Abstract
Background and Aims:
Global estimates of overweight and obesity prevalence are based on the World Health Organisation (WHO) body mass index (BMI) cut-off values of 25 and 30 kg m−2, respectively. To validate these BMI cut-offs for adiposity in the island population of Mauritius, we assessed the relationship between BMI and measured body fat mass in this population according to gender and ethnicity.
Methods:
In 175 young adult Mauritians (age 20-42 years) belonging to the two main ethnic groups—Indians (South Asian descent) and Creoles (African/Malagasy descent), body weight, height and waist circumference (WC) were measured, total body fat assessed by deuterium oxide (D2O) dilution and trunk (abdominal) fat by segmental bioimpedance analysis.
Results:
Compared to body fat% predicted from BMI using Caucasian-based equations, body fat% assessed by D2O dilution in Mauritians was higher by 3–5 units in Indian men and women as well as in Creole women, but not in Creole men. This gender-specific ethnic difference in body composition between Indians and Creoles is reflected in their BMI–Fat% relationships, as well as in their WC–Trunk Fat% relationships. Overall, WHO BMI cut-offs of 25 and 30 kg m−2 for overweight and obesity, respectively, seem valid only for Creole men (~24 and 29.5, respectively), but not for Creole women whose BMI cut-offs are 2–4 units lower (21–22 for overweight; 27–28 for obese) nor for Indian men and women whose BMI cut-offs are 3–4 units lower (21–22 for overweight; 26–27 for obese).
Conclusions:
The use of BMI cut-off points for classifying overweight and obesity need to take into account both ethnicity and gender to avoid gross adiposity status misclassification in this population known to be at high risk for type-2 diabetes and cardiovascular diseases. This is particularly of importance in obesity prevention strategies both in clinical medicine and public health.
Introduction
Although obesity has reached epidemic proportions worldwide,1 it has been increasing particularly rapidly in developing countries undergoing rapid economic growth associated with modernization, urbanization, globalization of food markets and a lifestyle of low physical activity.1, 2 The island nation of Mauritius, situated in the Southern Indian Ocean and east of Madagascar, is one such developing country—where the prevalence of coronary heart disease and type-2 diabetes is among the highest in the world, and higher than that observed for Caucasians at the same body mass index (BMI).3, 4 National Health Surveys conducted every five years over the past three decades3, 4, 5, 6, 7, 8, 9, 10 have indicated a steady increase in this island population's body mass index (BMI) associated with a surge in cardiovascular disease and type-2 diabetes that affect almost equally its two largest ethnic population groups (constituting >90% of the population), namely: Indian of South Asian ancestry and Creole of African/Malagasy ancestry. According to the results of the two latest National Surveys conducted in 2009 and 2015,9, 10 more than 50% of the adult population can be classified as overweight (preobese) or obese based on the World Health Organisation (WHO) BMI cut-offs of 25 and 30 kg m−2, respectively.
However, these WHO BMI cut-offs to delineate overweight and obesity were defined on the basis of the general trends in the relationship between BMI and morbidity and mortality rates,11 and not because BMI is an accurate measure of obesity. Indeed, BMI, a ratio of weight to height squared, is only a proxy of adiposity, and does not discriminate between fat mass and lean mass. As excess adiposity is believed to have a central role in the pathogenesis of type-2 diabetes and cardiovascular disease,12, 13 and body composition is influenced by gender and ethnicity,12, 13, 14, 15 it is therefore important to validate the extent to which the WHO BMI cut-offs for overweight and obese classification, based on Caucasians, are universal and valid for this multiethnic island population. To this end, the primary objectives of the study reported here were as follows:
to use the deuterium oxide (D2O) dilution technique in establishing the BM–body fat% relationship in a healthy cohort of young adult Mauritians according to gender and ethnicity;
to compare measured body fat% in this population sample with that predicted from Caucasian-based equations relating body fat% and BMI, 16, 17, 18 and to calculate the deviations from WHO (Caucasian-based) cut-offs.
A secondary objective was to apply segmental bioelectrical impedance analysis, using the ViScan (Tanita, Tokyo, Japan) device that measures both trunk (abdominal) fat% and waist circumference (WC), in establishing the relationship between WC and abdominal fat% in this same cohort, and to investigate potential gender and ethnic differences in this relationship for central adiposity.
Subjects and methods
Participants
Subject volunteers belonging to the two main ethnic groups on the island—those of South Asian ancestry (Indians) and those of Malagasy/African ancestry (Creoles)—were recruited from the Non Communicable Disease (NCD) 2009 survey cohort, via the NCD Health Promotion Unit at the Ministry of Health.9 Participants were chosen on the basis of general good health, pre-defined age 20–42 years, gender, ethnicity (Indians, Creoles), low physical activity occupation, non-regular smokers, non-regular alcohol consumers, and living in urban and suburban areas within an hour traveling distance by bus to the study screening centre located at the Central Health Laboratory Department, Victoria Hospital, Mauritius. The present study was conducted in accordance to the guidelines laid down in the Declaration of Helsinki, and received the approval of the Ethics Committee of the Ministry of Health and Quality of Life in Mauritius; written informed consent was obtained from all participants. The study measurements were performed with all subjects in the overnight-fasted state between 0800–1300 hours in a quiet and temperature-controlled room at the Central Health Laboratory Department, Victoria Hospital.
Anthropometry
Before the measurements, subjects were asked to empty their bladder and to remove all bulky clothing, jewelry and clothing accessories. Body weight was measured to the nearest 0.1 kg in light clothing and without shoes using an electronic weighing scale (Tanita Corporation) and taking into account the weight of clothes estimated to be ~1 kg. Standing height was measured to the nearest 1 mm using a portable stadiometer (Tanita Leicester Height Measure, Leicester, UK), and sitting height was then measured, using the same stadiometer, with the subject sitting on a chair with a flat and hard sitting board, and according to the Standardization Reference Manual of Lohman et al.19 BMI was calculated as the ratio of weight (kg) and height (m2), and the Cormic index as the ratio of sitting height and height. WC was measured to the nearest 1 mm at navel level using a non-stretchable tape, according to the Standardization Reference Manual of Lohman et al.;19 it was also measured by the ViScan device (as described below). All anthropometric parameters were measured by a single well-trained investigator, and the measurements were made in duplicate and the average value for each measurement was used in the analysis.
Body composition
Total body fat assessed by isotopic dilution technique
For each subject, a baseline saliva sample was obtained for the determination of background isotopic enrichment; the subject chewed a small piece of cotton wool for about 2 min, and the saliva from the cotton wool was then extracted and collected in a tube. This was followed by the administration of an oral 30 g dose of D2O (99.9% purity, Cambridge Isotope Laboratories, MA, USA); the bottle containing the D2O dose was rinsed with 100 ml drinking (bottle) water before its consumption so as to ensure complete ingestion of the tracer dose by the subject. A second and third saliva sample were obtained at 3 and 4 h after D2O ingestion, allowing equilibration with body water compartments. Saliva samples were collected into labeled plastic screw-capped test tubes and stored at −20 °C until analysis. Zip-lock bags were used to keep all samples for a single participant together and prevent cross-contamination. Enrichment in pre- and post-dose saliva samples was measured by Fourier Transformed Infrared Spectrophotometry using FTIR IR Affinity-1 (Shimadzu Corporation, Kyoto, Japan), after the saliva samples were centrifuged at 1000 r.p.m. for 10 min at room temperature, in accordance with the International Atomic Energy Agency (IAEA) guidelines.20 Each sample was measured in duplicate, and the mean value was used in the analysis. Before saliva measurement, the D2O standard was prepared by dilution of D2O with deionised water. The infrared spectra were measured in the range 2300–2800/cm. The magnitude of the response obtained from the FTIR was deducted from the deuterium absorption curve by an algorithm (Isotope Software, Shimadzu Corporation). Total body water (TBW) in kg was calculated from deuterium enrichment at time zero with the use of a correction factor for non-aqueous dilution of D2O, thereby accounting for the exchange of labile hydrogen that occurs in humans during the equilibration period. It is assumed that the deuterium space was 1.04 times TBW20 such that: TBW (kg)=VD /1.041, where VD (kg)=Dose D2O (mg)/enrichment 2H in saliva (mg kg−1). For each subject, the average of the 3 and 4 h values was used in the final calculation of TBW. Fat-free mass (FFM) was then calculated from TBW using a hydration constant of 0.73,20 fat mass was calculated as the difference between body weight and FFM, and total fat% was calculated as fat mass as a percentage of body weight.
Abdominal fat and WC by ViScan device
The ViScan Bioimpedance Analysis (BIA) device (ViScan AB-140, Tanita) was used for assessing trunk (abdominal) fat% by dual-frequency BIA. In addition, this device measures WC by laser technology. Subjects were placed supine on a suitable clinical table, relaxed and arms positioned on their chest. First, the laser was aligned with the subject's umbilicus and the apparatus measured the WC at this site by a combination of laser and infrared sensors located on both sides of the device. Second, a belt with in-built BIA electrodes was positioned on the abdomen in direct contact with the skin, verifying that the laser beam matched the middle of the belt. The ViScan device, which operates as a tetrapolar impedance method but applied specifically to the abdominal region, has been shown to be accurate for predicting total abdominal fat% when validated against MRI techniques.21, 22, 23 The reliability of this equipment for WC had also been previously validated by repeatability tests ran in triplicate and showing a low range of intra-individual coefficient of variation (CV%=0–1.5%) for WC measurement.24 Trunk Fat% and WC measurements were made in duplicate, repositioning the ViScan AB-140 between each measurement, and the average of the results was recorded.
Data analysis and statistics
Data analyses were performed using statistical software (STATISTIX version 8.0; Analytical Software, St Paul, MN, USA), the figures were made using Graphpad Prism Software (version 5.02 for Windows, San Diego, CA, USA), and all tabulated data are presented as mean±standard deviation (s.d.). The analysis of body composition parameters (total Fat%, Trunk Fat%) vs anthropometric surrogates of adiposity (BMI, WC) was performed by linear model procedures including Pearson's product-moment correlations for determining linear associations between variables, and statistical comparisons of the two regression lines for equality of variance, slopes and elevations (that is, y-intercepts). The analytical software for comparison of regression lines utilizes the analysis of covariance (ANCOVA). It first compares the variances for the two regression lines using Bartlett's test. Subsequently, assuming homogeneity of variance, it compares the slopes. Assuming homogenous variances and parallel lines, it then tests for differences in the y-intercept. For all tests, significance was set at P<0.05.
Results
Subjects characteristics
The subjects' mean age, weight, height and BMI, according to gender and ethnicity are provided in Table 1. The mean age (32.4–34.4 years), BMI (24.6–26.4 kg m−2) and WC (91.9–95.3 cm) of all groups and subgroups are similar, whereas weight and height are significantly higher in men than in women by 15.3 kg and 14 cm, respectively. Examination of the data according to ethnicity within each gender shows that compared with Creoles, the Indians are significantly shorter by 3 cm in men (P<0.05) and 5 cm in women (P<0.001), and weigh less by about 1.8 kg in men and 5.3 kg in women, respectively, with statistical significance for weight being reached only for females (P<0.05). The shorter stature of Indians than Creoles resides in a lower sitting height (and not in leg length), and hence in a lower ratio of sitting height and height, that is, in a lower Cormic index. On the basis of WHO BMI cut-offs, about 50% of the men and women participants in both ethnicities are classified as overweight or obese (Supplementary Table S1).
Table 1. Population sample physical characteristics (n=175) according to gender and ethnicity.
|
All |
Men |
Women |
||||
|---|---|---|---|---|---|---|
| Men, n=87 | Women, n=88 | Indians, n=41 | Creoles, n=46 | Indians, n=56 | Creoles, n=32 | |
| Age (years) | 33.7±5.7 | 32.7±6.5 | 32.8±5.1 | 34.4±6.2 | 32.4±6.4 | 33.2±6.9 |
| Weight (kg) | 77.7±15.7 | 62.4*** ±14.9 | 76.8±15 | 78.6±16.4 | 60.4±16.1 | 65.7†±12.2 |
| Height (m) | 1.72±0.07 | 1.58***±0.06 | 1.71±0.06 | 1.74†±0.07 | 1.56±0.06 | 1.61††† ±0.06 |
| BMI (kg m−2) | 26.2±5 | 24.9±5.3 | 26.4±5.3 | 26.0±4.7 | 24.6±5.9 | 25.3±3.9 |
| WC (cm) | 94.6±12.3 | 92.6±12.6 | 95.3±12.3 | 94.0±12.3 | 91.9±14 | 93.8±9.4 |
| Sitting height (m) | 0.78±0.07 | 0.71***±0.07 | 0.75±0.04 | 0.81††±0.08 | 0.69±0.05 | 0.74††±0.08 |
| Leg length (m) | 0.94±0.08 | 0.87***±0.06 | 0.96±0.05 | 0.93±0.10 | 0.87±0.05 | 0.87±0.07 |
| Cormic index (sitting height/height) | 0.454±0.039 | 0.449±0.034 | 0.441±0.019 | 0.465†±0.047 | 0.442±0.023 | 0.462±0.045 |
Abbreviations: BMI, body mass index; WC, waist circumference.
Values are means±s.d.; between subgroup comparisons (men vs women or Indians vs Creoles) by Mann–Whitney test, with statistical significance of differences as follows: gender difference: ***P<0.001; ethnic difference: †P<0.05; ††P<0.01; †††P<0.001.
BMI–Fat relationships
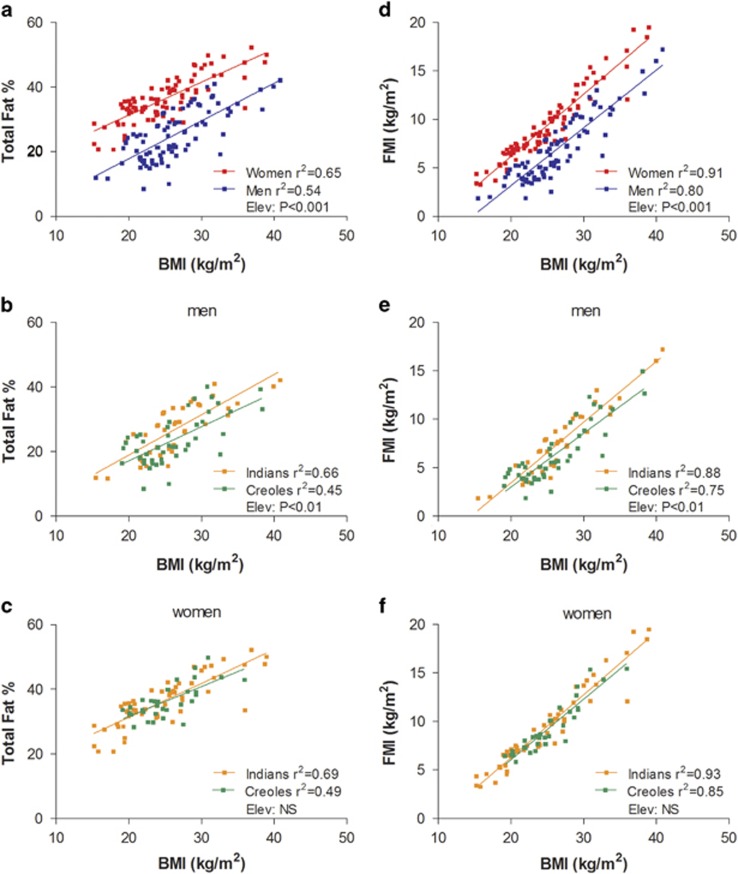
Using the D2O dilution technique to assess body fat%, the relationship between Fat%–D2O and BMI can be constructed using linear regression models on this population sample according to gender and ethnicity, as shown in Figure 1. Statistical comparison of the regression lines for men and women (Figure 1a) indicate no differences in the slope but significant differences in the y-intercept (P<0.001) indicating, as expected, higher body fat% in women than in men across the BMI range studied (by 13%). In men, comparison of the BMI–Fat% regression lines according to ethnicity indicates no significant differences in slope but significant differences in y-intercept (P<0.01) representing a difference in %fat of 2–4% units across the BMI range studied (Figure 1b). In other words, for the same BMI, Indian men have a higher fat% and lower FFM% than Creole men. The results of regression analysis according to ethnicity in women (Figure 1c), indicate no significant differences in slope or y-intercept. As body fat expressed as %fat mass has limitations as a weight-adjusted index of obesity with potential impact on the relationship between %fat and BMI,25 and the use of fat mass index (FMI) may be more appropriate in comparing the relationships between fatness and BMI,25 we also compared the relationships between BMI and FMI according to gender and ethnicity. As can be observed in Figure 1d–f, the various relationships between FMI and BMI are stronger (higher r values) than those between Fat% vs BMI. However, the outcome of the statistical differences in y-intercept between gender (panels a and d) and between ethnicity in men (panels b and e) and women (panels c and f) remain unchanged irrespective of whether the data are examined as BMI–Fat% or BMI–FMI relationships.
Figure 1.
Relationship between total body fat% measured by D2O dilution technique and BMI according to gender (a) and ethnicity in men (b) and women (c). (d–f) The relationship between body fat adjusted for height2, that is, FMI and BMI according to gender and ethnicity in men and women, respectively. Elev: statistical significance of differences in the elevation between regression lines, that is, in their y-intercepts; NS: no significant difference. The regression equations from BMI–Fat% relationship are as follows: (a) for men; %Fat=−5.52+(1.168 × BMI); for women, %Fat=10.868+(1.023 × BMI). (b) for men; Indian: %Fat=−6.06+(1.250 × BMI); Creole: %Fat=−3.98+(1.055 × BMI). (c) for women; Indian: %Fat=10.34+(1.05 × BMI); Creole: %Fat=13.06+(0.926 × BMI). The regression equations from BMI–FMI relationship are as follows: (d) for men; FMI=−8.75+(0.596 × BMI); for women, FMI=−6.64+(0.641 × BMI). (e) for men; Indian: FMI=−9.10+(0.627 × BMI); Creole: FMI=−8.09+(0.555 × BMI). (f) for women; Indian: FMI=−6.69+(0.648 × BMI); Creole: FMI=−6.39+(0.623 × BMI).
WC–Trunk Fat% relationships
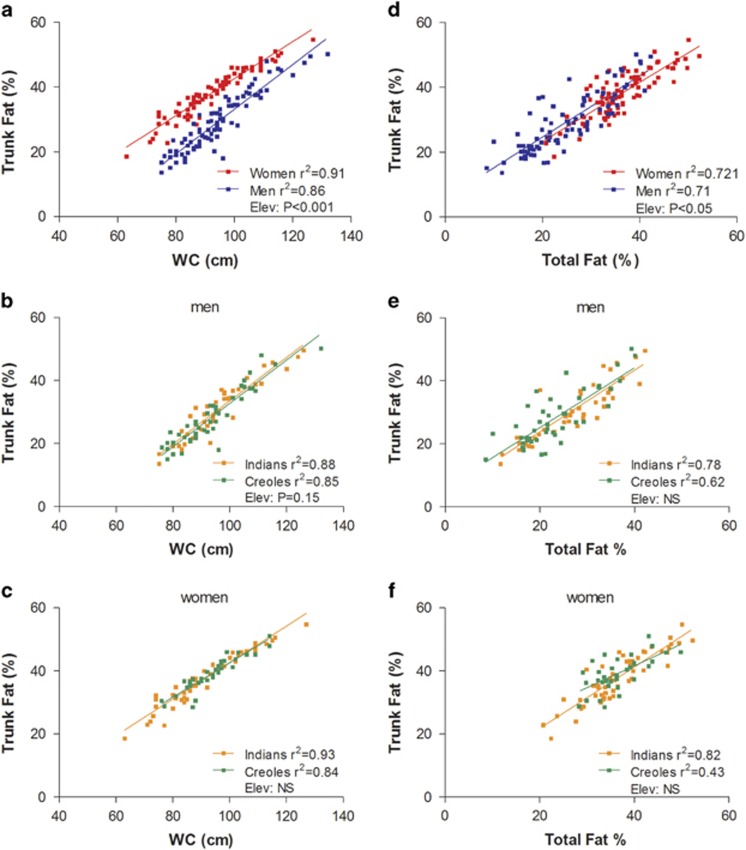
The data of Trunk Fat% vs WC (both assessed by ViScan) are plotted in Figure 2a–c. The regression lines show a high correlation for both men and women (r2: 0.86 and 0.91, respectively), with the regression line for women being significantly elevated relative to that for men by about 10% units (Figure 2a). In other words, women have significantly more Trunk Fat% than men across the range of WC studied (60–140 cm), with the difference slightly tapering at high WC. Regression analyses of the data according to ethnicity, that is, Creoles vs Indians, are presented for men and women in Figures 2b and c, respectively. Although there are no significant differences according to ethnicity in the WC–Trunk Fat% relationship in men or in women for the entire population sample, comparison of regression lines for males indicates a tendency for a difference in y-intercept (P=0.14) between Indians and Creoles. Indeed, sensitivity analysis indicates that this ethnic difference in men becomes statistically significant (P<0.05) across the BMI range of 18.5<BMI<30. In other words, for the same WC, Indian men across the normal and overweight categories of BMI have a higher Trunk Fat% than Creole men.
Figure 2.
Left column: relationship between Trunk Fat% (measured by ViScan) and waist circumference (WC)—measured by ViScan—by gender (a) and by ethnicity in men (b) and women (c). Right column: relationship between Trunk Fat% (measured by ViScan) and total body fat% measured by D2O dilution by gender (d) and by ethnicity in men (e) and women (f). Elev: statistical significance of differences in the elevation between regression lines, that is, in their y-intercepts; NS: no significant difference.
Trunk Fat%–Total Fat% relationship
The relationship between Trunk Fat% measured by ViScan and Total Fat% assessed by the D2O dilution technique is shown in Figure 2d–f. As shown in Figure 2d, there is a strong linear relationship between Trunk Fat% and total body fat% in both genders (r2= 0.71 and 0.72 for men and women, respectively). Although there is no difference in the slopes of these linear regressions, there is a significant difference in the y-intercept (P<0.05) with that for men being higher than for women (by 2% units). In other words, for the same total body fat%, men have a greater Trunk Fat% than women, suggesting a tendency for more central adiposity in men than in women. However, this small difference was no longer significant after the sensitivity analysis of data limited to a BMI range of 18.5<BMI<30. Furthermore, the analysis of these data according to ethnicity within each gender failed to reveal any significant difference in the relationship between Trunk Fat% and Total body fat% (Figures 2e and f).
Deviations from WHO (Caucasian-based) BMI cut-offs
Step 1
Body fat percentage of Mauritians measured by D20 dilution (Fat%MM) can be compared to that predicted from BMI for Caucasians (Fat%CP) using two independently derived predictive equations, namely:
(i) that of Gallagher et al.18 derived from healthy White adults at universities in UK and USA, and given as:
where, males=1 and females=0.
(ii) that of Deurenberg et al.16 which was developed and validated on several European Caucasian populations,16, 17 and given as:
where, males=1 and females=0.
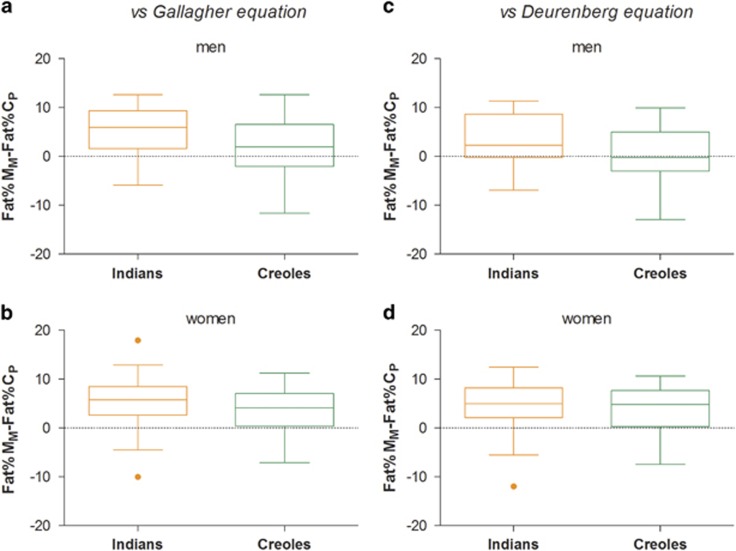
The distribution of data on the difference between Fat%MM and Fat%CP are presented as Box and Whisker plots (Figure 3). Analysis of the data by ethnicity indicates that among men (Figures 3a and c), only the Indians (and not Creoles) showed higher fat% than that predicted for Caucasians (by 3–5% units, P<0.001), whereas in women (Figures 3b and d), both Indians and Creoles showed equally higher Fat% compared to that predicted for Caucasians (4–5% units, P<0.001).
Figure 3.
Box and Whisker Plot showing distribution of values for differences between body fat (%) measured by D20 dilution technique in Mauritians (Fat%MM) with body fat% predicted from BMI (Fat%CP) using Caucasian-based equations of Gallagher et al.18 or Deurenberg et al.;16 data are presented according to ethnicity in men (a, c) and women (b, d). Each box encloses the data from the second and third quartiles and is bisected by a line at the median value. The tips of the vertical lines indicate the minimum and maximum values. The orange color closed circle symbol refers to probable outlier.
Step 2
From the above equations for Caucasians and from the equations derived from the linear regressions between BMI and Fat% for Mauritians according to gender and ethnicity (provided in legend to Figure 1), the following can be calculated:
(i) body fat% that corresponds to WHO BMI cut-offs of 25 and 30 kg m−2 according to ethnicity and gender (Supplementary Table S2), and
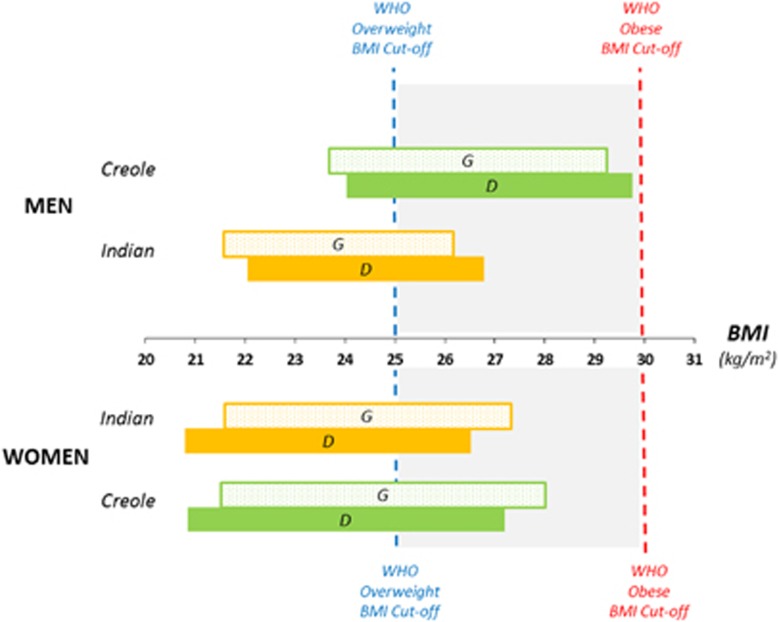
(ii) derived BMI cut-offs for each ethnic group (Indians, Creoles) according to gender for this calculated body fat% (Figure 4).
Figure 4.
Derived BMI cut-offs for overweight and obese in Mauritian Indians and Creoles compared with the WHO (Caucasian-based) BMI cut-offs of 25 and 30 kg m−2 for overweight and obese using the two Caucasian-based equations for fat% prediction from BMI, namely Gallagher et al.18 and Deurenberg et al.16 equations, and labeled as G and D, respectively.
Step 3
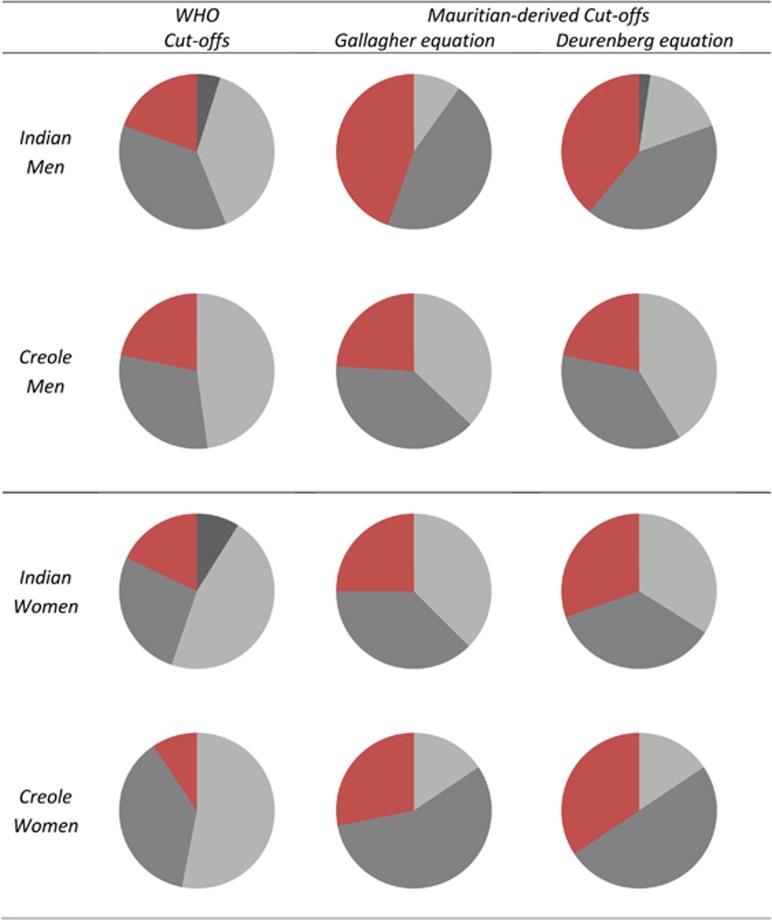
Re-classification of the population sample based on these derived BMI cut-offs for Mauritians according to gender and ethnicity indicate that the proportion of obese is greater by 1.5–2 fold in Indian women and men, and three times greater in Creole women, than that predicted by WHO (Caucasian-based) BMI cut-offs (Figure 5).
Figure 5.
Pie charts showing the proportions of the Mauritian population studied here (n= 175) who are underweight (UW, black shading), normal weight (NW, light gray shading), overweight (OW, dark gray shading) and obese (OB, red shading) according to WHO cut-offs for BMI or according to BMI cut-offs for BMI derived from Mauritian ethnic-specific BMI–Fat% relationship (with fat% measured by D20 dilution technique) and using either the Gallagher et al.18 equation or the Deurenberg et al.16 equation.
Discussion
The classification of this Mauritian population sample (n=175) according to WHO BMI cut-off values of 25 and 30 kg m−2 for overweight and obese, respectively, suggests that about 50% of the men and women participants across both ethnicities are overweight or obese. This is in line with the results of the last two National Surveys conducted in 2009 (n=6372) and 2015 (n= 3829) which, using the same WHO BMI cut-offs, found that 51 and 54% of the Mauritian adult population was overweight or obese.9, 10 These prevalence values are, however, likely to be markedly underestimated since, as our results here indicate, both Indian men and women as well as Creole women show measured body fat% (assessed by D2O dilution technique) that are substantially higher than that predicted from BMI using Caucasian-based equations relating body fat% and BMI. In these subgroups, the data obtained from measured body fat% show that the proportion of obese subjects is 1.5–3 times greater than that predicted by WHO BMI cut-offs, namely 1.5–2 times in Indian men and women, and tripled in Creole women (Figure 5).
Deviations in BMI cut-offs
Indeed, compared with body fat% predicted from BMI using age and sex-specific equations for Caucasians,16, 17, 18 our data show that measured body fat% was significantly higher by 3–5.5% units in Mauritian Indian men and women as well as in Creole women, but not in Creole men. The application of ethnic-specific regression equations derived from the BMI–Fat% relationships in this Mauritian population sample suggests that WHO BMI cut-offs of 25 and 30 kg m−2 seem valid only for Creole men (cut-off values of 24 and 29.7 kg m−2, respectively), but not for Creole women nor for Indian men and women whose BMI cut-offs values are 3–4 units lower (Figure 4). In particular, the BMI cut-offs of 21–22 kg m−2 for overweight and 27–28 kg m−2 for obese obtained for Mauritian Indian men and women are closer to the WHO recommended Asian cut-offs of 23 and 27.5 kg m−2 for identifying ‘increased risk' and ‘high-risk' individuals, respectively.26 Indeed, our findings in Mauritian Indians are consistent with numerous studies reporting that Asians in general, and South Asians in particular, have greater body fat% than Caucasians for the same BMI, thereby underscoring systematic bias when using the same BMI cut-offs derived from Caucasian populations to determine body composition.27 By contrast, Mauritian Creoles BMI cut-offs differ according to gender, with Creole men values being closer to the WHO cut-offs, whereas the creole women values are closer to the Asian cut-offs, thereby underlying potential underestimation of overweight and obese specifically in women Creoles when applying the WHO (Caucasian-based) cut-offs to this ethic group.
Gender-specific ethnic differences in adiposity
This gender influence on ethnic difference in body composition between Mauritian Indians and Creoles is reflected not only in their BMI–Fat%, but also in their WC–Trunk Fat% relationships, suggesting that the Indians showed, for the same WC, more abdominal fat% than Creoles in men but not in women. Several explanations for these gender-specific ethnic differences in adiposity can be put forward.
First, one could argue that the lack of difference in adiposity between Indian and Creole women may be a ‘false negative' that reside in the relatively small sample size of the population samples under study. We initially based our sample size on recruiting about 50 subjects per ethnic group for each gender, and while this was achieved for Creole men and Indian women, it was lower in Indian men (n=41) and Creole women (n=32) due to poor recruitment attendance. Nonetheless, power calculation indicates that, to detect a 5 unit difference in body fat% with a population s.d. of 7 at mean BMI, the required number of subjects per subgroup should be 30 (based on a statistical power of 80% and a 5% significance level). Furthermore, although the application of linear regression analysis provides a more sensitive approach than comparison of means to detect differences in %fat for the same BMI, there was no difference between Creole and Indian women in their BMI–Fat% or WC–Trunk Fat% relationships.
Second, as the age range of subjects in our study (that is, 20-42 years) captures women of reproductive age, and parity is known to influence trunk adiposity in young women, with a shift in fat distribution from lower to upper body independently of BMI,28, 29 a possible difference in parity status could be a confounding variable that limits our ability to detect differences between Creole and Indian women in Trunk Fat% for the same WC.
Third, the lack of difference in adiposity between Indian and Creole women may reside in the possibility of a high genetic admixture of Indian with African/Malagasy ancestry among Creole women. However, this latter contention seems unlikely as the well-known phenotypic difference in stature between these two ethnic groups—which has been repeatedly reported in large population samples during previous National Surveys in Mauritius3, 4, 5, 6, 7, 8, 9, 10—is also observed in our study, in which both in women and men, Creoles were found to be taller than Indians by about 5 cm. It is observed that the higher values for height in Creoles than in Indians are explained by a higher sitting height and not by differences in leg length. Consequently, as the ratio of sitting height and height (Cormic index)—a proxy of trunk to height ratio—has been implicated in ethnic differences in adiposity,30, 31 the greater Cormic index in Creoles than in Indians could hence potentially impact on the comparison of their BMI–Fat% or WC–Trunk Fat% relationships. However, in a multivariate analysis of body fat vs BMI, height and the Cormic index, neither height nor the Cormic index had a significant impact in explaining the variability in body fat% in men nor in women. Furthermore, the results of our analysis showing differences in the total fat% vs BMI relationship in men, but not in women, remain unchanged if total fat% is replaced by fat mass normalized by height2, that is, by FMI. Thus, our studies indicate that despite consistent findings of lower adult stature, sitting height and Cormic index in Indians than in Creoles of both sexes, only Indian men show greater adiposity (total and central) than the Creoles for the same BMI and WC, respectively. It should be noted, however, when Trunk Fat% is plotted against total body Fat% (Figure 2), no significant differences are observed between Indians and Creoles, whether in men or women, indicating that compared with the Creole men, the higher central adiposity in Indian men is a reflection of their higher total body fat% rather than a disproportionately elevated abdominal fat accumulation.
Study limitations and strengths
First, our study was conducted on subjects with low physical activity occupations, which may therefore have led a higher fat mass and lower lean mass per BMI. However, Mauritian adults have increasingly low physical activity occupations and the last two National Health Surveys9, 10 indicate that the vast majority (>70%) of men and women do not engage in regular leisure physical activity or undertake moderate physical activity to meet the national guidelines. Thus, the BMI–Fat relationships in our study are based on a population sample that reflects the sedentary lifestyle of most Mauritians.
Second, one may challenge the validity of assessing Trunk Fat% by segmental BIA using ViScan, a device whose algorithm was developed on Asian (Japanese) populations, for other populations. However, the estimation of Trunk Fat% by this device has been shown to be accurate against MRI in validation studies in several European populations.21, 22, 23 Thus, the application of BIA for adiposity assessment in the trunk area may be much less dependent on population-based specific equations.
Third, the values for total body fat% corresponding to WHO cut-offs were calculated from predictive equations, which were developed in Caucasian populations in whom body fat% was assessed by the four-compartment (4-C model) or by hydrodensitometry. Consequently, between-methods differences in measuring body fat may introduce errors in our calculations of differences between fat% measured in our Mauritian population sample using the D2O dilution technique and that predicted from BMI using the Gallagher equation using the 4-C model18 or the Deurenberg equation using hydrodensitometry.16 However, the use of 4-C model to develop body fat% prediction equations based on BMI in Caucasians in UK and USA revealed values for body fat% for WHO BMI cut-offs for overweight and obesity that were close to those obtained by the Deurenberg equation16 using hydrodensitometry in European Caucasians. These findings are in line with the close agreement in assessing body fat% by these two methods in Caucasians, that is, a mean bias of 0.6%.32 Furthermore, the mean bias in body fat% assessed by D20 dilution vs the 4-C model has been shown to be small (at~1% or less) in Caucasians32 as well as in Asians.33
Fourth, the assessment of body composition using the D2O dilution technique assumes that the proportion of water in FFM is a constant. However, variability in FFM hydration due to gender, ethnicity and menstrual cycle stage may lead to errors in the estimation of FFM, and hence contribute to differences in FFM and fat mass between population subgroups. Indeed, the FFM-hydration value of 73.5% for Caucasian adults may not be valid for other population groups. However, in reviewing the issue of FFM hydration, Deurenberg et al.34 concluded that most comparative studies found no differences in hydration of FFM between black and white Americans, and in studies investigating hydration of FFM of Caucasians and Asians (Chinese, Malays and Indians), these differences are too small to be biologically relevant. Furthermore, in a more recent study in India conducted on healthy weight stable young adults (20–40 years), the estimates of hydration of FFM using the 4-C model (in which there is no assumption about hydration of FFM) was found to be 73 and 74% in men and women, respectively,35 and hence similar to that values reported for European Caucasians.36
The D2O dilution method remains a well-accepted reference method for body composition assessment, and our study integrates within the international and regional projects of the International Agency on Atomic Agency (IAEA) on human body composition using this isotopic dilution approach.37, 38 The sample size of the cohort allowed investigations according to gender and two main ethnic groups (which together represent >90% of this island population). Furthermore, the large range of BMI and body fat% in our cohort allows for a meaningful investigation of the BMI–Fat% relationship, albeit in a young adult population. It remains to be investigated whether these gender-specific ethnic differences between Indians and Creoles are already present in children/adolescents and if they persist in the older (middle-age/elderly) population groups. In this context, it should be pointed out that studies which have compared the BMI–Fat% relationships in multiethnic adult populations across a wide range of ages (20–70 years) have often reported a higher body fat% in Asians than Caucasians for the same BMI after adjusting for gender and age.27, 39 However, none has specified whether these ethnic differences are more pronounced or less severe in the older compared with the young age-groups.
Conclusions
This study is the first investigation using a recognized reference method for assessing body composition in the two main ethnic groups in Mauritius, a population known to be at high risk for type-2 diabetes and cardiovascular disease.3, 4, 7, 9, 10 It suggests that European BMI cut-offs of 25 and 30 kg m−2 for overweight and obesity, respectively, seems valid only for Creole men, but not for Creole women nor for Indian men and women whose BMI cut-offs are 2–4 units lower. These gender-specific ethnic differences in body composition suggest that the cut-off points for classifying obesity based on BMI, or central obesity based on WC, need to take into account both gender and ethnicity, to avoid misclassification about adiposity excess and associated disease risks. This is particularly of importance in the development of obesity prevention strategies both in clinical medicine and for public health. To this end, the existence of facilities to assess body composition by the D2O dilution technique in a developing country like Mauritius opens the possibility of using this recognized reference method to validate cheaper and more rapid field techniques (for example, portable BIA systems) for advancing research and knowledge towards a better understanding of the relationships between anthropometry, body composition and health.
Acknowledgments
This research work was supported in part by the International Atomic Energy Agency (IAEA) (including training of the first author for the assessment of anthropometry and body composition in an expert laboratory in UK), by the Mauritian Ministry of Health & Quality of life, and the University of Fribourg Intramural funding. We are grateful to Dr (Mrs) Asha Deelchand, Coordinator of the Non Communicable Disease Unit, Victoria Hospital for providing facilities for this project and NCD Health Promotion Unit of the Ministry of Health & Quality of life.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WP. The fundamental drivers of the obesity epidemic. Obes Rev 2008; 9: 6–13. [DOI] [PubMed] [Google Scholar]
- Dowse GK, Gareeboo H, Zimmet PZ, Alberti KG, Tuomilehto J, Fareed D et al. High prevalence of NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Mauritius Noncommunicable Disease Study Group. Diabetes 1990; 39: 390–396. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Li N, Dowse G, Gareeboo H, Chitson P, Fareed D et al. The prevalence of coronary heart disease in the multi-ethnic and high diabetes prevalence population of Mauritius. J Intern Med 1993; 233: 187–194. [DOI] [PubMed] [Google Scholar]
- Hodge AM, Dowse GK, Zimmet PZ, Collins VR. Prevalence and secular trends in obesity in Pacific and Indian Ocean island populations. Obes Res 1995; 3: 77 s–87 s. [DOI] [PubMed] [Google Scholar]
- Hodge AM, Dowse GK, Gareeboo H, Tuomilehto J, Alberti KG, Zimmet PZ. Incidence, increasing prevalence, and predictors of change in obesity and fat distribution over 5 years in the rapidly developing population of Mauritius. Int J Obes Relat Metab Disord 1996; 20: 137–146. [PubMed] [Google Scholar]
- Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P et al. Increasing prevalence of T2 diabetes mellitus in all ethnic groups in Mauritius. Diabet Med 2005; 22: 61–68. [DOI] [PubMed] [Google Scholar]
- Nyamdorj R, Qiao Q, Söderberg S, Pitkäniemi JM, Zimmet PZ, Shaw JE et al. BMI compared with central obesity indicators as a predictor of diabetes incidence in Mauritius. Obesity 2009; 17: 342–348. [DOI] [PubMed] [Google Scholar]
- Mauritius Non-Communicable Disease Survey 2009. The Trends in Diabetes and Cardiovascular Disease Risk in Mauritius. The Mauritius Non Communicable Diseases Survey 2009. Available from: http://health.govmu.org/English/Documents/ncd-2009.pdf.
- Mauritius Non-Communicable Disease Survey 2015. The Trends in Diabetes and Cardiovascular Disease Risk in Mauritius. The Mauritius Non Communicable Diseases Survey 2015. Available from: http://health.govmu.org/English/Statistics/Documents/Mauritius%20NCD%20Survey%202015%20Report.pdf.
- World Health OrganizationObesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. World Health Organization: Geneva, 1998. [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes 2010; 34: S4–S17. [DOI] [PubMed] [Google Scholar]
- Blundell JE, Dulloo AG, Salvador J, Frühbeck G, , EASO SAB Working Group on BMI. Beyond BMI–phenotyping the obesities. Obes Facts 2014; 7: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurenberg P. Universal cut-off BMI points for obesity are not appropriate. Br J Nutr 2001; 85: 135–136. [DOI] [PubMed] [Google Scholar]
- Luke A. Ethnicity and the BMI-body fat relationship. Br J Nutr 2009; 102: 485–487. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr 1991; 65: 105–114. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Andreoli A, Borg P, Kukkonen-Harjula K, de Lorenzo A, van Marken Lichtenbelt WD et al. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr 2001; 55: 973–979. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000; 72: 694–701. [DOI] [PubMed] [Google Scholar]
- Lohmann TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- IAEA Report 2009. Using the deuterium dilution technique with analysis of saliva samples by Fourier Transform Infrared Spectroscopy; IAEA Human Health series no.12, 2009.
- Thomas EL, Collins AL, McCarthy J, Fitzpatrick J, Durighel G, Goldstone AP et al. Estimation of abdominal fat compartments by bioelectrical impedance: the validity of the ViScan measurement system in comparison with MRI. Eur J Clin Nutr 2010; 64: 525–533. [DOI] [PubMed] [Google Scholar]
- Browning LM, Mugridge O, Chatfield MD, Dixon AK, Aitken SW, Joubert I et al. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity 2010; 18: 2385–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamrazilová H, Hlavatý P, Dusátková L, Sedlácková B, Hainerová IA, Kunesová M et al. A new simple method for estimating trunk and visceral fat by bioelectrical impedance: comparison with magnetic resonance imaging and dual X-ray absorptiometry in Czech adolescents. Cas Lek Cesk 2010; 149: 417–422. [PubMed] [Google Scholar]
- Schutz Y, Sarafian D, Miles JL, Montani JP, Dulloo AG. Non-contact assessment of waist circumference: will tape measurements become progressively obsolete? Eur J Clin Nutr 2012; 66: 269–272. [DOI] [PubMed] [Google Scholar]
- Wells JC, Victora CG. Indices of whole-body and central adiposity for evaluating the metabolic load of obesity. Int J Obes Relat Metab Disord 2005; 29: 483–489. [DOI] [PubMed] [Google Scholar]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- Haldar S, Chia SC, Henry CJ. Body composition in Asians and Caucasians: comparative analyses and influences on cardiometabolic outcomes. Adv Food Nutr Res 2015; 75: 97–154. [DOI] [PubMed] [Google Scholar]
- Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol 2006; 131: 295–302. [DOI] [PubMed] [Google Scholar]
- Wells JC, Griffin L, Treleaven P. Independent changes in female body shape with parity and age: A life-history approach to female adiposity. Am J Hum Biol 2010; 22: 456–462. [DOI] [PubMed] [Google Scholar]
- Norgan NG. Relative sitting height and the interpretation of the body mass index. Ann Hum Biol 1994; 21: 79–82. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002; 3: 141–146. [DOI] [PubMed] [Google Scholar]
- Fogelholm M, van Marken Lichtenbelt W. Comparison of body composition methods: a literature analysis. Eur J Clin Nutr 1997; 51: 495–503. [DOI] [PubMed] [Google Scholar]
- Deurenberg-Yap M, Schmidt G, van Staveren WA, Hautvast JG, Deurenberg P. Body fat measurement among Singaporean Chinese, Malays and Indians: a comparative study using a four-compartment model and different two-compartment models. Br J Nutr 2001; 85: 491–498. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Deurenberg-Yap M. Validity of body composition methods across ethnic population groups. Forum Nutr 2003; 56: 299–301. [PubMed] [Google Scholar]
- Kuriyan R, Thomas T, Ashok S, Jayakumar J, Kurpad AV. A 4-compartment model based validation of air displacement plethysmography, dual energy X-ray absorptiometry, skinfold technique & bio-electrical impedance for measuring body fat in Indian adults. Indian J Med Res 2014; 139: 700–707. [PMC free article] [PubMed] [Google Scholar]
- Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci 1992; 82: 687–693. [DOI] [PubMed] [Google Scholar]
- IAEA PublicationIntroduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Saliva Samples by Fourier Transform Infrared Spectrometry. IAEA Human Health Series. No. 12. IAEA: Vienna, 2010. [Google Scholar]
- IAEA Co-ordinated Research ActivitiesNuclear Techniques to Assess Body Composition in Children and Adolescents as a Risk Factor in the Development of Chronic Diseases (E43024). Prevention and Control of NCDs: IAEA's Contribution Through Coordinated Research. IAEA: Vienna, 2014. [Google Scholar]
- Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998; 22: 1164–1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.