient datasets showed diagnostic image quality. Specifically, image quality was estimated to be excellent in the majority (34/42) of patients. Good image quality was found in 6/42 and moderate image quality in 2/42 patients, in such cases it was mainly due to mild respiratory motion artifacts.
Considering the relatively small number of individuals with low to moderate inflammatory activity score, we formed two patient groups (score = 0–2 vs. score = 3) for the tabular display of descriptive data (Table 2). All intestinal and extraintestinal MDCT findings except the mesenteric comb sign showed a tendency towards higher prevalence or extent in patients with high histological inflammatory activity score (Table 2). Notably, the extent of bowel wall thickness (6.0 mm vs. 3.5 mm), and the prevalence of mesenteric abscesses (32% vs. 0%) and mesenteric adenopathy (94% vs. 45%) tended to be higher in patients with a high inflammatory activity score (Table 2).
Table 2.
Prevalence or extent of CT findings in relation to histological inflammatory activity.
| CT-based biomarkers prevalence (%)/extent (mm) | Histological inflammatory activity score |
|
|---|---|---|
| 0–2 | 3 | |
| Wall thickness | 3.5 mm (Q1 = 1.3; Q3= 4.0) | 6.0 mm (Q1= 5.0; Q3= 7.0) |
| Mesenteric fat stranding | 73% | 94% |
| Mesenteric comb sign | 64% | 48% |
| Mesenteric lymphadenopathy | 45% | 94% |
| Mesenteric abscess | 0% | 32% |
| Intraperitoneal free fluid | 18% | 39% |
| Fistula | 27% | 38% |
| Skip lesions | 45% | 60% |
The prevalence of CT findings in the abdominal digestive tract including mesenteric fat stranding, mesenteric adenopathy, mesenteric abscess, intra-peritoneal free fluid, fistula and skip lesions as well as the extent of bowel wall thickening tended to be higher in patients with high histological inflammatory activity score (score = 3) compared to patients with low to moderate inflammatory activity (score = 0–2). The prevalence of the mesenteric comb sign tended to be lower in patients with high inflammatory activity.
Including all degrees of severity of the histologically assessed inflammatory activity, Spearman rank order correlation coefficient indicated a moderate correlation between the extent of bowel wall thickness and histological inflammatory activity score (r = 0.40, p < 0.05) (Table 3). Moreover, there was a moderate correlation between the prevalence of mesenteric adenopathy and histological inflammatory activity score (r = 0.54, p < 0.05) (Table 3). There was also a weak correlation between mesenteric abscesses and mesenteric fat stranding with histological inflammatory activity score (each r = 0.33, p < 0.05) (Table 3). Other MDCT findings related to CD did not show a significant correlation with the histological inflammatory activity score. Moreover, there was no statistically significant correlation between the localization of affected bowel segments and the inflammatory activity score. Specifically, lesions with low or moderate (score = 0–2) and high (score = 3) inflammatory activity scores were located predominantly in the ileocolic region (21% vs. 38%) and in the ileum (3% vs. 28%). Few lesions with low/moderate and high inflammatory activity were found in the colon (3% vs. 5%). Other bowel segments such as the Appendix were affected in 3% of cases with high inflammatory activity.
Table 3.
Correlation of CT findings with histological inflammatory activity score.
| Inflammatory activity vs. | Cases n | Rho | p |
|---|---|---|---|
| Wall thickness | 42 | 0.40 | <0.05 |
| Mesenteric fat stranding | 42 | 0.33 | <0.05 |
| Mesenteric adenopathy | 42 | 0.54 | <0.05 |
| Mesenteric abscess | 42 | 0.33 | <0.05 |
Spearman rank order correlation coefficient Rho calculated for the extent of bowel wall thickening, the presence of mesenteric fat stranding, adenopathy, and abscesses with histological inflammatory activity score in CD.
4. Discussion
In the present study, we aimed to explore the potential value of various intestinal and extraintestinal MDCT-based morphologic patterns to evaluate the extent of inflammatory activity in CD. Inflammatory activity determined by histological analysis was highly prevalent (95%) in this patient cohort. Imaging patterns of inflammatory bowel alterations were detected by MDCT in 41/42 subjects with active CD, confirming the ability of CT to detect inflammation in patients with inflammatory bowel disease [8]. This finding is supported by Chiorean et al. who found a high sensitivity of CT enteroclysis in the diagnosis of CD with CT and demonstrated the ability of CT to differentiate between inflammatory and fibrostenotic lesions [9].
The main localization of inflammation was found in the ileum and ileocolic region in 23% of lesions with low to moderate inflammatory activity and in 67% of lesions with high activity. This allows MDCT to play to its strengths, since small bowel loops are difficult to reach with endoscopy, accompanied by increased risk for bleeding, perforation and capsule retention if capsule endoscopy is performed [10]. Boudiaf et al. demonstrated the ability of CT to depict small bowel abnormalities with a sensitivity of 100%, a specificity of 95%, a positive predictive value of 94% and a negative predictive value of 100% [11].
We found a significant correlation of MDCT-based patterns of bowel inflammation and the histological inflammatory activity score. In this context, four different patterns appeared to be valuable predictors of inflammatory activity: mesenteric adenopathy (highest correlation), bowel wall thickness (moderate correlation) and mesenteric abscess/fat stranding (weak correlation) with the histological inflammatory activity score. Consequently, mesenteric adenopathy showed high prevalence in patients with high inflammatory activity (94%) compared to patients with no or low activity (45%). Mesenteric fat stranding showed high prevalence both in patients with high (94%) as well as moderate inflammatory activity (73%).
Abscesses were infrequent and found in subjects with high inflammatory activity. Bowel wall thickness was almost twice as high in patients with high inflammatory activity (6.0 vs. 3.5 mm). Results of Zissin et al. also showed CT findings of small bowel obstruction secondary to CD with a thickened small bowel wall and luminal narrowing that was evident at the transition zone in 12 of 14 examined patients [12].
Skip lesions or fistula did not correlate with the histological inflammatory activity score, since such findings reflect chronic inflammation [13] and our patient cohort suffered from acute onset inflammation. Additionally, mesenteric comb sign did not correlate with histological inflammation, since it is also mainly found in patients with chronic inflammation and only in few patients with acute inflammation. Comb sign is detected in up to 60% in patients with inactive ulcerative colitis and up to 8% of patients with Crohn disease [14]. It may also be seen in radiation enteritis, graft versus host disease, and chronic ischemic bowel [14]. The localization of CD did not correlate with inflammatory activity, which is not surprising because CD can potentially occur anywhere in the GI tract [13]. The predictive value of intestinal and extraintestinal CT findings for inflammatory activity of CD may be of relevance in subjects whose pathology is located mainly in small bowel segments, which are difficult to access by endoscopy. In such cases, MDCT may provide additional information about the status of inflammation and help to guide therapy. Moreover, the value of CT imaging in CD lies not only in the estimation of inflammatory activity. CT imaging offers the opportunity for detection of extraintestinal manifestations of CD, such as spondylarthritis and sacroiliitis [15]. Also extraluminal abscess or the extent of a perforation cannot be pre-estimated by endoscopy [8]. Moreover, ileoscopy offers a very limited access to the small bowel due to its localization to the terminal ileum [16].
MDCT provides a total overview of the small bowel. Moreover, MDCT allows for the detection of metastases of malignant diseases, which are hidden from endoscopy. This is of relevance since patients with CD are at increased risk of small bowel adenocarcinoma and lymphoma [17], which can result in liver metastasis and adenopathy [17].
Therefore the predictive value of MDCT for inflammatory activity will not lead to a replacement of endoscopy, but MDCT is able to provide complementary information with regard to small bowel inflammation, extraintestinal manifestations and possible malignant comorbidities.
There are several limitations of our study. First of all, the radiation exposure of patients and the cancer risk due to the cumulative radiation dose of CT in young patients are limiting factors. Furthermore MDCT has a lower spatial resolution compared to conventional enterography [8]. MRI is an alternative noninvasive imaging technique that can be used as a radiation-free alternative for evaluation of patients with CD [18]. But in an emergency setting CT is widely used in patients with abdominal pain [19]. In addition, MDCT is less stressful for the patient in the acute period of CD compared to MRI since it is faster and less confining [8]. Often a MDCT scan is performed as the first imaging method to rule out acute abdominal pathology resulting in the diagnosis of CD [19]. CT is also the fastest and most cost effective imaging method [20].
It is generally accepted that new CT techniques and advances in postprocessing, e.g. iterative reconstructions, are an excellent choice for radiation dose reduction without compromising image quality [20]. O’Neill et al. used an effective dose of approximating 1 mSv in patients with CD to confirm the feasibility of sub-millisievert abdominopelvic CT [21].
In conclusion, to prove the value of CT in the clinical assessment of CD prospective studies are needed to determine whether information gained from CT actually changes clinical decision-making and outcomes.
5. Conclusion
Intestinal and extraintestinal CT-based biomarkers of bowel inflammation including mesenteric adenopathy, wall thickness, mesenteric fat stranding, and mesenteric abscess positively correlated with the histological inflammatory activity score and can be regarded as weak to moderate predictors of inflammatory activity. Since inflammation of small bowel loops, extraintestinal manifestations or complications as well as CD-related malignancies are largely hidden from endoscopy; CT can provide complementary information for therapy stratification in patients with acute manifestations of CD.
Conflicts of interest
None.
Contributor Information
N. Paquet, Email: n.paquet@gmx.de.
J.N. Glickman, Email: jglickman@MiracaLS.com.
S.M. Erturk, Email: mehmeterturk@Superonline.com.
P.R. Ros, Email: Pablo.Ros@uhhospitals.org.
J.T. Heverhagen, Email: johannes.heverhagen@insel.ch.
M.A. Patak, Email: Michael.Patak@hirslanden.ch, michael@patak.ch.
References
- 1.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 2.Crohn B.B., Ginzburg L., Oppenheimer G.D. Regional ileitis; a pathologic and clinical entity. Am. J. Med. 1952;13:583–590. doi: 10.1016/0002-9343(52)90025-9. [DOI] [PubMed] [Google Scholar]
- 3.Satsangi J., Silverberg M.S., Vermeire S., Colombel J.F. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best W.R., Becktel J.M., Singleton J.W., Kern F., Jr. Development of a Crohn’s disease activity index: national cooperative Crohn’s disease study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 5.af Bjorkesten C.G., Nieminen U., Turunen U., Arkkila P., Sipponen T., Farkkila M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand. J. Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 6.Karlik S.J. Exploring and summarizing radiologic data. AJR Am. J. Roentgenol. 2003;180:47–54. doi: 10.2214/ajr.180.1.1800047. [DOI] [PubMed] [Google Scholar]
- 7.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- 8.Dambha F., Tanner J., Carroll N. Diagnostic imaging in Crohn’s disease: what is the new gold standard. Best Pract. Res. Clin. Gastroenterol. 2014;28:421–436. doi: 10.1016/j.bpg.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Chiorean M.V., Sandrasegaran K., Saxena R., Maglinte D.D., Nakeeb A., Johnson C.S. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am. J. Gastroenterol. 2007;102:2541–2550. doi: 10.1111/j.1572-0241.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 10.Moeschler O., Mueller M.K. Deep enteroscopy—indications, diagnostic yield and complications. World J. Gastroenterol. 2015;21:1385–1393. doi: 10.3748/wjg.v21.i5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudiaf M., Jaff A., Soyer P., Bouhnik Y., Hamzi L., Rymer R. Small-bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338–344. doi: 10.1148/radiol.2332030308. [DOI] [PubMed] [Google Scholar]
- 12.Zissin R., Hertz M., Paran H., Bernheim J., Shapiro-Feinberg M., Gayer G. Small bowel obstruction secondary to Crohn disease: CT findings. Abdom. Imaging. 2004;29:320–325. doi: 10.1007/s00261-003-0111-1. [DOI] [PubMed] [Google Scholar]
- 13.Freeman H.J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 2014;20:31–36. doi: 10.3748/wjg.v20.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madureira A.J. The comb sign. Radiology. 2004;230:783–784. doi: 10.1148/radiol.2303020645. [DOI] [PubMed] [Google Scholar]
- 15.Isene R., Bernklev T., Hoie O. Extraintestinal manifestations in Crohn’s disease and ulcerative colitis: results from a prospective, population-based European inception cohort. Scand. J. Gastroenterol. 2015;50:300–305. doi: 10.3109/00365521.2014.991752. [DOI] [PubMed] [Google Scholar]
- 16.Bodily K.D., Fletcher J.G., Solem C.A. Crohn disease: mural attenuation and thickness at contrast-enhanced CT Enterography—correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505–516. doi: 10.1148/radiol.2382041159. [DOI] [PubMed] [Google Scholar]
- 17.Hemminki K., Li X., Sundquist J., Sundquist K. Cancer risks in Crohn disease patients. Ann. Oncol. 2009;20:574–580. doi: 10.1093/annonc/mdn595. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.S., Kim A.Y., Yang S.K. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 19.Kerner C., Carey K., Baillie C. Clinical predictors of urgent findings on abdominopelvic CT in emergency department patients with Crohn’s disease. Inflamm. Bowel Dis. 2013;19:1179–1185. doi: 10.1097/MIB.0b013e31828133ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Hawary M.M., Kaza R.K., Platt J.F. CT enterography: concepts and advances in Crohn’s disease imaging. Radiol. Clin. N. Am. 2013;51:1–16. doi: 10.1016/j.rcl.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill S.B., Mc Laughlin P.D., Crush L. A prospective feasibility study of sub-millisievert abdominopelvic CT using iterative reconstruction in Crohn’s disease. Eur. Radiol. 2013;23:2503–2512. doi: 10.1007/s00330-013-2858-2. [DOI] [PubMed] [Google Scholar]