Abstract
Vaccines derived from totally synthetic carbohydrate antigens have been shown to elicit an immune response in both preclinical and clinical settings. The vaccines have been proven safe when administered in human clinical trials and are also competent at inducing antibodies that react with aberrant cells expressing the corresponding carbohydrate antigen. The most well studied vaccines have hitherto focused on single carbohydrate antigens, notwithstanding the known heterogeneity of transformed cells. Advances in synthetic organic chemistry have enabled the preparation and subsequent investigation of vaccines that contain several different tumor-associated carbohydrate antigens in a single molecule. These unimolecular constructs could, in principle, serve as superior mimics of cell surface antigens and hence, as multifaceted cancer vaccines. We report here the synthesis of a pentameric vaccine targeting a specific cancer. The new vaccine contains prostate tumor-associated antigens, Tn, TF, STn, Lewisy, and Globo-H. To reach our goal, antigen-containing amino acid monomers were assembled in a linear fashion to form a glycopeptide containing the five distinct carbohydrate antigen units. The attachment of a linker to the glycopeptide followed by an extraordinary global deprotection and subsequent conjugation to two different immunogenic carriers, keyhole limpet hemocyanin and N-α-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-l-cysteine, resulted in the vaccine constructs. The results described herein indicate that complex unimolecular multivalent vaccines can be efficiently produced in the laboratory. These fully synthetic vaccines have the potential to stimulate a multifaceted immune response against prostate cancer.
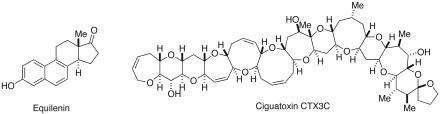
Some two decades ago, one of us had the occasion to offer some thoughts as to the history, current status, and future prospects of natural products-targeted synthesis (1). We would still argue that the launching point of this field can really be discerned, in retrospect, with the announcement of Bachmann and colleagues (2) at the University of Michigan that they had indeed accomplished the total synthesis of the equine-derived steroid hormone equilenin. Thus it was shown that the sophistication of organic chemistry had reached the point where it could deal with materials of this level of complexity. That equilenin was a member, albeit not a ranking member, of the then emerging field of steroid hormones certainly added to the luster of the accomplishment.
The development of the field of natural product-oriented total synthesis postequilenin exceeded the expectations and imagination of even its most venturesome enthusiasts. Who could have believed that in a matter of 65 years one could have advanced from equilenin (Fig. 1) to the point where a total synthesis of ciguatoxin (3) has recently been accomplished? That this level of advancement has been achieved is a confluence of many factors. Needless to say, first and foremost, the extraordinary progress can be traced to advances in synthetic methodology often discovered in the context of studying a particular transformation type rather than toward a well defined target molecule. Indeed there has been genuine progress at the level of synthetic strategy.
Fig. 1.
Natural products.
Certainly, the ways in which we look at problems in synthesis are much more sophisticated and polydimensional than was the case preequilenin. Moreover, synthetic strategy benefits enormously from spectacular advances in organic chemistry with particular emphasis on stereochemistry. Strategy in the field of synthesis has been of unique value in the growth of the science of chemistry. At its best, it goes beyond probing the depth of our current reservoir of knowledge. At its best, strategy asks the question “what if.” It suggests new horizons to which we can aspire if the methodology can be developed. The creative and synergistic interplay between strategy and methodology has brought us to the point where we can sensibly aspire to the synthesis of any new structure (within reason) that we can conceive totally independently or through hints from natural sources.
Where do we go from here? Of course, there are many unsolved problems out there and new, challenging targets are emerging continuously. Although such problems seem to be solvable in principle, it is well to be clear-headed that many of these goals are accomplished only after the utmost of travail and improvisation. Each solution to a difficult total synthesis problem constitutes, in effect, a tangible advance, which will, at some level, influence future thought processes and action plans.
We would posit, with even greater confidence than was asserted two decades ago, that with this new power in complex molecule synthesis (natural or unnatural total synthesis) comes major new opportunities. The field is well positioned to go beyond the classical-type challenge of reaching a fixed structural target. A particularly exciting possibility arises from drawing inferences from molecules first encountered from natural sources to design structures of potentially higher value.
Given appropriately inspired problem selection, in concert with the powers of total synthesis, the chemist whose curiosity remains robust can enter a whole and thus far uncharted universe. Hopefully in this now newly accessible space, the chemist in collaboration with other scientists can explore problems that could not have been imagined earlier. Synthesis allows us to merge the chemical and biological wisdom of the evolutionary forces that fashioned the kingdom of natural products with the unfettered imagination of the human mind. Chemical synthesis emerges as the “enabler” by which such synergies lead to evaluatable compounds, hopefully in a homogeneous state. In this article on the occasion of the much welcome focused PNAS initiative into chemical synthesis we describe such a problem and its solution.
Carbohydrate-Based Cancer Vaccines
After the first reports of carbohydrate-based structures associated with transformed cells, research has been aimed at developing cancer vaccines that focus on such moieties. These carbohydrate epitopes, overexpressed on the surfaces of cancer cells, may evoke a B cell response when introduced in an appropriate fashion to a host's immune system (4, 5). The antibodies produced could seek to recognize and bind to these antigens, thus initiating various cascades hopefully culminating in some level of immune-based selective elimination of cancer cells. Since the target cells include micrometastases and circulating tumor cells, this type of therapy would be adjuvant in nature and would serve the purpose of acting against tumor metastasis after primary therapy (chemotherapy, radiation, tumor extravasation) has relieved tumor burden.
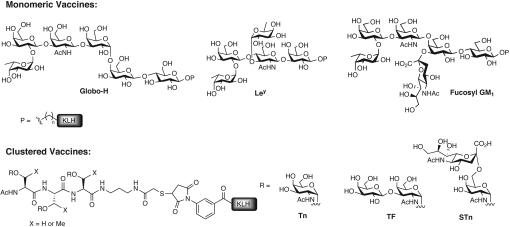
In the past two decades, research has focused on many aspects of vaccine construction: choice of antigen (6), development of procedures for the synthesis (7) or isolation of antigens, conjugation to an appropriate immunogenic carrier (8), and selection of immunological adjuvant for coadministration (9). The first-generation totally synthetic anticancer vaccines evaluated for their immunogenicity in human clinical settings were monomeric in nature, i.e., containing a single carbohydrate antigen appropriately conjugated to a carrier protein (Fig. 2). The carbohydrate antigens were synthesized with an allyl or pentenyl glycosidic appendage at the oligosaccharide reducing end that could be converted to an aldehyde for subsequent use in attachment to the immunogenic protein. Hexasaccharide Globo-H, first synthesized in 1996 (10) and the most advanced monomeric vaccine, has been involved in clinical trials investigating treatment for cancers of the breast, prostate, and ovary (11–13). It is currently projected for a phase II/III clinical trial against breast cancer. Similarly, this approach was also successful for constructing vaccines containing oligosaccharide antigens such as Fucosyl GM1 (L. M. Krug, G. Ragupathi, C. Hood, M. G. Kris, V. A. Miller, J. R. Allen, S.J.K., S.J.D., J. Gomez, L. Tyson, et al., unpublished work), Lewisy (Ley), and KH-1 (12).
Fig. 2.
Structures of monomeric and clustered vaccines in clinical trials.
We then turned our attention to vaccines comprised of smaller carbohydrate antigens (e.g., mono- and disaccharides), those typically associated with mucins. It was determined that multiple repeats or clustering of the carbohydrates was required for a robust and efficient immune response to be generated (14). The cassette approach to the synthesis of these antigenic glycosylamino acids allowed for facile construction of the requisite glycopeptides. Single-antigen vaccines, derived from both clustered (multiple copies of the same antigen displayed on a peptide backbone) and nonclustered (single copy of an antigen) carbohydrate antigens have been investigated for use in a variety of indications such as breast (13), prostate (11, 12, 15), and ovarian (16) cancers as well as small cell lung carcinoma (L. M. Krug, G. Ragupathi, C. Hood, M. G. Kris, V. A. Miller, J. R. Allen, S.J.K., S.J.D., J. Gomez, L. Tyson, et al., unpublished work and ref. 17). Although numerous vaccines of this type are being tested and are advancing through clinical trials, they are not directed to the multiplicity of antigens present in even a particular cancer type. With a view to maximizing the effectiveness of carbohydrate-based cancer vaccines we have undertaken research aimed at the synthesis and evaluation of vaccine constructs targeting several tumor-associated antigens simultaneously.
Transformed cells harbor a varying degree of heterogeneity in regard to the type and distribution of antigens expressed on their surfaces (18, 19). Both the variety and quantity of antigenic expression on cells may vary and fluctuate according to the different stages of cellular development. The inclusion of additional carbohydrate antigens closely associated with a particular cancer could well increase the percentage of cells targeted.
One could imagine two scenarios for implementing this idea of multiantigenic vaccines. The first approach, termed polyvalent, involves the administration of a mixture of existing monomeric (either clustered or nonclustered) conjugate vaccines (9, 20). Polyvalent vaccines are not a new idea, and outside the cancer field they have been used extensively, i.e., bacterial vaccines (21, 22). Preclinical trials concerning carbohydrate-based antigens for the treatment of cancer have demonstrated the viability of this method. Regardless of the mixing method and injection site, when coadministered with QS-21 or GPI-0100 adjuvant, these vaccines have elicited an immune response in which antibodies are produced that bind the antigens of interest as well as tumor cells that specifically express those carbohydrate antigens.
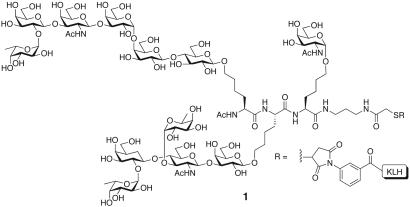
Although this approach seems attractive, its implementation must address several issues. First, the potential consequences of increased levels of carrier protein must be evaluated (23, 24). Additionally, from a regulatory perspective, one would need to obtain validation for each individual component of the polyvalent vaccine “mixture.” From a synthetic perspective, there are further disadvantages. In addition to synthesis, each carbohydrate antigen intended for inclusion would still require the often, low-yielding conjugation to carrier protein. A better alternative, and perhaps a more attractive vaccine, could arise from a unimolecular multivalent construct consisting of multiple carbohydrate antigens displayed on a single molecule that would undergo a single conjugation step to a carrier. Although a presumably low-yielding conjugation is still required, there is only one conjugation for the entire vaccine that is necessary, not one for each component. Moreover, this consolidation may also serve as an avenue for solving the potential for adverse immune suppression caused by carrier protein (25). A unimolecular trivalent vaccine shown (1, Fig. 3) has served as proof of principle for this approach (26). This vaccine, consisting of three different carbohydrate antigens (Tn, Ley, and Globo-H) displayed on a peptide backbone comprised of nonnatural amino acids, was able to elicit an immune response in murine hosts (27). ELISA-based antibody titers were found for each of the three different antigens. Additionally, fluorescence-activated cell sorting assay showed significant IgM reactivity and low IgG reactivity against MCF-7 cells and moderate reactivity against LSC cell lines. The presence of multiple carbohydrate epitopes in the construct did not appear to suppress the response against any of the constituent antigens. Accordingly, we undertook to construct and evaluate a unimolecular multiantigenic vaccine for use against a specific type of cancer.
Fig. 3.
Proof of principle: a unimolecular multivalent vaccine containing three different tumor-associated carbohydrate antigens.
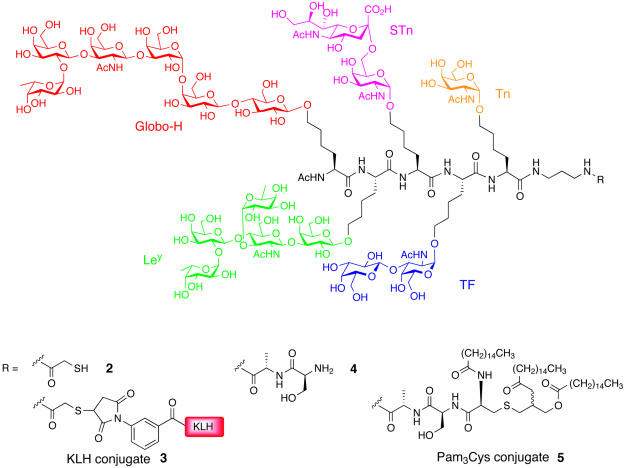
Prostate cancer provides a unique opportunity for us to explore the efficacy of multivalent vaccines since we have synthetic access to several of the different carbohydrate antigens that are associated with it. Many of these single-antigen vaccines have been included in early clinical trials with success. Tumors isolated from prostate cancer tissue collections have shown that the gangliosides, GM2 and Globo-H, along with the blood group-related antigens, STn, Tn, TF, and Ley, are found in abundance on the cell surface and to a lesser degree so are the Lea and sialyl Lea antigens (18, 19). We have designed two multivalent conjugate vaccines (3 and 5, Fig. 4) for prostate cancer that include five different carbohydrate antigens: Globo-H, STn, Tn, TF, and Ley. We hoped to evaluate as potential immuno carriers, the protein keyhole limpet hemocyanin (KLH) (28) and the macrolipid N-α-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-l-cysteine (Pam3Cys) (29). Although KLH, a molluscan protein, has been used extensively in many vaccines and provides for a favorable immune response (30), the Pam3Cys lipid, a known potent B cell stimulant, has only been minimally evaluated in preclinical and clinical settings (15, 31).
Fig. 4.
Multivalent vaccines designed for use in prostate cancer.
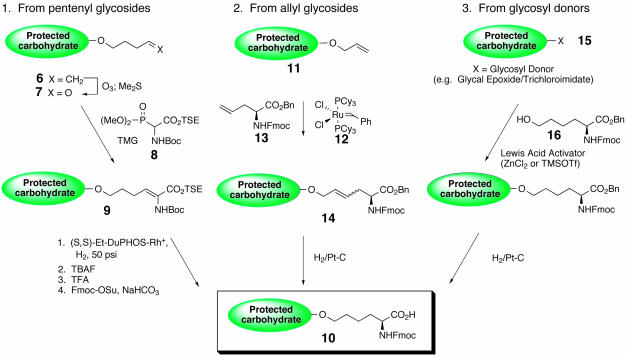
The strategy envisioned for the construction of the multivalent vaccines would require the fashioning of a pool of glycosylamino acids in which the carbohydrate entities would be completely protected and the amino terminus blocked with the fluorenylmethyl carbamate (Fmoc) group. Solution-phase Fmoc-based peptide chemistry would be used to assemble the glycopeptide, and the attachment of a linker domain followed by global deprotection would afford a vaccine construct that could then be conjugated to a carrier protein or lipid. There are currently three methodologies (Fig. 5) developed in our laboratory to construct the aforementioned, suitably protected glycosylamino acids. Starting from a pentenyl glycoside 6, ozonolytic cleavage of the terminal olefin followed by treatment with dimethyl sulfide produces an aldehyde 7. After reaction with phosphonate amino acid 8 in a Horner–Emmons reaction, a dehydroamino acid 9 is generated (26). Enantioselective reduction of the olefin in the presence of a chiral, nonracemic rhodium catalyst followed by protecting group manipulation provides the desired glycosylamino acids 10. Similarly, when allyl glycosides 11 are used as starting materials, an olefin cross-metathesis (using catalyst 12 and allyl glycine 13) followed by reduction of olefin 14 and benzyl ester hydrogenolysis results in the same type of glycosylamino acid (32). The third method we use allows for introduction of the amino acid functionality directly starting from glycal epoxide or trichloroacetimidate carbohydrate donors 15 by coupling with hydroxynorleucine 16 in the presence of a Lewis acid (33, 34). Regardless of the method used for introduction of the amino acid moiety, all of the resultant glycosylamino acids contain a four-carbon methylene side chain connecting the peptide backbone to the carbohydrate antigen. It should also be noted that carbohydrate domains depicted in Fig. 3 may consist of a single monosaccharide or a complex oligosaccharide.
Fig. 5.
Methods used to install the amino acid functionality to carbohydrate domains. TSE,2-(trimethylsilyl)ethyl ester; TMG, tetramethyl guanidine; TBAF, tetrabutylammonium fluoride; TFA, trifluoroacetic acid.
Materials and Methods
Synthetic procedures describing the peptide couplings, deprotection reactions, and conjugation methods shown in Schemes 1 and 2 are detailed in Supporting Methods, which is published as supporting information on the PNAS web site.
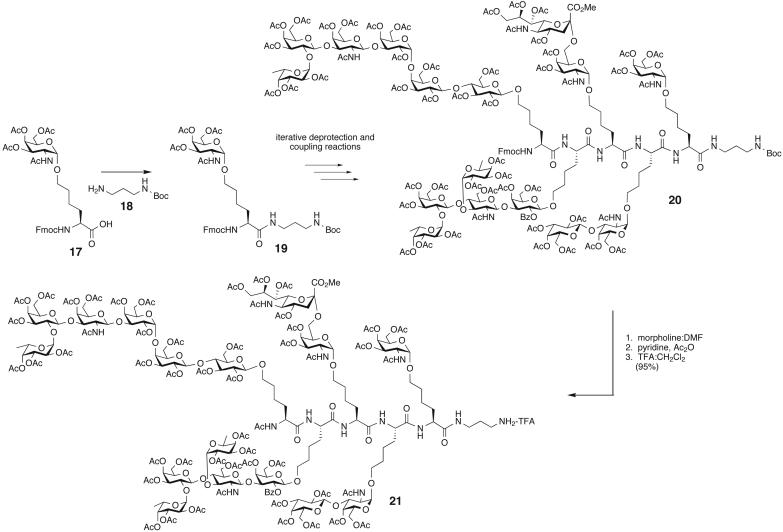
Scheme 1.
Glycopeptide synthesis. DMF, dimethylformamide; TFA, trifluoroacetic acid.
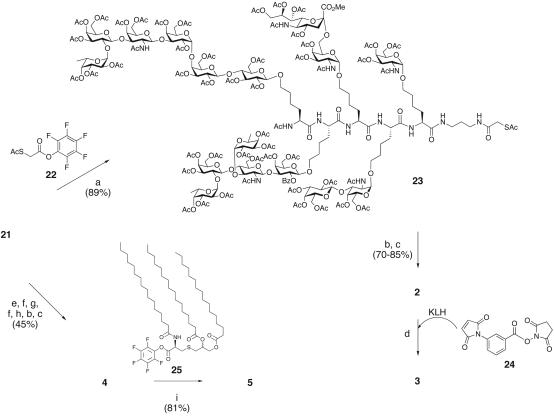
Scheme 2.
Synthesis of KLH conjugate 3 and Pam3Cys conjugate 5. Reagents and conditions used were: (a) pyridine, 22;(b) 0.1 M NaOH (aq), MeOH, 0°C24 h, 23°C 24 h, Amberlyst-15 H+; (c) MeOH:H2O:hydrazine hydrate, 24 h; (d) MBS 24, KLH; (e) dimethylaminopropylethyl carbodiimide hydrochloride, 1-hydroxybenzotriazole, triethylamine, CH2Cl2:dimethylformamide, Fmoc-Ala-OH; (f) morpholine:dimethylformamide; (g) dimethylaminopropylethyl carbodiimide hydrochloride, 1-hydroxybenzotriazole, triethylamine, CH2Cl2:dimethylformamide, Fmoc-Ser(OtBu)-OH; (h) trifluoroacetic acid, phenol, TES, H2O; (i) 25, NMP, O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate, 1-hydroxy-7-aza-benzotriazole, diisopropylethylamine, 23°C, 36 h.
Results
The five glycosylamino acids were obtained by using the three methodologies described above. The α-linked glycosylamino acids (Tn, TF, and STn) were synthesized by using the hydroxynorleucine cassette approach (33, 34). Ley glycosylamino acid was obtained from the n-pentenyl glycoside methodology (26), and Globo-H glycosylamino acid was obtained from the cross-metathesis reaction protocol (32). The peptide sequence we chose for the vaccine synthesis was based on the complexity of the carbohydrate antigens, which resulted in the Tn glycosylamino acid being at the C terminus of the glycopeptide followed by TF, STn, Ley, and finally Globo-H at the N terminus. The Tn glycosylamino acid 17 was first coupled to tert-butyl N-(3-aminopropyl) carbamate 18 to provide 19. The diaminopropyl unit serves as a partial linker that is further elaborated before the conjugation step. 19 was then elongated to the pentapeptide 20 via iterative Fmoc deprotection and coupling reactions (Scheme 1). The coupling steps for the mono- and disaccharide antigen amino acids were mediated by dimethylaminopropylethyl carbodiimide hydrochloride/1-hydroxybenzotriazole, whereas the more sterically encumbered ones used O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate/1-hydroxy-7-aza-benzotriazole coupling reagents. Each deprotection and coupling step proceeded in >75% yield with the overall yield for nine transformations being 28%. Modifications at the N and C termini of the peptide involved, respectively, Fmoc deprotection and capping with an acetyl group and then acid-mediated Boc cleavage generated glycopeptide 21, which required further derivatization commensurate to conjugation.
For the KLH conjugate vaccine, the primary amine from the aminopropyl linker of glycopeptide 21 was treated with S-acetylthioglycolic acid pentafluorophenyl ester 22 to install the protected thiol functionality (Scheme 2). At this juncture, the remaining steps necessary were shedding of the protecting groups and conjugation to the carrier protein. A two-step global deprotection facilitated the hydrolysis of all 47 blocking groups. Treatment of 23 with aqueous sodium hydroxide in methanol initially at 0°C and then at room temperature resulted in release of all of the protecting groups except for the benzoate and one undetermined acetate. The two hydroxyl groups that still remained in protected form were liberated by subsequent treatment with hydrazine. After derivatization of KLH with m-maleimidobenzoyl-N-hydroxysuccinimide 24, the thiol handle on the glycopeptide added, in a presumed Michael fashion, to the maleimide (35). The ratio of glycopeptide-to-protein for conjugate vaccine 3 was determined to be 228:1.
The lipid conjugation route commenced from 21, with sequential peptide coupling and Fmoc deblocking reactions, that installed alanine and serine-O-tert-butyl amino acids. Acid-mediated removal of the tert-butyl ether followed by global deprotection provided the penultimate construct 4. The final step occurred when the free amine was treated with an excess of the activated Pam3Cys pentafluorophenol ester 25. The unreacted lipid was simply removed from the desired vaccine conjugate 5 by extraction with organic solvent.
Discussion
Fully synthetic anticancer vaccines derived from entirely synthetic carbohydrate antigens have allowed for multiple preclinical and clinical trials to be undertaken and successfully completed (6). Since the natural abundance and availability of some of the key antigens is limited to minute nonusable quantities [the first isolation of the hexasaccharide Globo-H from tissue collection produced submilligram quantities of pure product (36, 37)], synthesis has clearly emerged as a key force in the evolution of oligosaccharide-based cancer vaccines. After advances in carbohydrate synthesis, such as are described herein, hundreds of milligrams of erstwhile rare antigens can be assembled in a viable time frame. With a wealth of carbohydrate chemistry transformations from which to choose, numerous complex carbohydrates armed with amino acid functionality at the reducing end give rise to valuable glycosylamino acids building blocks. The resulting antigen monomers are of value in the modular construction of different vaccines. We have illustrated in our previous proof of principle work (26, 38) and here that we can assemble these glycosylamino acids into glycopeptides. It has also been shown that these glycopeptides, when conjugated to an immunogenic carrier and introduced with an adjuvant, can induce an immune response (27).
The prostate cancer vaccines illustrated here, which contain five different carbohydrate antigens, demonstrate that the methods for making these types of molecules are robust. The yields for the coupling and deprotection steps in the glycopeptide synthesis are high and the reactions produce very clean compounds. This result is remarkable given that some of glycosylamino acids are quite sterically hindered and extraordinarily complex. Although the focus of this vaccine was primarily prostate cancer, it will be appreciated that this approach could be targeted toward other cancers. From our library of carbohydrate antigen-containing amino acids, vaccines for other cancers can be built in a modular fashion. We note in a more futuristic spirit that, in time, this approach could be applied to create tailor-made patient-driven cancer vaccines provided that rapid, automatable determinations of antigenic cell surface populations could be achieved.
Supplementary Material
Acknowledgments
We thank Dr. Kaustav Biswas and Dr. Jennifer R. Allen (Memorial Sloan–Kettering Cancer Center) for providing intermediate compounds, Dr. Govindaswami Ragupathi for KLH conjugation, Ms. Anna Dudkina for mass spectral analyses, and Dr. George Sukenick (NMR Core Facility, Sloan-Kettering Institute) for NMR assistance. This work was supported by National Institutes of Health Grants AI-16943 and CA-28824 (to S.J.D.). National Institutes of Health Postdoctoral Fellowship AI-51883 is gratefully acknowledged by S.J.K.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: KLH, keyhole limpet hemocyanin; Ley, Lewisy; Pam3Cys, N-α-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-l-cysteine; Fmoc, fluorenylmethyl carbamate.
References
- 1.Danishefsky, S. J. (1986) Aldrichim. Acta 19, 59-68. [Google Scholar]
- 2.Bachmann, W. E., Cole, W. & Wilds, A. L. (1939) J. Am. Chem. Soc. 61, 974-975. [Google Scholar]
- 3.Hirama, M., Oishi, T., Uehara, H., Inoue, M., Maruyama, M., Guri, H. & Satake, M. (2001) Science 294, 1904-1907. [DOI] [PubMed] [Google Scholar]
- 4.Livingston, P. O., Zhang, S. L. & Lloyd, K. O. (1997) Cancer Immunol. Immunother. 45, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston, P. O. & Ragupathi, G. (1997) Cancer Immunol. Immunother. 45, 10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danishefsky, S. J. & Allen, J. R. (2000) Angew. Chem. Int. Ed. Engl. 39, 836-863. [DOI] [PubMed] [Google Scholar]
- 7.Danishefsky, S. J. & Bilodeau, M. T. (1996) Angew. Chem. Int. Ed. Engl. 35, 1380-1419. [Google Scholar]
- 8.Keding, S. J. & Danishefsky, S. J. (2003) in Carbohydrate-Based Drug Discovery, ed. Wong, C.-H. (Wiley-VCH, Weinheim, Germany), Vol. 1, pp. 381-406. [Google Scholar]
- 9.Ragupathi, G., Koide, F., Sathyan, N., Kagan, E., Spassova, M., Bornmann, W., Gregor, P., Reis, C. A., Clausen, H., Danishefsky, S. J., et al. (2003) Cancer Immunol. Immunother. 52, 608-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park, T. K., Kim, I. J., Hu, S. H., Bilodeau, M. T., Randolph, J. T., Kwon, O. & Danishefsky, S. J. (1996) J. Am. Chem. Soc. 118, 11488-11500. [Google Scholar]
- 11.Ragupathi, G., Slovin, S. F., Adluri, S., Sames, D., Kim, I. J., Kim, H. M., Spassova, M., Bornmann, W. G., Lloyd, K. O., Scher, H. I., et al. (1999) Angew. Chem. Int. Ed. Engl. 38, 563-566. [DOI] [PubMed] [Google Scholar]
- 12.Slovin, S. F., Ragupathi, G., Adluri, S., Ungers, G., Terry, K., Kim, S., Spassova, M., Bornmann, W. G., Fazzari, M., Dantis, L., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 5710-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilewski, T., Ragupathi, G., Bhuta, S., Williams, L. J., Musselli, C., Zhang, X. F., Bencsath, K. P., Panageas, K. S., Chin, J., Hudis, C. A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 3270-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, S. L., Walberg, L. A., Ogata, S., Itzkowitz, S. H., Koganty, R. R., Reddish, M., Gandhi, S. S., Longenecker, B. M., Lloyd, K. O. & Livingston, P. O. (1995) Cancer Res. 55, 3364-3368. [PubMed] [Google Scholar]
- 15.Slovin, S. F., Ragupathi, G., Musselli, C., Olkiewicz, K., Verbel, D., Kuduk, S. D., Schwarz, J. B., Sames, D., Danishefsky, S., Livingston, P. O., et al. (2003) J. Clin. Oncol. 21, 4292-4298. [DOI] [PubMed] [Google Scholar]
- 16.Sabbatini, P. J., Kudryashov, V., Ragupathi, G., Danishefsky, S. J., Livingston, P. O., Bornmann, W., Spassova, M., Zatorski, A., Spriggs, D., Aghajanian, C., et al. (2000) Int. J. Cancer 87, 79-85. [PubMed] [Google Scholar]
- 17.Dickler, M. N., Ragupathi, G., Liu, N. X., Musselli, C., Martino, D. J., Miller, V. A., Kris, M. G., Brezicka, F. T., Livingston, P. O. & Grant, S. C. (1999) Clin. Cancer Res. 5, 2773-2779. [PubMed] [Google Scholar]
- 18.Zhang, S., Cordon-Cardo, C., Zhang, H. S., Reuter, V. E., Adluri, S., Hamilton, W. B., Lloyd, K. O. & Livingston, P. O. (1997) Int. J. Cancer 73, 42-49. [DOI] [PubMed] [Google Scholar]
- 19.Zhang, S., Zhang, H. S., Cordon-Cardo, C., Reuter, V. E., Singhal, A. K., Lloyd, K. O. & Livingston, P. O. (1997) Int. J. Cancer 73, 50-56. [DOI] [PubMed] [Google Scholar]
- 20.Ragupathi, G., Cappello, S., Yi, S. S., Canter, D., Spassova, M., Bornmann, W. G., Danishefsky, S. J. & Livingston, P. O. (2002) Vaccine 20, 1030-1038. [DOI] [PubMed] [Google Scholar]
- 21.Lee, C. J., Lee, L. H. & Koizumi, K. (2002) Infect. Med. 19, 127-133. [Google Scholar]
- 22.Lee, C. J., Lee, L. H. & Koizumi, K. (2002) Infect. Med. 19, 179-182. [Google Scholar]
- 23.Dagan, R., Eskola, J., Leclerc, C. & Leroy, O. (1998) Infect. Immun. 66, 2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fattom, A., Cho, Y. H., Chu, C., Fuller, S., Fries, L. & Naso, R. (1999) Vaccine 17, 126-133. [DOI] [PubMed] [Google Scholar]
- 25.Herzenberg, L. A. & Tokuhisa, T. (1980) Nature 285, 664-667. [DOI] [PubMed] [Google Scholar]
- 26.Allen, J. R., Harris, C. R. & Danishefsky, S. J. (2001) J. Am. Chem. Soc. 123, 1890-1897. [DOI] [PubMed] [Google Scholar]
- 27.Ragupathi, G., Coltart, D. M., Williams, L. J., Koide, F., Kagan, E., Allen, J., Harris, C., Glunz, P. W., Livingston, P. O. & Danishefsky, S. J. (2002) Proc. Natl. Acad. Sci. USA 99, 13699-13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markl, J., Lieb, B., Gebauer, W., Altenhein, B., Meissner, U. & Harris, J. R. (2001) J. Cancer Res. Clin. Oncol. 127, R3-R9. [DOI] [PubMed] [Google Scholar]
- 29.Bessler, W. G., Cox, M., Lex, A., Suhr, B., Wiesmuller, K. H. & Jung, G. (1985) J. Immunol. 135, 1900-1905. [PubMed] [Google Scholar]
- 30.Musselli, C., Livingston, P. O. & Ragupathi, G. (2001) J. Cancer Res. Clin. Oncol. 127, R20-R26. [DOI] [PubMed] [Google Scholar]
- 31.Kudryashov, V., Glunz, P. W., Williams, L. J., Hintermann, S., Danishefsky, S. J. & Lloyd, K. O. (2001) Proc. Natl. Acad. Sci. USA 98, 3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswas, K., Coltart, D. M. & Danishefsky, S. J. (2002) Tetrahedron Lett. 43, 6107-6110. [Google Scholar]
- 33.Keding, S. J., Atsushi, E., Biswas, K., Zatorski, A., Coltart, D. M. & Danishefsky, S. J. (2003) Tetrahedron Lett. 44, 3413-3416. [Google Scholar]
- 34.Keding, S. J., Endo, A. & Danishefsky, S. J. (2003) Tetrahedron 59, 7023-7031. [Google Scholar]
- 35.Zhang, S. L., Graeber, L. A., Helling, F., Ragupathi, G., Adluri, S., Lloyd, K. O. & Livingston, P. O. (1996) Cancer Res. 56, 3315-3319. [PubMed] [Google Scholar]
- 36.Kannagi, R., Levery, S. B., Ishigami, F., Hakomori, S.-I., Shevinsky, L. H., Knowles, B. B. & Solter, D. (1983) J. Biol. Chem. 258, 8934-8942. [PubMed] [Google Scholar]
- 37.Bremer, E. G., Levery, S. B., Sonnino, S., Ghidoni, R., Canevari, S., Kannagi, R. & Hakomori, S.-I. (1984) J. Biol. Chem. 259, 14773-14777. [PubMed] [Google Scholar]
- 38.Williams, L. J., Harris, C. R., Glunz, P. W. & Danishefsky, S. J. (2000) Tetrahedron Lett. 41, 9505-9508. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.