Abstract
This study examined the association between salivary oxytocin (sOT) levels and generosity in preschoolers. Fifty preschoolers played two dictator games (DG) by deciding how to allocate 10 chocolates between themselves and another child, who was either from the same class as the participant (ingroup member), or an unknown child from another class (outgroup member). sOT levels were assessed in saliva collected from the children immediately prior to the DG tasks. While sOT levels were negatively associated with allocations made to both ingroup and outgroup members by boys, among girl sOT levels were positively related to allocations made to ingroup members, and unrelated to allocations made to outgroup members. These results suggest sex differences in the association between salivary oxytocin and generosity.
Ingroup favouritism is a fundamental psychological mechanism whereby people tend to be more generous and cooperative to ingroup members than to outgroup members1,2,3. Ingroup favouritism is observed in various situations and cultures around the world4,5,6,7, and many studies suggest that humans have evolved several tendencies that enable them to quickly identify and cooperate with group members, such as attending to reputation8,9 and reciprocity10,11. These ingroup favouring tendencies become adaptive in cases of intergroup conflict (e.g., war)12 and may lead to the creation of cultural groups13.
Recent studies have started to reveal the biological foundations of ingroup favoritism14,15,16,17,18. One focus of such research is the role of oxytocin, a peptide hormone produced in the hypothalamus and secreted from the posterior pituitary gland. Oxytocin has been shown to be associated with social cognition, in addition to social stress/anxiety reduction19. Recent research suggests that oxytocin plays a pivotal role not only as a hormone but also as a neurotransmitter in the brain, enhancing various aspects of human sociality, such as trusting behaviour20,21, theory of mind22, facial expression recognition23, parenting behavior24, and social bonding25,26.
Oxytocin also appears to play a role in increasing ingroup favouritism (for a review, see ref. 27). For instance, De Dreu et al.15 found that intranasal oxytocin administration enhances individuals’ cooperativeness with other ingroup members when playing economic games. Interestingly, oxytocin did not affect cooperativeness with outgroup members. Another study found that intranasal administration of oxytocin increased the liking of symbols representing national identity28, and yet other studies suggest that genetic variants associated with the oxytocin receptor gene (OXTR) influence processes related to ingroup favouritism. For instance, one study found that individuals who were homozygous for the G-allele of OXTR rs53576 were more likely to show empathetic responses, which were assessed by measuring activity in the anterior cingulate and supplementary motor area in response to racial ingroup members and compared to responses to racial outgroup members29. Thus, oxytocin appears to play a role in increasing social bonding and affiliation within groups.
Sex differences in the effects of oxytocin on social behaviour
A large number of studies have shown that the effects of oxytocin on mammalian social behaviour differ between males and females (for reviews, see refs 30, 31). Oxytocin is implicated in a number of sex-specific process involved in gestation, lactation, and maternal social behaviour in mammalian females, and oxytocin receptors are induced by estradiol, a female sex hormone, which enhances responsiveness to oxytocin32,33. For instance, studies examining sex differences in the impact of oxytocin on social behaviour in non-human animals have found that intranasal oxytocin administration enhances gazing behaviour toward the owner only in female dogs34 and the anxiolytic effect of oxytocin differs between male and female rodents, possibly due to differences in sex steroid hormones19.
In humans, similar evidence suggests sex differences in the role of oxytocin on social behaviour. For instance, Rilling and colleagues35,36 examined sex differences in the effect of intranasal oxytocin administration on cooperative behaviour, and found that oxytocin influenced cooperative behaviour only among female participants. In their study, participants played a repeated prisoner’s dilemma game, and female participants who received the intranasal administration of oxytocin tended to detect others’ betrayal when the opponents were computer-controlled36. However, this effect was not observed among male participants35. Likewise, Scheele et al.37 found that oxytocin differentially affected moralistic behaviour in men and women, whereby the administration of oxytocin enhanced self-beneficial choices in a moral dilemma task in males, but inhibited self-beneficial choices in females.
This study sought to examine the relation between oxytocin and social behavior in preschool children. To date, a great deal of research has focused on the emergence of ingroup favoritism in childhood. Recent developmental research has shown that even 6-year-old children can distinguish ingroup members from outgroup members, and tend to be more generous toward ingroup members38,39,40. However, no study has examined the association between ingroup favouritism and oxytocin levels in children. Thus, we sought to examine whether the impact of oxytocin on social behaviour, in this case ingroup favouritism, can be observed in children, and if so, whether there are sex differences in the association of oxytocin to ingroup favouritism.
Investigating the relation between social behavior and oxytocin in children is an important key to understanding the biological development of behaviour related to social norms, as well as developmental disorders. As research has suggested that oxytocin may be implicated in developmental disorders in childhood such as Autism Spectrum Disorders (ASD), examining the development of social behavior in childhood is important to gain a better understanding of how social behavior relates to oxytocin levels. Furthermore, if ASD in children is related to oxytocin levels, we would expect an association between social behavior and oxytocin levels in children.
To assess oxytocin levels, we examined oxytocin concentration in saliva. Recent studies have demonstrated a positive relationship between sociality and salivary oxytocin (sOT) in preschool-aged children41,42,43. Furthermore, because saliva collection is simple and painless, it is better suited to assessing oxytocin levels in children than other methods, such as using plasma. In order to assess generosity shown towards ingroup and outgroup members, we used a simple two-person economic game known as the dictator game (DG), in which children decided how many resources to allocate between themselves and an ingroup and an outgroup member, respectively.
As oxytocin is implicated in social behaviours that are particularly relevant within the context of ingroups familial relationships, we suspected that sOT levels may be positively related to generosity expressed toward ingroup members in particular. Furthermore, as previous studies have found evidence for sex-specific effects of the OT system on social behaviour, we sought to examine whether sex effects would be observed even in preschool children.
Results
Behavioural Data
To examine the effect of condition order on allocations made in the DG, we conducted a two-way analysis of variance (ANOVA) with recipient affiliation (ingroup vs. outgroup) as a within-subject factor and condition order as a between-subject factor. Results showed no main effect of the affiliation of the recipient (F (1, 48) = 1.35, p = 0.2517, ηp2 = 0.027), condition order (F (1, 48) = 0.153, p = 0.6971, ηp2 = 0.003), and an interaction effect (F (1, 48) = 3.47, p = 0.0688, ηp2 = 0.067). Thus, we concluded that an effect of condition order on allocations was not observed in this study.
The mean levels of the allocation amounts by condition, and sex for older (age 5–6) and younger (age 3–4) children are shown in Fig. 1. We conducted an analysis of covariance (ANCOVA) model on the amounts allocated, with recipient affiliation (ingroup vs. outgroup) as a within-subject factor and sex as a between-subject factor. Because previous studies have shown an effect of age on allocations made by children44,45,46,47,48, we used age in months as a covariate. Results indicated an interaction effect of recipient affiliation × sex (F (1, 47) = 7.30, p = 0.0096, ηp2 = 0.134) and a main effect of age (F (1, 47) = 11.34, p = 0.0015, ηp2 = 0.194). However, the main effects of sex (F (1, 47) = 0.52, p = 0.4736, ηp2 = 0.011), recipient affiliation (F (1, 47) = 0.88, p = 0.3523, ηp2 = 0.018), and the recipient affiliation × age interaction effect (F (1, 47) = 0.53, p = 0.4708, ηp2 = 0.011) were not significant. When we added the interaction term of recipient affiliation × sex × age in this model, we did not find significant interaction effect (F (1, 46) = 1.63, p = 0.208, ηp2 = 0.034).
Figure 1. Mean level of the amount of offer in each condition by sex.
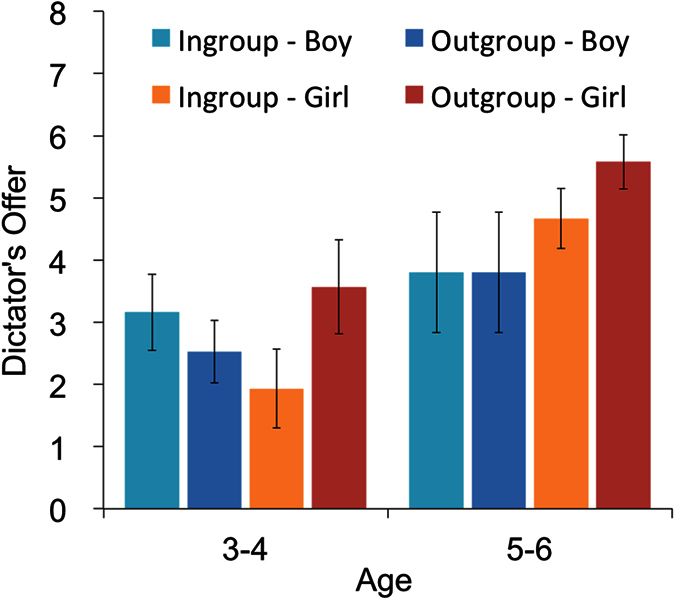
Error bars represent the standard error of the mean.
Hormonal Data
First, we examined whether sOT levels were related to sex or age. There was no significant difference in sOT levels between boys (M = 3.24 pg/ml, SD = 1.52, range = 0.94–6.31, n = 21) and girls (M = 3.20 pg/ml, SD = 1.65, range = 0.80–6.09, n = 23, t (42) = 0.08, p = 0.9340, d = 0.03), and sOT levels were not correlated with age in months in either boys or girls (boys: r = 0.048, p = 0.8379; girls: r = −0.075, p = 0.7342).
Next, we conducted an ANCOVA examining amounts allocated, with recipient affiliation (ingroup vs. outgroup) as a within-subject factor, participant sex as a between-subjects factor, and age in months and sOT levels as covariates. Results showed a significant interaction between sOT level × sex (F (1, 39) = 14.12, p = 0.0006, ηp2 = 0.266) in addition to the significant main effects of age (F (1, 37) = 10.21, p = 0.0028, ηp2 = 0.207), sex (F (1, 39) = 8.59, p = 0.0056, ηp2 = 0.180), and sOT levels (F (1, 39) = 4.61, p = 0.0381, ηp2 = 0.106). No significant effects were observed for recipient affiliation (F (1, 39) = 0.81, p = 0.3728, ηp2 = 0.020), recipient affiliation × sOT (F (1, 37) = 1.22, p = 0.2758, ηp2 = 0.030), recipient affiliation × sex (F (1, 39) = 3.91, p = 0.0550, ηp2 = 0.091) recipient affiliation × age (F (1, 39) = 0.18, p = 0.6778, ηp2 = 0.004), or recipient affiliation × sex × sOT (F (1, 39) = 1.26, p = 0.2679, ηp2 = 0.031) were observed.
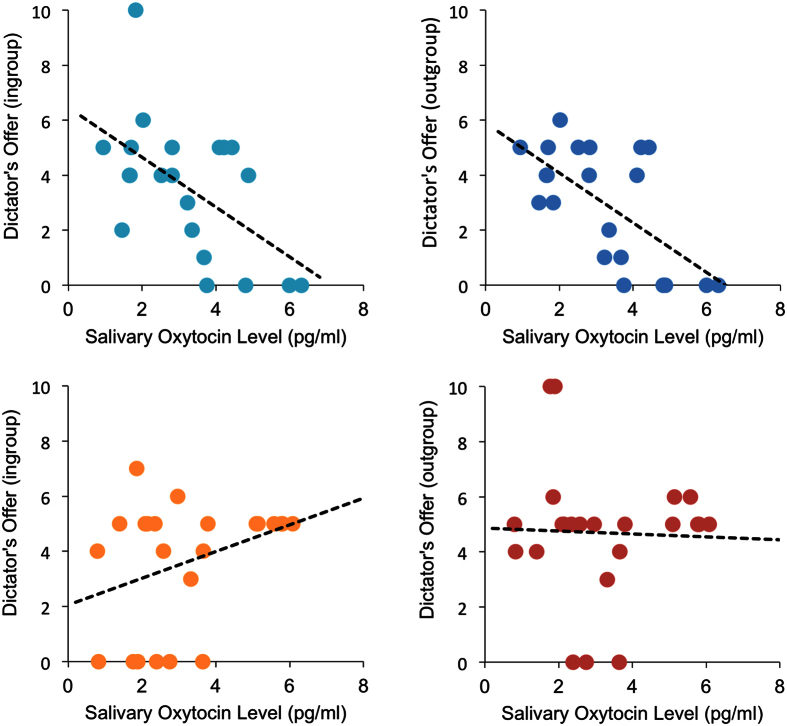
As we observed a significant sOT level × sex interaction, we examined the correlations between sOT levels and DG offers made by girls and boys. As the results indicated a main effect of age on allocations, we examined partial correlations controlling for the effect of age. Among boys, sOT levels correlated negatively with allocations made to ingroup (r (18) = −0.568, p = 0.0090) (Fig. 2a) and outgroup members (r (18) = −0.704, p = 0.0005) (Fig. 2b). For girls, sOT levels were positively correlated with allocations made to ingroup members (r (20) = 0.444, p = 0.0387) (Fig. 2c), but not to outgroup members (r (20) = −0.016, p = 0.9420) (Fig. 2d). Although the recipient affiliation × sOT × sex ANCOVA reported above did not find a significant three-way interaction, a sOT × sex ANCOVA on allocations made to ingroup members with age as a covariate found a significant interaction between sOT and sex (F (1, 39) = 13.70, p = 0.0007, ηp2 = 0.260), in addition to significant main effects of sex (F (1, 39) = 12.75, p = 0.0010, ηp2 = 0.246) and age (F (1, 39) = 7.92, p = 0.0076, ηp2 = 0.169).
Figure 2. Association between sOT levels and the amount of offer in each condition by sex.
In boys, sOT levels were negatively associated with the dictator’s offer in the ingroup (a) and outgroup conditions (b). In girls, while sOT levels were positively associated with the dictator’s offer in the ingroup (c), sOT was not associated with the dictator’s offer in the outgroup conditions (d).
Finally, we examined the association between sOT levels and the differences in the allocations made to ingroup and outgroup members, for both boys and girls. The results indicated no significant correlation in the ingroup-outgroup difference scores and sOT levels made by boys (r = 0.000, p = 0.9989) or girls (r = 0.298, p = 0.1675).
Discussion
In this study, we examined the association between sOT level and generosity toward ingroup and outgroup members in preschoolers in the context of a DG. While sOT levels observed in boys were negatively correlated with allocations made to both ingroup and outgroup members, sOT levels in girls significantly correlated with allocations made to the ingroup only. These results suggest that sOT may negatively influence generosity in boys, regardless of whether the target of generosity is an ingroup member or an outgroup member.
While a previous study showed a positive association between oxytocin levels and the extent to which children engaged in social interaction with their parents42, to our knowledge this is the first study to show an association between oxytocin level and generosity in children. Although several studies have demonstrated a relationship between oxytocin and ingroup favouritism in adults14,15,16,17,18, our study did not find such a pattern among preschoolers. Furthermore, this study suggests the existence of sex differences in the influence of oxytocin on generosity in preschoolers. This finding is notable given the number of findings in the literature suggesting that oxytocin may function to influence social behaviour in sex-specific ways35,36,37.
A number of possible neuro-psychological processes may underlie the negative relationship between sOT levels and generosity observed in boys. We suspect that high levels of sOT might be associated with a reduction in social risk taking (i.e., socially defensive tendencies) in boys. Prosocial behaviour, including behaviours such as interpersonal trust, often correspond to social risk taking49,50,51; this may be because prosocial behaviour may be beneficial for oneself, but only in the long run (prosocial/generous behaviour often does not pay off immediately, but is rather returned through reciprocity). In line with this claim, a recent study reported that oxytocin was related to risk aversion in males (but not in females) only under social conditions52. Another recent study of preschool children showed that children became more generous when they were monitored by others53, supporting the notion that increases in generosity observed across development in childhood may reflect strategic behavior rather than pure increases in prosociality54,55. That is, preschoolers may expect future reciprocity from those who are aware of their pro-social behavior, and behave in a generous manner when they are observed by others in order to elicit such reciprocal behavior. In this study, because children determined the amounts they wanted to allocate in the DG while they were being watched by the experimenter, children with low sOT levels may have been more willing in engage in risk seeking behavior, and therefore allocated more chocolate to others. Thus, it is possible that the association between sOT levels and amounts allocated by boys in the DG may be reduced in completely anonymous situations.
Another potential interpretation of these results is that oxytocin is associated with increased perception of social competition in boys. This interpretation is consistent with a previous study showing that oxytocin was related to the perception of competitiveness in men, but kinship in women56.
In this study, while a significant three-way interaction between SOT, target affiliation, and participant sex was not observed, the direction of the effects for boys and girls were opposite in direction: sOT levels were negatively correlated with allocations to both ingroup and outgroup members amoung boys, and positively correlated with allocations to ingroup members among girls. Future studies should examine whether sOT relates to underlying factors associated with ingroup favoritism that have been shown to vary based on gender, such as social dominance orientation57. Furthermore, as it is possible that the current study did not have enough power to reliably show such an effect among girls, future studies should include a larger sample size to investigate sex differences in the effect of oxytocin on generosity in preschool aged children.
Future studies should further examine these and other hypotheses to understand how oxytocin relates to generosity, risk taking, and competition in boys and girls. In addition, because the DG used in this study cannot differentiate between ingroup favoritism and outgroup derogation, future studies should use more diverse paradigms to further investigate the relation between salivary oxytocin and behavior toward ingroup and outgroup members in preschool aged boys and girls.
While the amounts allocated by participants overall in this study are on par with amounts typically offered in DG paradigms by adults participants in identifiable situations58, notably, this study did not replicate findings of previous studies reporting ingroup favouritism in children38,39,40, finding no evidence of ingroup favoritism among boys, and evidence of outgroup favoritism in girls. These inconsistent results might stem from the differing experimental situations used across these studies. Although the recipients were randomly chosen from group photos in our study, in a previous study the recipients in the ingroup condition were specific friends of the children40. Thus, children in the ingroup condition in this previous study40 may have been more strongly motivated to give their own resources to the recipients than were those in our study. Furthermore, paradigms used in previous studies did not incur a cost to participants to provide ingroup members with resources38,39; this study required children to give up their own resources for the recipients, which may have diminished the desire to provide ingroup members with rewards. Consistent with this hypothesis, Fehr et al.48 did not find ingroup favouritism in preschoolers using a sharing game similar to the paradigm used in our study, in which sharing was costly to participants. Furthermore, a recent neuroimaging study59 showed that the cortical thickness of the dorsolateral prefrontal cortex, an area that plays an important role in executive function, positively relates to both age as well as the inhibition of the desire for economic interests in elementary school-aged children. Thus, it is likely that when children must allocate their own resources in order to provide resources to a recipient, it may be more difficult to observe effects of generosity or ingroup favouritism.
In summary, this study found that sOT levels relate negatively to generosity shown toward both ingroup and outgroup members in boys. Further research is needed to examine sex differences in the association between oxytocin and social behaviour in children, as well as developmental changes in the relation between oxytocin and social behaviour. Future studies should examine the effect of other neuropeptides such as vasopressin, a neuropeptide structurally similar to oxytocin that is thought to moderate a number of social behaviours shown by mammalian males (for a review, see ref. 30). Furthermore, since the ability to measure sOT in saliva is a relatively recent development60, it is necessary to confirm whether the findings in our study can be replicated using other oxytocin indicators (e.g., urine sOT levels)34,61 and genetic variations in oxytocin receptor genes62.
Methods
Participants
Fifty Japanese preschoolers (26 girls and 24 boys) participated in the study. The mean age in months of participants was 56.9 (SD = 11.6, range: 38–81). The experimenter explained the outline of the research to all children and their guardians. Informed consent was obtained from guardians of all participants, and participants provided their assent to take part in the study. The ethical committee at Tamagawa University approved this study and the methods in this study were carried out in accordance with the approved guidelines.
Dictator Game
Children played the DG in a vacant room with a male experimenter present. The experimenter sat next to the child and explained the DG to him/her. After the description, the experimenter confirmed the child’s understanding of the game. Following this confirmation, the child decided how to allocate 10 coin chocolates between him/herself and another child (the recipient). The recipient’s affiliation was manipulated such that participants played either with an ingroup member (a child from the same class) or an outgroup member (an unknown child from a different class). A group photo was placed on the table and the children were told that the recipient would be randomly chosen from the members in the group photo after the experiment (Fig. 3). In the ingroup condition, a photo of the participant’s classmates was placed on the table. However, in the outgroup condition, a photo of preschoolers who attended a different kindergarten from the participants was placed on the table. Both pictures included both boys and girls. These two conditions were assigned as a within-subjects factor, and the order of these conditions was randomized and performed subsequently. The task was completed once children had decided how many chocolates to allocate to another child in both conditions.
Figure 3. A photo of the experiment environment.

Children sat at a desk and decided how to allocate ten chocolates. A male experimenter sat next to the child and explained the rules of the game to the children.
Collection and Assessment of Salivary Oxytocin
In order to assess salivary oxytocin levels, we collected saliva from participants with the Saliva Collection Aid (Salimetrics, Inc., State College, PA) using the passive drool method. Children were asked to produce at least 1 ml of saliva, which was collected in a cryogenic vial (2 ml). A total of six children were unable to produce more than 1 ml of saliva, resulting in a total of 44 saliva samples. Saliva was collected before the DG between 9:00 am and 11:00 am. Cryovials containing the saliva were immediately frozen in a storage box and stored at −80 °C until assay, which was conducted at a professional analysis agency (MACROPHI Inc., Japan, http://www.macrophi.co.jp/english/index.htm). The process of preparing each sample was as follows: First, the sample (1 ml) underwent column-based purification. Second, the column-purified liquid was frozen at −80 °C and freeze-dried. Third, the sample was lyophilized by adding an Assay Buffer (concentration ratio was four times). Finally, the sample was centrifuged for 5 min (800 × g) and the supernatant was subjected to ELISA assay. The assay was conducted using a commercially available Oxytocin ELISA kit (Enzo Life Sciences, Inc., Farmingdale, NY). Each sample was prepared in duplicate, and concentrations were calculated using the SpectraMax® (Molecular Device, Sunnyvale, LLC, Sunnyvale, CA) microplate reader according to relevant standard curves.
Additional Information
How to cite this article: Fujii, T. et al. Relationship between Salivary Oxytocin Levels and Generosity in Preschoolers. Sci. Rep. 6, 38662; doi: 10.1038/srep38662 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number JP15H05730. We thank Toshiaki Sakurai, Kimiko Sakamoto, and Asami Yoshimura for their support in conducting this study.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.T., T.F., K.N., H.O., and J.S. designed the research. T.F. and K.N. performed the research. H.T., T.F., and J.S. analyzed the data. H.T., T.F., J.S., and T.T. wrote the paper.
References
- Tajfel H., Billig M. G., Bundy R. P. & Flament C. Social categorization and intergroup behaviour. Eur. J. Soc. Psychol. 1, 149–178 (1971). [Google Scholar]
- Tajfel H. & Turner J. C. An integrative theory of intergroup conflict in The Social Psychology of Intergroup Relations (eds Austin W. G. & Worchel S.) 33–47 (Monterey, 1979). [Google Scholar]
- Brewer M. B. In-group bias in the minimal intergroup situation: A cognitive-motivational analysis. Psychol. Bull. 86, 307 (1979). [Google Scholar]
- Balliet D., Wu J. & De Dreu C. K. Ingroup favoritism in cooperation: A meta-analysis. Psychol. Bull. 140, 1556–1581 (2014). [DOI] [PubMed] [Google Scholar]
- Yamagishi T. & Mifune N. Does shared group membership promote altruism? Fear, greed, and reputation. Ration. Soc. 20, 5–30 (2008). [Google Scholar]
- Yamagishi T. & Mifune N. Social exchange and solidarity: In-group love or out-group hate? Evol. Hum. Behav. 30, 229–237 (2009). [Google Scholar]
- Yamagishi T., Mifune N., Liu J. H. & Pauling J. Exchanges of group-based favours: Ingroup bias in the prisoner’s dilemma game with minimal groups in Japan and New Zealand. Asian J. Soc. Psychol. 11, 196–207 (2008). [Google Scholar]
- Mifune N., Hashimoto H. & Yamagishi T. Altruism toward in-group members as a reputation mechanism. Evol. Hum. Behav. 31, 109–117 (2010). [Google Scholar]
- Yamagishi T. & Kiyonari T. The group as the container of generalized reciprocity. Soc. Psychol. Q. 63, 116–132 (2000). [Google Scholar]
- Alexander R. D. The Biology of Moral Systems (Transaction Publishers, 1987). [Google Scholar]
- Nowak M. A. & Sigmund K. The dynamics of indirect reciprocity. J. Theor. Biol. 194, 561–574 (1998). [DOI] [PubMed] [Google Scholar]
- Choi J. K. & Bowles S. The coevolution of parochial altruism and war. Science 318, 636–640 (2007). [DOI] [PubMed] [Google Scholar]
- Efferson C., Lalive R. & Fehr E. The Coevolution of Cultural Groups and Ingroup Favoritism. Science 321, 1844–1849 (2008). [DOI] [PubMed] [Google Scholar]
- De Dreu C. K. Oxytocin modulates cooperation within and competition between groups: an integrative review and research agenda. Horm. Behav. 61, 419–428 (2012). [DOI] [PubMed] [Google Scholar]
- De Dreu C. K. et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411 (2010). [DOI] [PubMed] [Google Scholar]
- De Dreu C. K., Greer L. L., Van Kleef G. A., Shalvi S. & Handgraaf M. J. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci. 108, 1262–1266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C. K., Shalvi S., Greer L. L., Van Kleef G. A. & Handgraaf M. J. Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PLoS One 7, e46751 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van IJzendoorn M. H. & Bakermans-Kranenburg M. J. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrino. 37, 438–443 (2012). [DOI] [PubMed] [Google Scholar]
- Neumann I. D. & Slattery D. A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiat. 79, 213–221 (2015). [DOI] [PubMed] [Google Scholar]
- Kosfeld M., Heinrichs M., Zak P. J., Fischbacher U. & Fehr E. Oxytocin increases trust in humans. Nature 435, 673–676 (2005). [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Heinrichs M., Vonlanthen A., Fischbacher U. & Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58, 639–650 (2008). [DOI] [PubMed] [Google Scholar]
- Domes G., Heinrichs M., Michel A., Berger C. & Herpertz S. C. Oxytocin improves “mind-reading” in humans. Biol. Psychiat. 61, 731–733 (2007). [DOI] [PubMed] [Google Scholar]
- Kirsch P. et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. The adaptive human parental brain: implications for children’s social development. Trends Neurosci. 38, 387–399 (2015). [DOI] [PubMed] [Google Scholar]
- Insel T. R. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768–779 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391 (2012). [DOI] [PubMed] [Google Scholar]
- De Dreu C. K. & Kret M. E. Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiat. 79, 165–173 (2015). [DOI] [PubMed] [Google Scholar]
- Ma X. et al. Oxytocin increases liking for a country’s people and national flag but not for other cultural symbols or consumer products. Front. Behav. Neurosci. 8, 266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S. et al. Oxytocin receptor gene and racial ingroup bias in empathy-related brain activity. NeuroImage 110, 22–31 (2015). [DOI] [PubMed] [Google Scholar]
- Bales K. L. Comparative and developmental perspectives on oxytocin and vasopressin in Mechanisms of social connection: From brain to group (eds Mikulincer M. & Shaver P. R.) 15–31 (American Psychological Association, 2014). [Google Scholar]
- Dumais K. M. & Veenema A. H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrin. 40, 1–23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F., Diorio J., Sharma S. & Meaney M. J. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. 98, 12736–12741 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C. A., Caldwell J. D., Walker C., Ayers G. & Mason G. A. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci. 108, 1163–1171 (1994). [DOI] [PubMed] [Google Scholar]
- Nagasawa M. et al. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science 348, 333–336 (2015). [DOI] [PubMed] [Google Scholar]
- Rilling J. K. et al. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrino. 37, 447–461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J. K. et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrino. 39, 237–248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D. et al. Opposing effects of oxytocin on moral judgment in males and females. Hum. Brain Mapp. 35, 6067–6076 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttelmann D. & Böhm R. The ontogeny of the motivation that underlies in-group bias. Psychol. Sci. 25, 921–927 (2014). [DOI] [PubMed] [Google Scholar]
- Schug M. G., Shusterman A., Barth H. & Patalano A. L. Minimal group membership influences children’s responses to novel experience with group members. Dev. Sci. 16, 47–55 (2013). [DOI] [PubMed] [Google Scholar]
- Moore C. Fairness in children’s resource allocation depends on the recipient. Psychol. Sci. 20, 944–948 (2009). [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I. & Zagoory Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: Considering stress and affiliation components of human bonding. Dev. Sci. 14, 752–761 (2011). [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T. & Ebstein R. P. Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacol. 38, 1154–1162 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T. X., Tanaka S., Saito D. N., Kosaka H. & Tomoda A. Visual attention for social information and salivary oxytocin levels in preschool children with autism spectrum disorders: an eye-tracking study. Front. Neurosci. 8, 295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi H. et al. The development of the effect of peer monitoring on generosity differs among elementary school-age boys and girls. Front. Psychol. 6, 895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagishi H., Kameshima S., Schug J., Koizumi M. & Yamagishi T. Theory of mind enhances preference for fairness. J. Exp. Child Psychol. 105, 130–137 (2010). [DOI] [PubMed] [Google Scholar]
- Takagishi H. et al. The role of cognitive and emotional perspective taking in economic decision making in the ultimatum game. PLoS One 9, e108462 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson J. F., Pascoe J. & Radmore N. Children’s altruistic behavior in the dictator game. Evol. Hum. Behav. 28, 168–175 (2007). [Google Scholar]
- Fehr E., Bernhard H. & Rockenbach B. Egalitarianism in young children. Nature 454, 1079–1083 (2008). [DOI] [PubMed] [Google Scholar]
- Bohnet I., Greig F., Herrmann B. & Zeckhauser R. Betrayal Aversion: Evidence from Brazil, China, Oman, Switzerland, Turkey, and the United States. Am. Econ. Rev. 98, 294–310 (2008). [Google Scholar]
- Bohnet I., Herrmann B. & Zeckhauser R. Trust and the Reference Points for Trustworthiness in Gulf and Western Countries. Q. J. Econ. 125, 811–828 (2010). [Google Scholar]
- Molm L. D., Takahashi N. & Peterson G. Risk and Trust in Social Exchange: An Experimental Test of a Classical Proposition. Am. J. Sociol. 105, 1396–1427 (2000). [Google Scholar]
- Patel N. et al. Oxytocin and vasopressin modulate risk-taking. Physiol. Behav. 139, 254–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Takagishi H., Koizumi M. & Okada H. The effect of direct and indirect monitoring on generosity among preschoolers. Sci. Rep. 5, 9025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli I., Massaro D., Sanfey A. G. & Marchetti A. “What is fair for you?” Judgments and decisions about fairness and Theory of Mind. Eur. J. Dev. Psychol. 11, 49–62 (2014). [Google Scholar]
- Schug J., Takagishi H., Benech C. & Okada H. The development of theory of mind and positive and negative reciprocity in preschool children. Front. Psychol. 7, 888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Shofty M., Levkovitz Y. & Shamay-Tsoory S. G. Oxytocin facilitates accurate perception of competition in men and kinship in women. Soc. Cogn. Affect. Neur. 8, 313–317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidanius J., Levin S., Liu J. & Pratto F. Social dominance orientation, anti-egalitarianism and the political psychology of gender: An extension and cross-cultural replication. Eur. J. Soc. Psychol. 30, 41–67. (2000). [Google Scholar]
- Engel C. Dictator games: A meta study. Exp. Econ. 14, 583–610 (2011). [Google Scholar]
- Steinbeis N., Bernhardt B. C. & Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron 73, 1040–1051 (2012). [DOI] [PubMed] [Google Scholar]
- Carter C. S. et al. Behavioral associations and potential as a salivary biomarker. Ann. N.Y. Acad. Sci. 1098, 312–322 (2007). [DOI] [PubMed] [Google Scholar]
- Seltzer L. J., Ziegler T. E. & Pollak S. D. Social vocalizations can release oxytocin in humans. Proc. Biol. Sci. 277, 2661–2666 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis O. A., McQuaid R. J., Matheson K. & Anisman H. The moderating role of an oxytocin receptor gene polymorphism in the relation between unsupportive social interactions and coping profiles: Implications for depression. Front. Psychol. 6, 1133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]