Abstract
Background:
A membrane-penetrating cation, dodecyltriphenylphosphonium (C12TPP), facilitates the recycling of fatty acids in the artificial lipid membrane and mitochondria. C12TPP can dissipate mitochondrial membrane potential and may affect total energy expenditure and body weight in animals and humans.
Methods:
We investigated the metabolic effects of C12TPP in isolated brown-fat mitochondria, brown adipocyte cultures and mice in vivo. Experimental approaches included the measurement of oxygen consumption, carbon dioxide production, western blotting, magnetic resonance imaging and bomb calorimetry.
Results:
In mice, C12TPP (50 μmol per (day•kg body weight)) in the drinking water significantly reduced body weight (12%, P<0.001) and body fat mass (24%, P<0.001) during the first 7 days of treatment. C12TPP did not affect water palatability and intake or the energy and lipid content in feces. The addition of C12TPP to isolated brown-fat mitochondria resulted in increased oxygen consumption. Three hours of pretreatment with C12TPP also increased oligomycin-insensitive oxygen consumption in brown adipocyte cultures (P<0.01). The effects of C12TPP on mitochondria, cells and mice were independent of uncoupling protein 1 (UCP1). However, C12TPP treatment increased the mitochondrial protein levels in the brown adipose tissue of both wild-type and UCP1-knockout mice. Pair-feeding revealed that one-third of the body weight loss in C12TPP-treated mice was due to reduced food intake. C12TPP treatment elevated the resting metabolic rate (RMR) by up to 18% (P<0.05) compared with pair-fed animals. C12TPP reduced the respiratory exchange ratio, indicating enhanced fatty acid oxidation in mice.
Conclusions:
C12TPP combats diet-induced obesity by reducing food intake, increasing the RMR and enhancing fatty acid oxidation.
Introduction
Obesity and its comorbidities represent a global health threat and a rapidly increasing burden to economic prosperity. The development of pharmacologic agents for the treatment of obesity has been challenged by both low efficacy and serious adverse side effects, leading to the removal of these agents from the market.1 Despite the checkered past of obesity drug development, the search remains focused on an elusive molecule that might specifically target obesity and effectively prevent its consequences.
Mitochondria occupy a key position in cellular metabolism. The proton motive force across the mitochondrial inner membrane drives ATP synthesis. In addition, the energy stored in the proton motive force can be dissipated by proton leak through the inner membrane, contributing to the basal metabolic rate and thermogenesis.2, 3, 4 Protonophores such as 2, 4-dinitrophenol (DNP) increase mitochondrial proton leakage and have been used to treat obesity.5, 6 However, a slight increase in the DNP concentration above the therapeutically effective individual dose is toxic and DNP was consequently withdrawn from clinical use.7 Thus, considerable interest remains in the development safer protonophores with a wider range of therapeutic concentrations.
Several design concepts for the development of alternative cationic protonophores have been used.8, 9, 10, 11 We recently demonstrated that one such cationic protonophore, a short-chain alkyl derivative of rhodamine 19, exhibits metabolic effects in obese mice and consequently may be a promising anti-obesity agent.12
Alternatively, mitochondrially targeted uncouplers can be designed by linking the uncoupler moiety to the triphenylphosphonium cation.9, 10 MitoDNP, which was designed using this approach, exhibited extensive mitochondrial uptake but did not uncouple mitochondria, as evidenced by its inability to either increase their respiration rate or to decrease membrane potential.9 In contrast, another mitochondrially targeted compound conjugated to triphenylphosphonium cation, dodecyltriphenylphosphonium (C12TPP), exhibits protonophorous activity in the lipid bilayer membrane and isolated mitochondria.10 The uncoupling activity of C12TPP was proposed to be mediated by the recycling of endogenous fatty acids, which are natural uncoupling agents in the mitochondrial membrane.10 In this work, we studied the potential efficacy of C12TPP as an anti-obesity agent.
Materials and methods
Animals and treatment
Uncoupling protein 1 (UCP1)-ablated mice that were progeny of mice described in Enerbäck et al.13 were backcrossed to the C57Bl/6 mouse strain, which was used as the wild-type mouse strain. Male mice were singly housed, had free access to water and were maintained on a 12:12 h light:dark cycle (0800–2000 h) at 21 °C or 30 °C (thermoneutrality). The mice were fed either a chow diet (R70 Standard Diet, Lactamin AB, Vadstena, Sweden; 4.5 gm% fat, 14.5 gm% protein, 60 gm% carbohydrates) or a high-fat diet (HFD; D12451, Research Diets Inc., New Brunswick, NJ, USA; 24 gm% fat, 24 gm% protein and 41 gm% carbohydrate). At age 12 weeks, mice were randomly assigned to ether control or C12TPP-treated group. Both treated and control mice received food ad libitum. Each mouse in the pair-fed group was paired to a mouse from the treated group according to their body weight and received the same amount of food eaten by the corresponding treated mouse during the previous day. Pair-fed mice received one meal every 24 h at 2000 h to prevent disturbance of circadian rhythm.14 Water and food intake, as well as body weight, were measured always at the same time between 1900 and 2000 h. The C12TPP-treated mice were provided with water supplemented with one of two doses of C12TPP (5 or 50 μmol per (day•kg body weight)). Control and pair-fed mice received tap water supplemented with the corresponding dose of vehicle (NaBr and 0.07 to 0.1% ethanol, because C12TPP is a bromide salt dissolved in ethanol). The water was changed every 4 days. The body composition (body fat and lean mass) was measured with in vivo magnetic resonance imaging using an EchoMRI-100 instrument (EchoMRI LLC, Houston, TX, USA).
The animal protocols were conducted in accordance with the guidelines for the humane treatment of animals and were approved by the Animal Ethics Committee of the North Stockholm Region.
Indirect calorimetry and energy expenditure
Indirect calorimetry was performed principally as described in Abreu-Vieira et al.15 using a Somedic INCA apparatus (Horby, Sweden). The resting metabolic rate (RMR) was calculated by determining the minimal stable (during at least 10–15 min) oxygen consumption rate during the light phase. The respiratory exchange ratio (RER) was calculated by determining the ratio of the rate of CO2 production to the rate of O2 consumption. The total energy expenditure (TEE) was calculated using two methods: (1) based on indirect calorimetry (TEEic) using the Weir equation: TEEic, kJ=(16.3 × volume O2+4.57 × volume CO2)/1000(ref. 16) and (2) based on food intake and changes in body composition using the energy balance technique (TEEbal), kJ=energy intake (kJ)−39.33 × fat mass change (g)−4.184 × lean mass change (g)).17
Fecal energy and lipid analysis
All feces produced by each mouse were collected, weighed and stored at −20 °C until being subjected to bomb calorimetry (Oxygen Bomb Calorimeter 6300 (Parr Instrument, Molin, IL, USA)) or a lipid analysis (gravimetric following a Folch extraction, as in Zadravec et al.18).
Isolation and oxygen consumption of brown-fat mitochondria
Brown-fat mitochondria were isolated by differential centrifugation as described in Cannon et al.19 The oxygen consumption was measured with a Clark-type oxygen electrode (Yellow Springs Instrument Co., Yellow Springs, OH, USA) in a sealed chamber at 37 °C, as described previously.20 The mitochondria (0.25 mg protein per ml) were incubated in medium consisting of 125 mM sucrose, 20 mM K+-Tes (pH 7.2), 2 mM MgCl2, 1 mM EDTA, 4 mM KPi and 0.1% fatty-acid-free bovine serum albumin and 3 mM malate.
Primary brown adipocyte culture and oxygen consumption
Primary brown adipocytes were cultured in medium supplemented with 1 μM rosiglitazone maleate (Alexis Biochemicals, San Diego, CA, USA) as described previously.21 C12TPP was added to brown adipocytes cultured for 7 days at a concentration of 1 μM and control cells were treated with 0.1% ethanol only. After a 3 h incubation, the cells were collected and the oxygen consumption was monitored with a Clark-type oxygen electrode as described previously.22
Western blottings
Western blottings were principally performed as described in Shabalina et al.23 The levels of mitochondrial respiratory chain proteins (Complex I (NDUFB8), Complex II (SDHB), Complex III (UQCRC2), Complex IV (COX2) and Complex V (α-subunit FoF1-ATP-syntase) were examined using the Total OXPHOS Mouse Antibody cocktail from MitoSciences (#MS601, Eugene, OR, USA)) (diluted 1:15 000). UCP1 and voltage-dependent anion channel (VDAC) expressions were examined using either a UCP1 antibody produced in rabbit against the C-terminal UCP1 decapeptide at a dilution of 1:20 000 (in-house product) or a VDAC antibody from Cell Signaling (#4661S) diluted 1:2000. The immunoblottings were visualized in a charge-coupled device camera and expression was quantified using the Image Gauge V3.45 program (Fuji Film Co., Tokyo, Japan).
Statistics
The detailed statistics is provided in Supplementary Information. KaleidaGraph 4.5.2 Synergy Software (Reading, PA, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA) were used for the graphs and statistical analysis. Mann–Whitney test was used for comparison of samples with n=4. Groups with normal distribution were compared with Student's two-tailed t-test (for comparing two groups), a one-way analysis of variance (for comparing more than two groups with equal variance) or a two-way analysis of variance (when comparing two parameters). Analysis of variance was followed by Bonferroni post-hoc test for multiple comparisons. All data were expressed as mean±s.e.m. Significance was accepted at the level of P<0.05 (indicated in the graph by one symbol), P<0.01 (two symbols) and P<0.001 (three symbols).
Results
C12TPP combats HFD-induced obesity in mice
In the first experimental series, we tested the metabolic effects of C12TPP in C57Bl/6 mice consuming a standard chow diet and living at an ambient temperature of 21 °C. The drinking water was supplemented with 0.04 mM or 0.4 mM C12TPP; the water intake was ~4 ml per day, which resulted in C12TPP doses of 5 or 50 μmol per (day•kg body weight). One week of treatment with the high dose induced a minor but significant reduction in body weight (Figure 1a), whereas the low dose was ineffective (not shown).
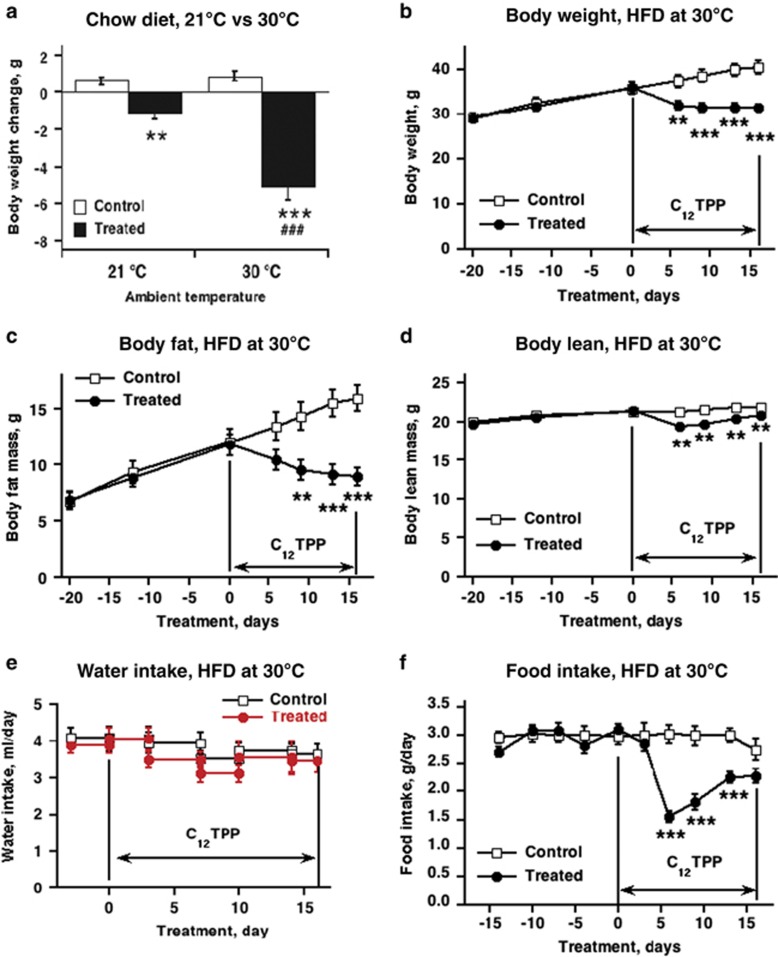
Figure 1.
Body weight and food intake of wild-type mice treated with C12TPP. (a) Body weight change in mice maintained on a chow diet acclimated at 21 or 30 °C and treated with vehicle (control) or C12TPP (treated). The C12TPP dose was 50 μmol per (day•kg body weight) for 7 days. The values are the means±s.e.m. of five to six mice in each group. Student t-test for unpaired data with unequal variance was used for comparison. *Significant differences between the control and C12TPP-treated groups; #significant difference between 21 and 30 °C. Time course of body weight (b), body fat mass (c), body lean mass (d), water (e) and food intake (f) of mice on a HFD before C12TPP treatment and during 16 days of C12TPP treatment. The period of treatment is indicated by arrows. The values are the means±s.e.m. (n=8 in each group). The statistical analysis of effects was conducted with a repeated measures 2-way analysis of variance (ANOVA): in b, c and f (time: P<0.001; treatment: P<0.001; interaction P<0.001); in d (time: P<0.01; treatment: P<0.001; interaction P<0.05). Asterisks in graphs indicate significant differences between the control and C12TPP-treated groups.
The stimulation of metabolism by C12TPP at 21 °C may have been masked by increases in facultative thermogenesis due to the cold environment.24, 25 To explore this possibility, we tested the effects of C12TPP at 30 °C (thermoneutrality). At thermoneutrality, the body weight loss of mice treated with C12TPP was fourfold increased compared with that observed at 21 °C (Figure 1a). In all further experiments, the mice were maintained at 30 °C and the chow diet was exchanged for a HFD to induce obesity.
The time-dependent effect of C12TPP was studied in obese mice maintained on a HFD for 6 weeks before the C12TPP treatment and during the 16 days of treatment (Figures 1b–f). The body weights of control mice, which received vehicle, increased during the experiment, as expected. In contrast, C12TPP-treated mice displayed a remarkable decrease in body weight, which was observed as early as during the first 6 days of the treatment (Figure 1b). On the 16th day of the treatment, the treated mice lost 12% of the initial body weight. The decrease in body weight was paralleled by a decrease in body fat. The mice lost 24% of their body fat (Figure 1c), whereas body lean mass was only slightly affected (Figure 1d). Thus, C12TPP counteracts diet-induced obesity. Therefore, we continued to investigate the mechanism underlying this reduction in body weight/body fat by studying various parameters, such as food intake and energy expenditure.
C12TPP reduces food intake
C12TPP did not affect water intake and water palatability (Figure 1e and Supplementary Figures S1a and b). However, C12TPP treatment significantly reduced food intake. Food intake was lowest (50% reduction) between the second and the sixth day of treatment and was spontaneously restored thereafter (Figure 1f). During the posttreatment period, food intake was normal and the body weight slowly reached the control level (Supplementary Figures S1c and d). Thus, the reduction in food intake contributes to the body weight loss observed in C12TPP-treated mice.
C12TPP recruits BAT and uncouples brown-fat mitochondria and adipocytes via an UCP1-independent mechanism
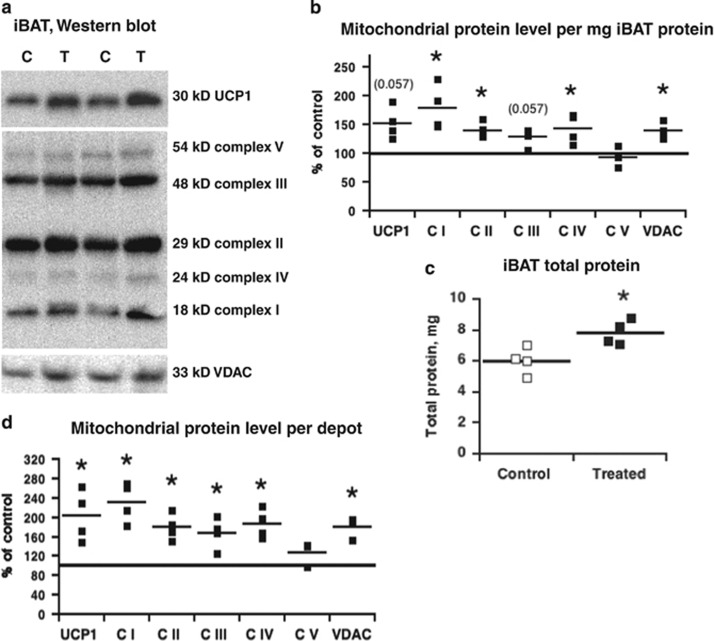
Brown adipose tissue (BAT) affects metabolic efficiency via non-shivering thermogenesis by combusting a fraction of the food, which attenuates body weight gain via the activity of UCP1.26 The total mitochondrial supplement and UCP1 content increased in the interscapular BAT of C12TPP-treated mice (Figure 2). Thus, mitochondria and UCP1 activity may be involved in the mechanism by which C12TPP reduces body weight in mice.
Figure 2.
Recruitment of BAT in wild-type mice treated with C12TPP for 16 days on a HFD at thermoneutrality. (a) Western blotting of mitochondrial proteins performed on interscapular BAT (iBAT) protein extract. (b) Mitochondrial protein content per mg tissue protein taken from quantification of western blotting as in a. (c) Total iBAT protein content. (d) Mitochondrial protein content per iBAT depot. In b–d, the mean and individual data points of four independent tissue extracts of each group are presented. For graphic presentation on b and d, the mean protein level of control iBAT was defined as 100% and the levels in iBAT from C12TPP-treated mice expressed relatively to this value. For statistics on b–d, the raw data were analyzed with Wilcoxon–Mann–Whitney test. Asterisks indicate significant differences between the control and C12TPP-treated groups.
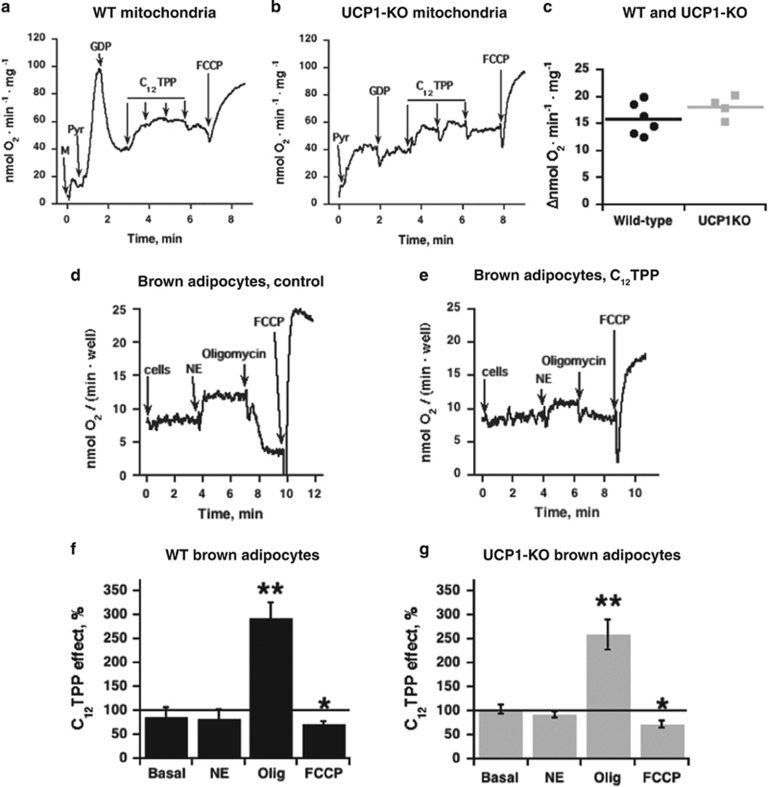
In a series of in vitro experiments, we examined the ability of C12TPP to activate isolated brown-fat mitochondria and brown adipocytes (Figure 3). In situ, the innate activity of UCP1 is inhibited by high cellular levels of purine nucleotides.27, 28 To mimic this in situ condition, we first inhibited respiration with high amounts of the UCP1 inhibitor GDP and then added C12TPP. C12TPP re-activated UCP1-containing mitochondria (Figure 3a). To examine whether this C12TPP effect was mediated by UCP1, we examined the effects of C12TPP in UCP1 knockout (KO) mitochondria (Figure 3b). In contrast to the wild-type mitochondria (Figure 3a), pyruvate-supported respiration was low in brown-fat mitochondria lacking UCP1 and the addition of GDP did not affect respiration (Figure 3b). These results are consistent with Matthias et al.;29 Monemdjou et al.30 However, the C12TPP concentration–response curves were similar in wild-type and UCP1-KO mitochondria; maximal responses were observed at the same C12TPP concentration (22 μM). The magnitudes of these responses were the same in wild-type and UCP1-KO mitochondria (Figure 3c). Thus, remarkably, C12TPP can activate brown fat mitochondria. However, the mechanism of activation was different from that associated with the classical activators of UCP1 fatty acids. The concentration–response curves of the structural analogue of the C12TPP tail, lauric acid, clearly differed between wild-type and UCP1-KO mitochondria (Supplementary Figure 2); these results are consistent with Shabalina et al.28, 31
Figure 3.
C12TPP-stimulated oxygen consumption in brown-fat mitochondria and brown adipocytes isolated from wild-type and UCP1-KO mice. Representative trace depicting titration with C12TPP of brown-fat mitochondria from wild-type (a) or UCP1-KO (b) mice. C12TPP was successively added at concentrations ranging from 11 to 44 μM (the concentration was increased by 11 μM in each step). Additions were 5 mM pyruvate (Pyr), 1 mM GDP and 2.1 μM carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP). (c) Comparison of the effects of C12TPP in wild-type and UCP1-KO brown-fat mitochondria. The mitochondria were examined as shown in a and b, and the values were obtained by estimating the maximal responses. The mean and individual data points of four to six independent mitochondrial preparations for each group are presented. Representative traces depicting the oxygen consumption of control (d) and C12TPP-pretreated (e) brown adipocytes. Primary cultures of brown adipocytes were treated with 0.1% ethanol (control) or 1 μM C12TPP for 3 h before harvesting. Additions were 1 μM β-adrenergic agonist norepinephrine (NE), 2 μM oligomycin and 60 μM FCCP. C12TPP effects on oxygen consumption rates in brown adipocytes originating from wild-type mice (f) or UCP1-ablated mice (g). Control and C12TPP-treated adipocytes were examined in parallel as shown in d and e. The oxygen consumption rate of control adipocytes on a given day was defined as 100% and oxygen consumption rate of C12TPP-treated adipocytes from parallel examination is expressed relative to that of these control adipocytes (as % of basal, NE, oligomycin and FCCP). The bars represent the means±s.e.m. of five independent cell cultures of each genotype. The raw data were analyzed with Student's t-test and asterisks indicate significant differences between control and C12TPP-treated cells.
To test the ability of C12TPP to activate cellular thermogenesis, we examined the effects of C12TPP in brown adipocytes in culture (Figures 3d–g). The basal and norepinephrine-stimulated oxygen consumption rates were the same in the control and C12TPP-treated wild-type brown adipocytes (Figures 3d and e). However, when mitochondrial oxidative phosphorylation was inhibited by oligomycin, the oxygen consumption of C12TPP-treated brown adipocytes was considerably increased compared with control adipocytes (Figure 3f), which reflects increased mitochondrial membrane proton conductance in the treated cells. The same pattern was also observed in UCP1-KO brown adipocytes (Figure 3g).
Thus, we concluded that C12TPP induced uncoupling in isolated brown-fat mitochondria and intact brown adipocytes in an UCP1-independent manner.
The level of this uncoupling was mild and did not significantly inhibit or disrupt mitochondrial oxidative capacity. The artificial protonophore carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone applied to mitochondria after C12TPP exerted a high stimulatory effect (Figures 3a and b), indicating that the mitochondria retained their high oxidative capacity. In cells, the carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone response was only slightly affected (Figures 3f and g).
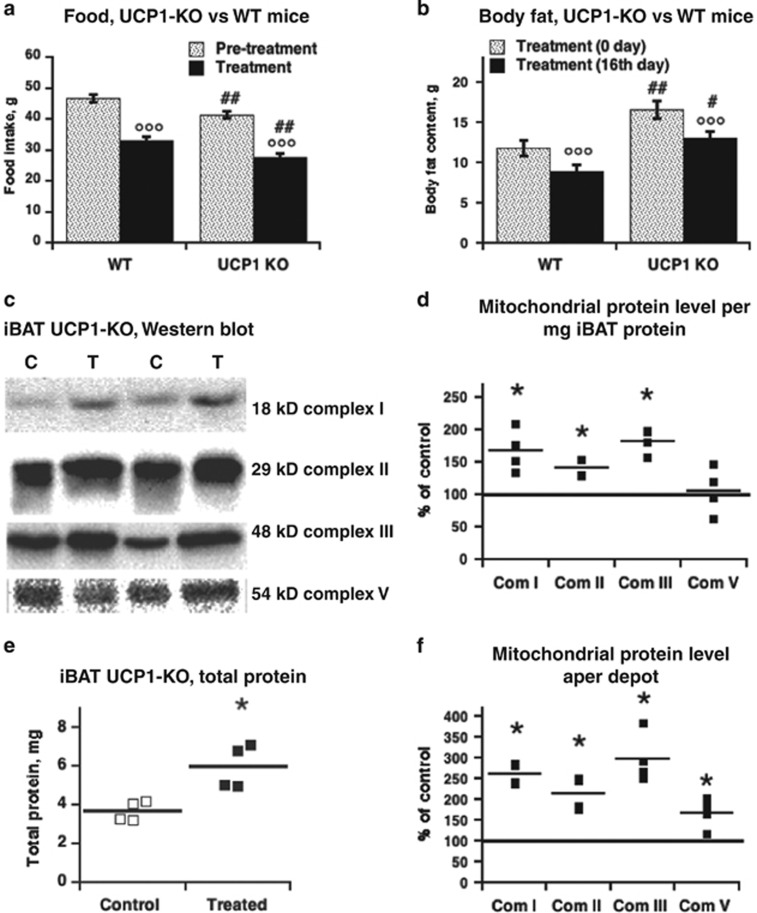
Although we demonstrated that the in vitro uncoupling effect of C12TPP in isolated mitochondria and cultured brown adipocytes was independent of the presence of UCP1, we could not exclude the possibility that the effects of C12TPP in vivo were mediated by UCP1. For example, a reduction in food intake may depend on UCP1, as recently demonstrated.32 Therefore, we compared food intake in wild-type and UCP1-KO mice. The C12TPP-induced reduction in food intake in UCP1-KO mice was the same as that observed in wild-type mice (Figure 4a and Supplementary Figure S3a), which strongly suggests that C12TPP affected food intake independently of the presence of UCP1. However, UCP1-KO mice generally ate less than wild-type mice (Figure 4a). Despite this lower food intake, UCP1-KO mice were significantly fatter than wild-type mice (Figure 4b), as demonstrated in Feldmann et al.24 Importantly, the C12TPP-induced decreases in body weight and fat weight were similar in UCP1-KO and wild-type mice (Figure 4b and Supplementary Figures S3b and c). Changes in lean body mass were also independent of UCP1 (data not shown).
Figure 4.
UCP1-independent effects of C12TPP in mice. (a) Food intake for 16 days of pretreatment and treatment period in the wild-type and UCP1-KO mice on HFD at thermoneutrality. The data for wild-type mice were obtained from Figure 1f. (b) Body fat mass on day 0 (the start of C12TPP treatment) and on the 16th (last) day of treatment in wild-type and UCP1-KO mice. The data for wild-type mice were obtained from Figure 1c. In a and b, the values are means±s.e.m. (n=8 in each group). The data were statistically analyzed with a two-way mixed analysis of variance (ANOVA) with treatment as within-subjects factor and genotype as between-subjects factor: in a, (genotype: P<0.01; treatment: P<0.001; interaction ns); in b, (genotype: P<0.01; day: P<0.001; interaction ns). °Significant differences between days (pretreatment and treatment, or day 0 and day 16); #Significant differences between genotypes. (c) Western blotting of mitochondrial proteins in the total interscapular BAT (iBAT) protein extract from UCP1-KO mice treated with C12TPP for 16 days on a HFD at thermoneutrality. (d) Mitochondrial protein content per mg tissue extract taken from quantification of western blotting as in c. (e) iBAT total protein content. (f) Mitochondrial protein content per total iBAT depot. In d–f, the mean and individual data points of four independent tissue extracts of each group are presented. For graphic presentation on d–f, the mean protein level of control iBAT was defined as 100% and the levels in iBAT from C12TPP-treated mice expressed relatively to this value. For statistics on d–f, the raw data were analyzed with Wilcoxon–Mann–Whitney test. Asterisks indicate significant differences between the control and C12TPP-treated groups.
The increases in the levels of total interscapular BAT protein and individual mitochondrial protein complexes (Figures 4c–f) were similar in UCP1-KO and wild-type mice (Figure 2).
BAT is a powerful tissue for releasing energy as heat.33 Furthermore, BAT can contain a high level of endogenous fatty acids under physiological conditions, which favors C12TPP uncoupling by facilitating the cycling of endogenous fatty acids in mitochondria.10 Skeletal muscle is another metabolically active tissue that contributes to energy expenditure. However, C12TPP did not affect the mitochondrial protein content in the skeletal muscle of mice treated with C12TPP (Supplementary Figure S4), which supports the hypothesis that BAT may have an important role in the mechanism of C12TPP.
The anti-obesity effect of C12TPP is partially attributable to decreased food intake
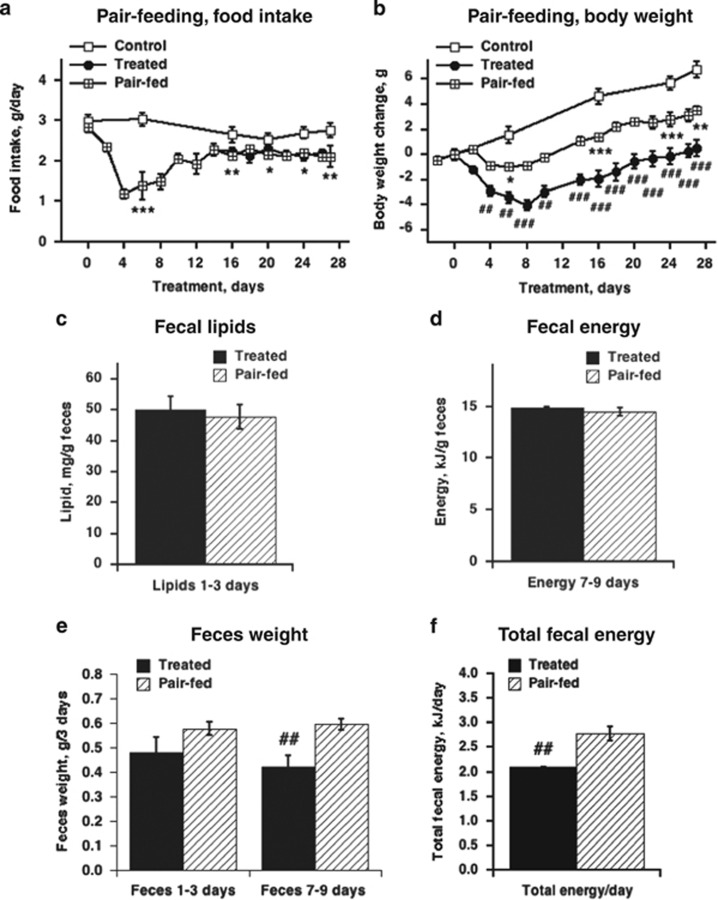
To estimate the extent to which the effects of C12TPP were determined by a decrease in food intake, we introduced a pair-fed group of mice and increased the duration of the treatment for up to 27 days. Obvious differences were observed in the body weights of C12TPP-treated and pair-fed mice (Figures 5a and b). When food intake was lowest (between the fourth and the eighth day of treatment), C12TPP-treated mice lost threefold more body weight than pair-fed mice (Figure 5b). Thus, the C12TPP-induced loss in body weight is due to two mechanisms: food intake dependent and food intake independent. The food intake-dependent component contributes to a maximum of 30% of the body weight loss. Thus, most of the body weight loss must be explained by another mechanism. To highlight the impact of food intake-independent mechanisms, we selected the pair-fed group as the proper control for the C12TPP-treated group. All further parameters were compared between treated and pair-fed mice.
Figure 5.
Food intake-independent effects of C12TPP in wild-type mice on a HFD at thermoneutrality. Time course of food intake (a) and body weight change relative to day 0 (b) of control, C12TPP-treated mice and mice pair-fed with the treated group. Treatment and pair-feeding started on day 0 and finished on day 27. The values are the means±s.e.m. of six to seven mice per group. The effects for overlapping time points with the control were statistically analyzed with a repeated measures two-way analysis of variance (ANOVA): for both a and b (time: P<0.0001; treatment: P<0.0001; interaction P<0.001). The effects for overlapping time points for treated and pair-fed mice was statistically analyzed with a repeated measures two-way ANOVA for a: the food intake in pair-fed group was pre-defined and cannot be included in the ANOVA; for b (time: P<0.0001; treatment: P<0.0001; interaction P<0.001). *Significant differences between the pair-fed and control groups. #Significant differences between the pair-fed and treated groups. The difference between the control and treated groups is not shown. (c and d) Lipid and energy contents of feces. A lipid analysis (c) was performed on feces from the first 3 days of treatment and bomb calorimetry was used to estimate the energy (d) content of feces from 7 to 9 days of treatment. Bars represent the mean±s.e.m. of four to six mice per group. (e) Amount of feces produced during the first three days (1 to 3) and 7 to 9 days of treatment. Bars represent the mean±s.e.m. of five to six mice per group. The data were statistically analyzed with a repeated measures two-way ANOVA: (treatment: P<0.001; days ns; interaction ns). #Significant differences between the pair-fed and treated groups. (f) Total fecal energy per day of C12TPP treatment. The fecal energy (as in d) was multiplied by the amount of feces (as in e) and this amount was divided by 3 days. Bars represent the mean±s.e.m. of five to six mice per group. The data were analyzed with Student's t-test and # indicates significant differences between the pair-fed and treated groups.
The effect of C12TPP on body weight may be attributed to an adverse effect on the intestines, for example, impaired digestion and/or food absorption. However, a pathomorphological examination of the alimentary system did not reveal any sign of digestive tract impairment in C12TPP-treated mice (data not shown). The energy content and amount of lipid per gram of feces were also similar in treated and pair-fed mice (Figures 5c and d). Importantly, both the amount of feces and the total fecal energy were reduced in treated mice (Figures 5e and f). From the seventh to the ninth day of treatment, treated mice excreted 5.9±0.4% of the energy consumed, whereas pair-fed mice excreted 8.2±0.4% (P<0.01).
Thus, C12TPP simultaneously reduces food intake and excreted energy, which together explain ~20–30% of mouse body weight loss.
High RMR in C12TPP-treated mice
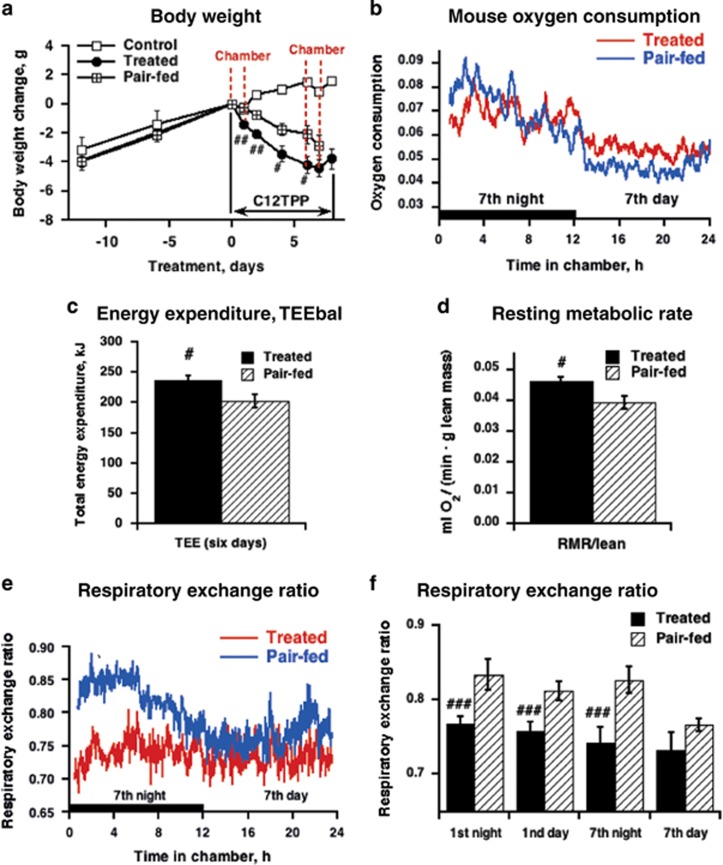
Indirect calorimetry was applied to examine the oxygen consumption, TEE (TEEic), RMR and RER. The mice were placed into indirect calorimetry chambers during the first and the seventh day of treatment (Figure 6a). The oxygen consumption rates were higher during dark phase than during the light phase (Figure 6b), reflecting circadian rhythm of mice.
Figure 6.
Effects of C12TPP on the rates of oxygen consumption and RER in wild-type mice on a HFD at thermoneutrality. (a) Change in body weight over time relative to day 0 in control, C12TPP-treated and pair-fed to treated mice. Treatment started on day 0 and finished on day 7, as is indicated by arrows. The mice were exposed twice to indirect calorimetry measurements on day 1 and 7 of treatment. The periods inside the chamber are indicated by red lines. The values are the means±s.e.m. of five to six mice per group. The effects were statistically analyzed using a repeated measures two-way analysis of variance (ANOVA) (time: P<0.01; treatment: P<0.01; interaction P<0.01). #Significant differences between the treated and pair-fed groups. Significant differences from the control are not shown. (b) Rate of oxygen consumption (ml O2 per min·g lean body mass) of treated and pair-fed mice on the seventh day of treatment. Nighttime is indicated by the black box on the x axis. The values are the means of six mice per group. (c) TEE (TEEbal) estimated using the energy balance method for the 6 days of treatment. (d) RMR in C12TPP-treated and pair-fed mice during the seventh light phase of day of treatment. In c and d, the values are the means±s.e.m. (n=6 for each group). The data were analyzed with a paired Student's t-test and the # indicates significant differences between the pair-fed and treated groups. (e) RER traces during the seventh day of treatment. Each trace is the mean of n=6 mice per group. (f) Average RER of treated and pair-fed mice. The values are the means±s.e.m. (n=5–6 for each group). The effects were statistically analyzed with a two-way ANOVA matched for both time and mouse (separate for day 1 and day 7 due to not being the same animals): day 1 (time: P<0.05; treatment: P<0.05; interaction P<0.05); day 7 (time: P<0.05; treatment: P<0.05; interaction P<0.01). #Significant differences between the treated and pair-fed groups.
The TEEic did not differ between the treated and pair-fed group (Supplementary Table). However, the TEE estimated over 6 days using an energy balance method17 (TEEbal) was 15% increased in treated compared with pair-fed mice (Figure 6c). Surprisingly, these two methods of TEE estimation yielded different results. The TEEbal was measured at standard conditions in the ‘home cage,' which provided an accurate integrated long-term measurement of energy expenditure, whereas the TEEic may have been affected by potentially confounding stress that may accompany the use of an indirect calorimetry system.17 Pair-fed mice lost more body weight in the indirect calorimetry chamber compared with a standard environment, whereas C12TPP-treated mice were minimally affected by the new environment (Supplementary Figure S5a).
Basal mitochondrial proton leakage (uncoupling) significantly contributes to the RMR in mice.7, 34 To identify the uncoupling activity of C12TPP in vivo, we determined the RMR of mice treated with C12TPP. The RMR per mouse was 6% (day 1) and 11% (day 7) increased in C12TPP-treated mice compared with pair-fed mice (Supplementary Table). The normalization of the RMR by the lean body mass35 (Supplementary Figure S5b) resulted in an 18% increased RMR in treated mice compared with pair-fed mice (Figure 6d).
Thus, both an increased RMR and reduced energy intake could explain the anti-obesity activity of C12TPP. Importantly, a comparison with pair-fed animals minimizes the impact of the thermic effect of food on the RMR and consequently enables the estimation of the uncoupling activity of C12TPP in vivo.
C12TPP treatment enhances fatty acid utilization
The RER of pair-fed mice was ≈0.85 and slightly higher during the dark than during the light phase (Figures 6e and f). The RER of the C12TPP-treated mice was significantly reduced compared with pair-fed mice, which was noted as early as the first day of the treatment and remained low until the seventh night (Figure 6f). In the latter case, C12TPP decreased the RER to a minimal level (0.7), indicating a total switch to lipid catabolism on C12TPP treatment. Thus, C12TPP treatment enhanced fatty acid utilization via a mechanism independent of decreases in food consumption.
Discussion
C12TPP induces mitochondrial uncoupling
In this study, we demonstrated the uncoupling effects of C12TPP in isolated brown-fat mitochondria, brown adipocyte culture and in mice in vivo. Although these effects were independent of UCP1, the effect of C12TPP on the metabolic rate may be mediated by brown-fat mitochondria. Free fatty acids are released in BAT on adrenergic stimulation. C12TPP may facilitate the flip-flopping of fatty acid anions in the mitochondrial membrane, which dissipates the energy of the transmembrane potential. This mechanism may be additionally stimulated by the observed upregulation of mitochondrial protein synthesis in the BAT on C12TPP treatment. Interestingly, this effect of C12TPP contrasts the effect of the classical uncoupler DNP, which reduces the thermogenic capacity of BAT.25 C12TPP does not affect skeletal muscle mitochondriogenesis, which is also in the contrast to the effect of DNP.36
The effect of C12TPP on body weight was more evident at thermoneutrality than 21 °C. The anti-obesity effects of the artificial uncoupler DNP and natural uncoupler UCP1 were strongly dependent on environmental temperature.24, 25, 37, 38 Thus, the present study confirms a general principle—the potential anti-obesity agents should be studied at thermoneutrality in mice. A recent study of the anti-obesity effect of a mitochondria-targeting mitoQ, which can uncouple mitochondrial respiration in vitro,39 did not reveal an increase in the metabolic rate of mitoQ-treated mice.40 However, the study was performed in mice maintained at 22 °C, which could explain the lack of an effect of mitoQ on the metabolic rate of the mice.
The C12TPP-induced increase in metabolic rate was small. It is equal to the change in metabolism rate, which occurs in mice on change of only 3 °C of ambient temperature.33 Such a small increase in metabolism should not detrimentally affect the function of the cardiovascular, renal and endocrine systems. It has been demonstrated that the slightly higher metabolism observed after exercise or after short exposure to cold ameliorates the symptoms of diabetes and atherosclerosis, and reduces the risk of cardiovascular diseases.41, 42, 43, 44, 45 Thus, a beneficial effect of C12TPP on these pathologies may also be proposed.
C12TPP enhances fatty acid utilization
C12TPP significantly reduced the RER, which indicates enhanced fatty acid utilization in mice. Several mechanisms are hypothesized to underlie this enhanced fatty acid utilization. The first hypothesis relies on the suggestion that mitochondrial uncoupling generally increases mitochondrial fatty acid oxidation, which ultimately results in a low RER throughout the body. Natural uncoupling by UCP1 in brown/brite adipose tissues typically correlates well with enhanced mitochondrial fatty acid utilization and lipid droplet remodeling.23, 41, 46 Furthermore, the mild mitochondrial uncoupler niclosamide ethanolamine reduced the RER consistently with a significant reduction in adipose tissue depots.47
Enhanced fatty acid utilization is also related to increased lipid availability in the systemic circulation,48, 49 improved fatty acid uptake into cells and/or the enhanced transport of fatty acid-derived substrates into mitochondria.50, 51 The C12TPP cation might facilitate the delivery of fatty acid anions to fatty acyl-CoA synthetases, which is an attractive prospect.52
The effect of C12TPP on RER was large and under resting condition, it could be entirely explained by the utilization of fatty acids in BAT. Several facts favorite this hypothesis: (1) C12TPP uncouples mitochondria by means of endogenous fatty acids 10 and BAT is the only tissue enriched by both free fatty acids and mitochondria;33 (2) C12TPP recruits BAT; and (3) BAT actively controls the plasma triglycerides clearance.41 However, under strenuous exercise, a fatty acid utilization is highly increased in skeletal muscle53 and whether C12TPP could facilitate this process is currently unknown.
C12TPP affects mouse appetite
C12TPP potentially reduced food intake in mice by affecting the production (or functioning) of hormone-like compounds that affect appetite. Indeed, the effects of C12TPP on food intake resemble the effects of the anorexic gut peptide YY.54 Development of tolerance to appetite suppression appears to be a common feature of many anti-obesity drugs: for example, rimonabant, sibutramine and tesofensine (reviewed in Fernstrom et al.55).
Other mitochondria-targeting compounds such as mitoQ40 and C4R1(ref. 12) also influence food intake. The ability of all three mitochondria-targeting compounds that have been studied to influence appetite is intriguing; however, the mechanism underlying this influence remains elusive. Nevertheless, the direct action of mitoQ on the satiety center in the central nervous system has been excluded, because mitoQ does not enter the central nervous system.56
C12TPP is a potential therapeutic route to combat obesity
Three anti-obesity effects of C12TPP were observed: decreased energy intake, elevated RMR and an increased utilization of fatty acids. This combination of several modes of action appears to be a desirable feature of C12TPP as an anti-obesity agent, because a poly-therapeutic strategy against obesity (targeting different metabolic pathways) often yields better results than strategies that modify one pathway.1
C12TPP did not affect water intake or the palatability and morphology of the alimentary tract. Treatment with C12TPP (27 days in total) also did not induce visible toxic effects in mice. Mice behavior was normal, even during periods of substantially reduced food intake. This C12TPP study was performed under humanized conditions (that is, human metabolism was modeled in mice), namely at thermoneutrality and with high fat and high sugar diets resembling obesity-inducing Western diets. Therefore, the anti-obesity properties of C12TPP are expected to be reproduced in man.
Thus, C12TPP appears to be promising as an effective and safe anti-obesity drug.
Acknowledgments
The studies were supported by Russian Scientific Foundation grant No 14-24-00107, by the Institute of Mitoengineering and by Mitotech SA. AVK was supported by a salary from the Academic Initiative of Stockholm University. We thank Jan Nedergaard and Barbara Cannon for valuable comments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Rodgers RJ, Tschop MH, Wilding JP. Anti-obesity drugs: past, present and future. Dis Models Mech 2012; 5: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta 1998; 1363: 100–124. [DOI] [PubMed] [Google Scholar]
- Brand MD. The contribution of the leak of protons across the mitochondrial inner membrane to standard metabolic rate. J Theor Biol 1990; 145: 267–286. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 2001; 1504: 82–106. [DOI] [PubMed] [Google Scholar]
- Tainter ML, Cutting WC, Stockton AB. Use of dinitrophenol in nutritional disorders: a critical survey of clinical results. Am J Public Health Nations Health 1934; 24: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola J. Dinitrophenol and bioenergetics: an historical perspective. Mol Cell Biochem 1974; 5: 69–77. [DOI] [PubMed] [Google Scholar]
- Harper JA, Dickinson K, Brand MD. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2001; 2: 255–265. [DOI] [PubMed] [Google Scholar]
- Trendeleva TA, Sukhanova EI, Rogov AG, Zvyagilskaya RA, Seveina II, Ilyasova TM et al. Role of charge screening and delocalization for lipophilic cation permeability of model and mitochondrial membranes. Mitochondrion 2013; 13: 500–506. [DOI] [PubMed] [Google Scholar]
- Blaikie FH, Brown SE, Samuelsson LM, Brand MD, Smith RA, Murphy MP. Targeting dinitrophenol to mitochondria: limitations to the development of a self-limiting mitochondrial protonophore. Biosci Rep 2006; 26: 231–243. [DOI] [PubMed] [Google Scholar]
- Severin FF, Severina II, Antonenko YN, Rokitskaya TI, Cherepanov DA, Mokhova EN et al. Penetrating cation/fatty acid anion pair as a mitochondria-targeted protonophore. Proc Natl Acad Sci USA 2010; 107: 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko YN, Avetisyan AV, Cherepanov DA, Knorre DA, Korshunova GA, Markova OV et al. Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J Biol Chem 2011; 286: 17831–17840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinovich AV, Shabalina IG. Novel mitochondrial cationic uncoupler C4R1 is an effective treatment for combating obesity in mice. Biochemistry (Mosc) 2015; 80: 620–628. [DOI] [PubMed] [Google Scholar]
- Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper M-E et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997; 387: 90–94. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Shimizu H, Kobayashi I, Kobayashi S. Importance of feeding time in pair-fed, ovariectomized rats. Physiol Behav 1989; 45: 1197–1200. [DOI] [PubMed] [Google Scholar]
- Abreu-Vieira G, Fischer AW, Mattsson C, de Jong JM, Shabalina IG, Ryden M et al. Cidea improves the metabolic profile through expansion of adipose tissue. Nat Commun 2015; 6: 7433. [DOI] [PubMed] [Google Scholar]
- Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol 2012; 303: R459–R476. [DOI] [PubMed] [Google Scholar]
- Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. Int J Obes 20052013; 37: 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadravec D, Brolinson A, Fisher RM, Carneheim C, Csikasz RI, Bertrand-Michel J et al. Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J 2010; 24: 4366–4377. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Studies of thermogenesis and mitochondrial function in adipose tissues. Methods Mol Biol 2008; 456: 109–121. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Vrbacky M, Pecinova A, Kalinovich AV, Drahota Z, Houstek J et al. ROS production in brown adipose tissue mitochondria: the question of UCP1-dependence. Biochim Biophys Acta 2014; 1837: 2017–2030. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARgamma agonist. Am J Physiol Endocrinol Metab 2008; 295: E287–E296. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Landreh L, Edgar D, Hou M, Gibanova N, Atanassova N et al. Leydig cell steroidogenesis unexpectedly escapes mitochondrial dysfunction in prematurely aging mice. FASEB J 2015; 29: 3274–3286. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5: 1196–1203. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009; 9: 203–209. [DOI] [PubMed] [Google Scholar]
- Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, Reitman ML. The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. J Biol Chem 2014; 289: 19341–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 2010; 11: 268–272. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. The bioenergetics of brown adipose tissue mitochondria. FEBS Lett 19761976; 61: 103–110. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Jacobsson A, Cannon B, Nedergaard J. Native UCP1 displays simple competitive kinetics between the regulators purine nucleotides and fatty acids. J Biol Chem 2004; 279: 38236–38248. [DOI] [PubMed] [Google Scholar]
- Matthias A, Jacobsson A, Cannon B, Nedergaard J. The bioenergetics of brown fat mitochondria from UCP1-ablated mice. Ucp1 is not involved in fatty acid-induced de-energization (‘uncoupling'). J Biol Chem 1999; 274: 28150–28160. [DOI] [PubMed] [Google Scholar]
- Monemdjou S, Kozak LP, Harper ME. Mitochondrial proton leak in brown adipose tissue mitochondria of Ucp1-deficient mice is GDP insensitive. Am J Physiol 1999; 276: E1073–E1082. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Kalinovich AV, Cannon B, Nedergaard J. Metabolically inert perfluorinated fatty acids directly activate uncoupling protein 1 in brown-fat mitochondria. Arch Toxicol 2016; 90: 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Kramarova TV, Mattsson CL, Petrovic N, Rahman Qazi M, Csikasz RI et al. The environmental pollutants perfluorooctane sulfonate and perfluorooctanoic acid upregulate uncoupling protein 1 (UCP1) in brown-fat mitochondria through a UCP1-dependent reduction in food intake. Toxicol Sci 2015; 146: 334–343. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359. [DOI] [PubMed] [Google Scholar]
- Rolfe DF, Brand MD. Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol 1996; 271 (4 Pt 1): C1380–C1389. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 2011; 214 (Pt 2): 242–253. [DOI] [PubMed] [Google Scholar]
- Schlagowski AI, Singh F, Charles AL, Gali Ramamoorthy T, Favret F, Piquard F et al. Mitochondrial uncoupling reduces exercise capacity despite several skeletal muscle metabolic adaptations. J Appl Physiol (1985) 2014; 116: 364–375. [DOI] [PubMed] [Google Scholar]
- Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab 2009; 9: 111–112. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 2014; 20: 396–407. [DOI] [PubMed] [Google Scholar]
- Fink BD, Herlein JA, Yorek MA, Fenner AM, Kerns RJ, Sivitz WI. Bioenergetic effects of mitochondrial-targeted coenzyme Q analogs in endothelial cells. J Pharmacol Exp Ther 2012; 342: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BD, Herlein JA, Guo DF, Kulkarni C, Weidemann BJ, Yu L et al. A mitochondrial-targeted coenzyme q analog prevents weight gain and ameliorates hepatic dysfunction in high-fat-fed mice. J Pharmacol Exp Ther 2014; 351: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011; 17: 200–205. [DOI] [PubMed] [Google Scholar]
- Asano RY, Sales MM, Browne RA, Moraes JF, Coelho Junior HJ, Moraes MR et al. Acute effects of physical exercise in type 2 diabetes: a review. World J Diabetes 2014; 5: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralova Lesna I, Rychlikova J, Vavrova L, Vybiral S. Could human cold adaptation decrease the risk of cardiovascular disease? J Therm Biol 2015; 52: 192–198. [DOI] [PubMed] [Google Scholar]
- Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med 2015; 21: 863–865. [DOI] [PubMed] [Google Scholar]
- Berbee JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 2015; 6: 6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Cui L, Wang W, Na H, Zhu X et al. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim Biophys Acta 2015; 1853: 918–928. [DOI] [PubMed] [Google Scholar]
- Tao H, Zhang Y, Zeng X, Shulman GI, Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat Med 2014; 20: 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Tan GD. Adipose tissue function in the insulin-resistance syndrome. Biochem Soc Trans 2005; 33 (Pt 5): 1045–1048. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci 1998; 3: D1011–D1027. [DOI] [PubMed] [Google Scholar]
- Carley AN, Severson DL. What are the biochemical mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice? Cardiovasc Drugs Ther 2008; 22: 83–89. [DOI] [PubMed] [Google Scholar]
- Bebernitz GR, Schuster HF. The impact of fatty acid oxidation on energy utilization: targets and therapy. Curr Pharm Des 2002; 8: 1199–1227. [DOI] [PubMed] [Google Scholar]
- Poppelreuther M, Rudolph B, Du C, Grossmann R, Becker M, Thiele C et al. The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake. J Lipid Res 2012; 53: 888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom M, Bakkman L, Tonkonogi M, Shabalina IG, Rozhdestvenskaya Z, Mattsson CM et al. Reduced efficiency, but increased fat oxidation, in mitochondria from human skeletal muscle after 24- h ultraendurance exercise. J Appl Physiol (1985) 2007; 102: 1844–1849. [DOI] [PubMed] [Google Scholar]
- Adams SH, Lei C, Jodka CM, Nikoulina SE, Hoyt JA, Gedulin B et al. PYY[3-36] administration decreases the respiratory quotient and reduces adiposity in diet-induced obese mice. J Nutr 2006; 136: 195–201. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Choi S. The development of tolerance to drugs that suppress food intake. Pharmacol Ther 2008; 117: 105–122. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, Prime TA, Rose C et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med 2010; 48: 161–172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.