Abstract
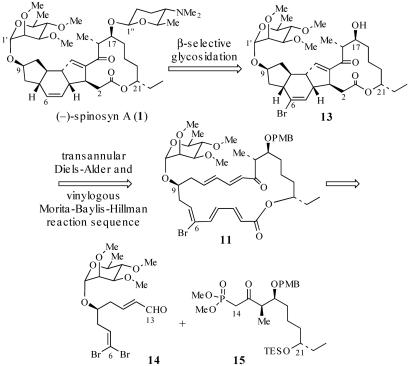
A convergent, highly stereoselective total synthesis of (–)-spinosyn A (1) is described. Key features of the synthesis include the transannular Diels–Alder reaction of macrocyclic pentaene 11 and the transannular Morita–Baylis–Hillman cyclization of 12 that generates tetracycle 26. The total synthesis of (–)-spinosyn A was completed by a sequence involving the highly β-selective glycosidation reaction of 13 and glycosyl imidate 30.
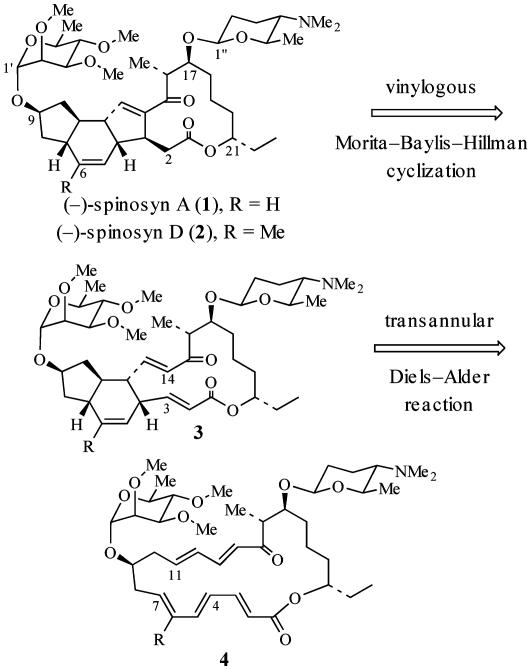
The spinosyns are a family of polyketide natural products that possess extraordinary insecticidal activity. The biosynthetic mixture, generated by Saccharopolyspora spinosa, comprises mostly spinosyn A (1) (Scheme 1) (≈85%) and spinosyn D (≈10–15%) (1–8). This mixture is currently marketed for use as an insecticide against a variety of insects (6). Total syntheses of spinosyn A have been reported by Evans and Black (9) and Paquette et al. (10, 11).
Scheme 1.
Biomimetic strategy for synthesis of 1.
Synthetic Strategy
Diels–Alder reactions have been proposed as key steps in the biogenesis of several natural products, including lovastatin, solanapyrone, nargenicin, and ikarugamycin (12). Kirst et al. (3) suggested that the biosynthesis of spinosyn A may involve a transannular Diels–Alder (TDA) (13) reaction of a macrocyclic pentaene to form the C(4)—C(12) and C(7)—C(11) bonds (see 4 → 3; Scheme 1). Kirst also suggested that a transannular cyclization of a 1,3-dicarbonyl nucleophile may generate the C(3)—C(14) bond (3). Alternatively, we speculated that the C(3)—C(14) bond may be formed by a vinylogous Morita–Baylis–Hillman (MBH) reaction mediated by an enzymatic nucleophile (compare 3 → 1; Scheme 1). Based on these biosynthetic considerations, we sought to assemble spinosyn A (1) via a TDA and MBH cyclization sequence of an appropriately functionalized macrocyclic pentaene 4.
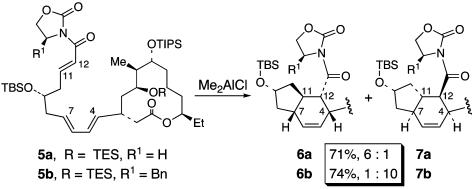
Diastereoselectivity of the Diels–Alder Reaction. Paramount to the success of this synthetic strategy is the control of the diastereoselectivity of the TDA reaction (4 → 3). It is known from the work of Evans and Black (9) that the intrinsic diastereofacial selectivity of the intramolecular Diels–Alder (IMDA) reaction of 5a favors the incorrect C(7)—C(11) trans-fused diastereomer 6a with 6:1 selectivity (Scheme 2). Although Evans and Black (9) addressed this issue by incorporating a chiral auxiliary (13) in the dienophile (see 5b → 7b), we would not have recourse to this strategy for the TDA cyclization of 4. Thus, some means for controlling the stereochemistry at the C(7)—C(11) ring fusion relative to the C(9)—alkoxy substituent in the Diels–Alder reaction was required.
Scheme 2.
Results of IMDA reactions of trienes 5a and 5b (9).
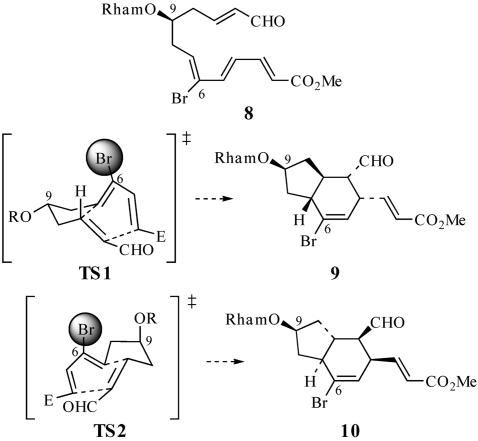
We initially hoped to use the steric directing-group strategy to control the diastereoselectivity of the Diels–Alder reaction (15–17), which could involve appending a bromine steric directing group at C(6) of the IMDA or TDA substrate to control the stereochemical outcome of the cycloaddition, leading to the desired C(7)—C(11) trans-fused diastereomer. It was anticipated that TS2, which leads to the undesired C(7)—C(11) trans-fused isomer 10 and is favored in the absence of the C(6)—Br steric directing group, may be destabilized relative to TS1 by a steric interaction between C(6)—Br and the C(9)—alkoxy substituent (Scheme 3). However, the IMDA reaction of 8 under Lewis acid catalysis provides only an ≈2:1 mixture of C(7)—C(11) trans-fused diastereomers 9 and 10 (18).‡ Thus, although the presence of the C(6)—Br directing group in 8 counteracts the intrinsic diastereofacial bias imparted by the C(9) alkoxy substituent in 5a, it was apparent that some additional means of increasing the diastereoselectivity of the TDA reaction of 4 would be required.
Scheme 3.
Transition states for the IMDA reaction of 8.
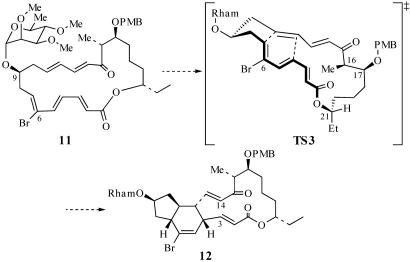
It is well established that conformational preferences of macrocyclic systems can be used to control the stereochemical outcome of synthetic transformations (19–26). In this connection, we anticipated that the C(21) stereocenter, adjacent to the macrolactone carbonyl, might be a stereochemical control element capable of enhancing the diastereoselectivity of the TDA reaction (11 → 12; Scheme 4). In the preferred conformation of the macrolactone linkage, C(21)—H should eclipse the carbonyl group (27). Analysis of the transition states of the TDA reaction of 11 indicates that the macrolactone linkage would possess this favorable conformation in TS3, which leads to the desired cycloadduct 12 (Scheme 4).
Scheme 4.
Transition-state analysis of the TDA reaction of 11.
Based on this analysis, we targeted macrocyclic pentaene 11 as a substrate for the assembly of spinosyn A (Scheme 5). It was envisaged that 11 would be accessible via Horner–Wadsworth–Emmons coupling of aldehyde 14 and β-ketophosphonate 15. A TDA and transannular MBH reaction sequence from 11 should lead to 13. Installation of the forosamine unit then should afford the natural product. We report herein the successful application of this strategy to the total synthesis of (–)-spinosyn A (1).
Scheme 5.
Retrosynthetic strategy for synthesis of 1.
Chemistry
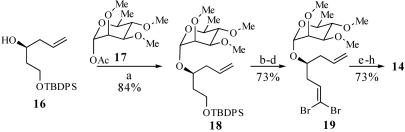
Synthesis of Aldehyde 14. The synthesis of 14 began with readily available alcohol 16 (28) (Scheme 6). Glycosidation of 16 with α-l-rhamnopyranosyl (Rham) acetate 17 (29) in the presence of tert-butyldimethylsilyl (TBS) trifluoromethanesulfonate afforded the α-glycoside 18 in 84% yield. Removal of the tert-butyldiphenylsilyl group with tetrabutylammonium fluoride proceeded in 84% yield, and oxidation of the resulting alcohol under Dess–Martin conditions afforded the corresponding aldehyde in 92% yield (30, 31). Treatment of this aldehyde with Ph3P and CBr4 then afforded 1,1-dibromoolefin 19 in 94% yield (32). Selective ozonolysis of the primary olefin (33) and Wittig olefination of the resulting aldehyde with Ph3P CHCO2Me generated the unsaturated ester with the required (E)-configuration in 82% yield and with ≥95:5 selectivity. Reduction of the ester with diisobutylaluminum hydride and oxidation of the allylic alcohol with SO3·pyridine then provided aldehyde 14 (34).
CHCO2Me generated the unsaturated ester with the required (E)-configuration in 82% yield and with ≥95:5 selectivity. Reduction of the ester with diisobutylaluminum hydride and oxidation of the allylic alcohol with SO3·pyridine then provided aldehyde 14 (34).
Scheme 6.
Synthesis of aldehyde 14. a, TBS trifluoromethanesulfonate, 4 Å MS, CH2Cl2, 23°C, 15 min, 84%; b, tetrabutylammonium fluoride, THF, 0°C → 23°C, 1.5 h, 84%; c, Dess–Martin periodinane, pyridine, wet CH2Cl2, 0°C → 23°C, 2.5 h, 92%; d, CBr4, Ph3P, CH2Cl2, 0°C → 23°C, 30 min, 94%; e, O3, 4:1 CH2Cl2/MeOH, KHCO3, -78°C → 23°C, 3 h; f, Ph3P CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.
CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.
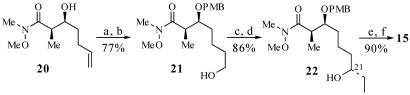
Synthesis of β-Ketophosphonate 15. We elected to use readily available alcohol 20 (9) as the starting material for the synthesis of β-ketophosphonate 15 (Scheme 7). Treatment of 20 with potassium hexamethyldisilazane and p-methoxybenzyl bromide (PMB—Br) afforded the PMB ether in 91% yield. Hydroboration of the olefin with disiamylborane then provided primary alcohol 21 in 85% yield. Oxidation of 21 with SO3·pyridine gave the corresponding aldehyde in 91% yield (34). Treatment of this aldehyde with diethylzinc in the presence of (–)-N,N-dibutylnorephedrine then gave the C(21) alcohol 22 as a 9:1 mixture of diastereomers in 94% yield (35–37). Protection of alcohol 22 as the triethylsilyl ether proceeded in 94% yield. Exposure of this triethylsilyl ether to dimethyl lithiomethylphosphonate then afforded β-ketophosphonate 15 (25, 38).
Scheme 7.
Synthesis of β-ketophosphonate 15. a, PMB—Br, Et3N, potassium hexamethyldisilazane, THF, -78°C → 23°C, 13 h, 91%; b, BH3·SMe2, 2-methyl-2-butene, THF, 0°C, 5 h, then H2O2, NaHCO3, 85%; c, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 30 min, 91%; d, Et2Zn (1 M in hexanes), (–)-N,N-dibutylnorephedrine, toluene, 0°C → 23°C, 76 h, 94%, ds = 90:10; e, triethylsilyltrifluoromethanesulfonate, 2,6-lutidine, CH2Cl2, 0°C, 1 h, 94%; f, (MeO)2P(O)CH3, BuLi, THF, -78°C, 30 min, 96%.
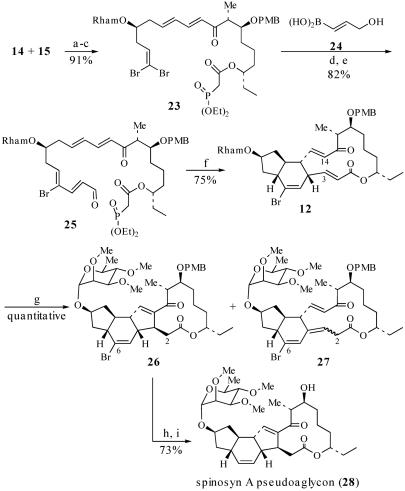
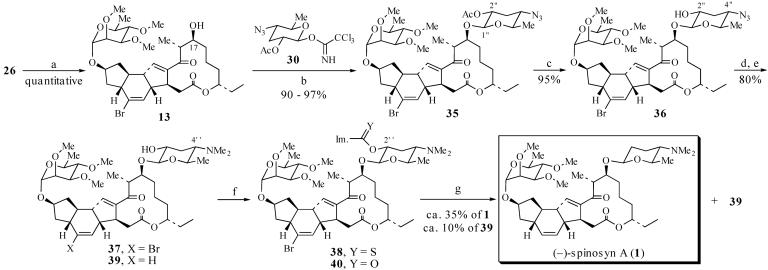
Synthesis of Pseudoaglycon 28. Aldehyde 14 and β-ketophosphonate 15 were coupled by treatment of 15 with activated Ba(OH)2 followed by the addition of 14 (39) (Scheme 8), which afforded the corresponding triene in 93% yield over two steps. Removal of the C(21)—triethylsilyl group and acylation of the resulting alcohol with diethyl phosphonoacetic acid then generated phosphonate 23 in 98% yield. Suzuki coupling of 23 with vinyl boronic acid 24 (40) provided the allylic alcohol in 82% yield. Oxidation of this alcohol with SO3·pyridine provided the aldehyde 25 (34). We anticipated that macrocyclization of 25 would give macrocycle 11 (41–43); however, treatment of 25 with i-Pr2NEt and LiCl in CH3CN (44) directly afforded Diels–Alder cycloadduct 12 in 75% yield (over two steps) as a 73:12:9:6 mixture of two trans- and two cis-fused diastereomers. Cycloadduct 12 presumably arises from a tandem macrocyclization and TDA reaction sequence (25 → 11 → 12). Based on the observation that the TDA reaction of macrocycle 11 (11 → 12) is more diastereoselective than the corresponding IMDA reaction of triene 8 (8 → 9), it seems that conformational preferences of 11 play a role in determining the stereoselectivity of the TDA reaction, as predicted in TS3 (Scheme 4). To establish the stereochemistry of the major cycloadduct 12, this intermediate was converted to the spinosyn A pseudoaglycon 28. Thus, treatment of 12 with Me3P effected the vinylogous MBH cyclization (18, 45, 46) and provided an 88:7:5 mixture of the desired product 26, the olefin migration product 27, and the C(3) epimer of 26 (structure not shown) in quantitative yield. After HPLC purification of 26, reductive removal of the C(6)—Br directing group was accomplished by treatment of 26 with (tristrimethylsilyl)silane and azobisisobutyronitrile (AIBN) (47, 48). Finally, removal of the PMB group with 2,3-dichloro-4,5-dicyano-1,4-benzoquinone (DDQ) afforded the spinosyn A pseudoaglycon 28, which was identical in all respects to natural 28. Because the published syntheses of (–)-spinosyn A (1) by Paquette et al. (10, 11) and (+)-spinosyn A by Evans and Black (9) proceed by way of the pseudoaglycon 28 (or ent-28 in the case of Evans and Black), this synthesis of 28 constitutes a formal synthesis of (–)-spinosyn A (1).
Scheme 8.
Synthesis of pseudoaglycon 28. a, Ba(OH)2, 40:1 THF/H2O, 23°C, 8 h, 93% over two steps; b, 8:8:1 THF/HOAc/H2O, 23°C, 4 h, quantitative; c, (EtO)2P(O)CH2CO2H, N-ethyl, N′-(3-dimethylaminopropyl)-carbodiimide·MeI, DMAP, CH2Cl2, 23°C, 1.5 h, 98%; d, 24, Pd(PPh3)4, Tl2CO3, 3:1 THF/H2O, 2 h, 82%; e, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 30 min; f, i-Pr2NEt, LiCl, CH3CN (1 mM), 23°C, 19 h, 75%, (E)/(Z) = ≥95:5, ds = 73:12:9:6; g, Me3P (8 eq), tert-amyl alcohol (0.005 M), 23°C, 6 h, quantitative; h, (trimethylsilyl)3SiH, AIBN, dioxane, 80°C, 1.5 h; i, DDQ, CH2Cl2/pH 7 buffer, 0°C, 3 h, 73%.
Synthesis of Glycosyl Donor 30. The remaining challenge in completing a total synthesis of (–)-spinosyn A was the installation of the forosamine unit at C(17)—OH. This glycosidation proved to be a significant problem in the syntheses of spinosyn A by both Evans and Black (9) and Paquette et al. (11). Evans and Black obtained a 70% yield for the installation of the forosamine unit in their synthesis; however, the selectivity for the formation of the required β-glycoside was 1:6 β/α. In the synthesis of spinosyn A by Paquette et al., installation of the forosamine unit proceeded in 17% yield and with 2:3 β/α selectivity. Thus, both the efficiency and selectivity of this glycosidation step needed to be addressed.
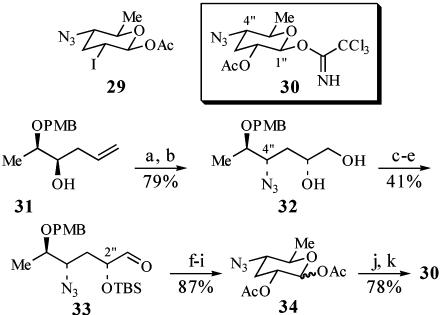
Highly selective methods for the synthesis of 2-deoxy-β-glycosides by using 2-iodo-glycosyl acetate, trichloroacetimidate, and fluoride donors were recently reported from this laboratory (49–53). These glycosidation reactions afford the β-glycosides in high yield and with excellent β-selectivity. We considered applying this technology to the installation of the forosamine unit in the present work; however, we recognized that stereoselective preparation of the requisite 2-iodo-glycosyl acetate donor 29 would be difficult. Therefore, we elected to target glycosyl imidate (54, 55) 30, which contains a C(2″)—OAc directing group for stereocontrol of the glycosidation step (56–60) and a C(4″)—N3 for protection of the amino functionality (61) (Scheme 9).
Scheme 9.
Synthesis of glycosyl imidate donor 30. a, (PhO)2P(O)N3, Ph3P, diethyl azodicarboxylate, THF, 0°C, 2 h, 82%; b, K2OsO4(OH)2, (dihydroquinidine)2-pyr, K3Fe(CN)6, K2CO3, 0°C, 7.5 h, 96%, ds = 86:14; c, TBSCl, imidazole, dimethylformamide, 4 h; d, HOAc/THF/H2O (3:3:1), 23°C, 55 h, 62%; e, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 15 min, 66%; f, DDQ, CH2Cl2/pH 7 buffer, 0°C, 4 h; g, Ac2O, Et3N, DMAP, CH2Cl2; h, tetrabutylammonium fluoride, THF, 0°C, 2 h; i, Ac2O, Et3N, DMAP, CH2Cl2, 87% from 33, 1.3:1 β/α; j, ethylenediamine, HOAc, THF, 23°C, 24 h, 78%; k, 1,8-diazabicyclo[5.4.0]undec-7-ene, CH2Cl2/Cl3CCN (1:1), 0°C, 1.5 h, 5:1 β/α.
The synthesis of 30 began with homoallylic alcohol 31 (62). A Mitsunobu reaction of 31 with diphenylphosphoryl azide was used to install the C(4″)—N3 (63, 64). Sharpless asymmetric dihydroxylation of the terminal olefin then afforded diol 32 in 96% yield as an 86:14 mixture of inseparable diastereomers (65). Silylation of the diol mixture with TBSCl followed by treatment of the resulting bis-silyl ether with aqueous acetic acid (1:3) in tetrahydrofuran afforded the corresponding primary alcohol in 62% yield over two steps. Oxidation of this alcohol with SO3·pyridine (34) then gave the aldehyde 33 in 66% yield after HPLC purification. Purified 33 had diastereomeric purity of 93:7. Deprotection of the PMB ether with DDQ afforded the lactol, which then was acylated with acetic anhydride. Removal of the C(2″) TBS ether and acylation of the resulting alcohol with Ac2O provided glycosyl acetate 34 in 87% yield from 33. Removal of the anomeric acetate with ethylenediamine and HOAc in THF gave the corresponding lactol in 78% yield (66). Finally, treatment of the lactol with 1,8-diazabicyclo[5.4.0]undec-7-ene in Cl3CCN/CH2Cl2 (1:1) afforded trichloroacetimidate donor 30 with >95:5 diastereomeric purity at C(2″) (55).
Because of its instability, 30 was used immediately in the glycosidation reaction with acceptor 13, itself prepared from 26 in quantitative yield via removal of the PMB group with DDQ (Scheme 10). Treatment of 13 with 2.1 eq of 30 in the presence of trimethylsilytrifluoromethanesulfonate afforded the β-glycoside 35 in 90–97% yield. The stereochemistry of 35 was assigned based on the observed J1″-2″ coupling constant of 7.8 Hz.
Scheme 10.
Completion of the total synthesis of (–)-spinosyn A (1). a, DDQ, CH2Cl2/pH 7 buffer, 0°C, 4 h, quantitative; b, 30 (2.1 eq), trimethylsilytrifluoromethanesulfonate (30 mol %), CH2Cl2, -78°C, 1h, 90–97%; c, guanidinium nitrate, NaOMe, MeOH, CH2Cl2, 2 h, 95%; d, SnCl2, PhSH, Et3N, THF, 15 min, 92%; e, NaBH3CN, CH2O, MeOH, HOAc, NaOAc, 87%; f, thiocarbonyldiimidazole, DMAP, Ph—CH3, 65°C, 2 h; g, Bu3SnH (25 eq), AIBN, dioxane, 100°C, 20 min, ≈35% of 1, ≈10% of 39 after HPLC.
Completion of the Total Synthesis of 1. Deoxygenation at C(2″) and conversion of the C(4″)—N3 to the C(4″)—NMe2 of glycoside 35 were now required to complete a synthesis of 1. The C(2″)—OAc was removed by treatment of 35 with guanidinium nitrate (67), which afforded alcohol 36 in 95% yield (Scheme 10). Deoxygenation at C(2″) was attempted first. Conversion of 36 to the corresponding thioimidazolide was accomplished by treatment with thiocarbonyldiimidazole (68); however, exposure of the thioimidazolide to (trimethylsilyl)3SiH (47, 48) and AIBN in dioxane at 80°C afforded only products in which the azide had been reduced to the primary amine.
Consequently, reduction of the azide before radical deoxygenation was explored next. Exposure of the azide to Me3P or Ph3P in THF followed by the addition of water did not afford the primary amine; only decomposition of 36 was observed (69–71). Fortunately, reduction of the azide could be accomplished with SnCl2 in the presence of PhSH and Et3N (72, 73). This reduction protocol gave the amine in 92% yield. Reductive amination with formaldehyde then afforded dimethylamine 37 in 87% yield (9).
Conversion of alcohol 37 to thioimidazolide 38 proceeded smoothly in the presence of thiocarbonyldiimidazole and dimethylaminopyridine (DMAP); however, reduction of 38 with (trimethylsilyl)3SiH and AIBN was unsuccessful. The major products observed under these conditions were alcohol 37, desbromo alcohol 39, and imidazolide 40 resulting from an unexpected thiocarbonyl-to-carbonyl exchange. Attempted deoxygenation of the thionochloroformate and xanthate derivatives of 37 gave similar results (68). Fortunately, treatment of 38 with the more reactive hydrogen atom donor Bu3SnH (25 eq) and AIBN in dioxane afforded ≈3:1 mixture of (–)-spinosyn A (1) and alcohol 39. The isolated yield of synthetic (–)-spinosyn A (1) was ≈35% (from 37) after reverse-phase HPLC purification. The yield of recovered alcohol 39 was ≈10%. Synthetic (–)-spinosyn A was identified by comparison to a sample of natural (–)-1 by 1H NMR, IR, high-resolution MS, optical rotation, and TLC mobility.
Conclusions
This synthesis of (–)-spinosyn A features a tandem macrocyclization and TDA reaction in addition to a complex application of the vinylogous MBH reaction for construction of the spinosyn A pseudoaglycon 28. Installation of the forosamine sugar was accomplished by means of a highly β-selective glycosidation of C(6)—bromo pseudoaglycon 13 with glycosyl imidate 30. Conditions then were developed for removal of the C(6) and C(2″) directing groups and the installation of the C(4″) tertiary amine functionality from 35. This synthesis of (–)-spinosyn A involved 23 steps in the longest linear sequence (31 steps total) and proceeded in 3% overall yield. Full experimental details are provided in the supporting information, which is published on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Professor Anna K. Mapp and Aaron R. Minter for assistance in purifying synthetic (–)-spinosyn A; Professor Leo A. Paquette for providing a sample of natural spinosyn A pseudoaglycon; and Dr. Herbert A. Kirst for providing a sample of both the natural pseudoaglycon and natural spinosyn A. This work was supported by National Institutes of Health Grant GM26782.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TDA, transannular Diels–Alder; MBH, Morita–Baylis–Hillman; IMDA, intramolecular Diels–Alder; TBS, tert-butyldimethylsilyl; PMB, p-methoxybenzyl; AIBN, azobisisobutyronitrile; DDQ, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; THF, tetrahydrofuran; DMAP, dimethylaminopyridine; Rham, rhamnopyranosyl.
Footnotes
Diastereomers 9 and 10 were inseparable by HPLC. Consequently, the stereochemistry of 9 and 10 was not rigorously established.
References
- 1.Kirst, H. A., Michel, K. H., Martin, J. W., Creemer, L. C., Chio, E. H., Yao, R. C., Nakatsukasa, W. M., Boeck, L., Occolowitz, J. L., Paschal, J. W., et al. (1991) Tetrahedron Lett. 32, 4839-4842. [Google Scholar]
- 2.Kirst, H. A., Michel, K. H., Chio, E. H., Yao, R. C., Nakatsukasa, W. M., Boeck, L., Occolowitz, J. L., Paschal, J. W., Deeter, J. B. & Thompson, G. D. (1991) in Microbial Metabolites, ed. Nash, C. (William C. Brown, Dubuque, IA), Vol. 32, pp. 109. [Google Scholar]
- 3.Kirst, H. A., Michel, K. H., Mynderase, J. S., Chio, E. H., Yao, R. C., Nakasukasa, W. M., Boeck, L. D., Occlowitz, J. L., Paschal, J. W., Deeter, J. B., et al. (1992) in Synthesis and Chemistry of Agrochemicals III, eds. Baker, D. R., Fenyes, J. G. & Steffens, J. J. (Am. Chem. Soc., Washington, DC), Vol. ACS Symposium Series 504, pp. 214-225.
- 4.Martynow, J. G. & Kirst, H. A. (1994) J. Org. Chem. 59, 1548-1560. [Google Scholar]
- 5.Creemer, L. C., Kirst, H. A. & Paschal, J. W. (1998) J. Antibiot. (Tokyo) 51, 795-800. [DOI] [PubMed] [Google Scholar]
- 6.Dagani, R. (1999) Chem. Eng. News 77, 30-32. [Google Scholar]
- 7.Crouse, G. D., Sparks, T. C., Schoonover, J., Gifford, J., Dripps, J., Bruce, T., Larson, L. L., Garlich, J., Hatton, C., Hill, R. L., et al. (2001) Pest Manag. Sci. 57, 177-185. [DOI] [PubMed] [Google Scholar]
- 8.Sparks, T. C., Crouse, G. D. & Durst, G. (2001) Pest Manag. Sci. 57, 896-905. [DOI] [PubMed] [Google Scholar]
- 9.Evans, D. A. & Black, W. C. (1993) J. Am. Chem. Soc. 115, 4497-4513. [Google Scholar]
- 10.Paquette, L. A., Gao, Z. L., Ni, Z. J. & Smith, G. F. (1998) J. Am. Chem. Soc. 120, 2543-2552. [Google Scholar]
- 11.Paquette, L. A., Collado, I. & Purdie, M. (1998) J. Am. Chem. Soc. 120, 2553-2562. [Google Scholar]
- 12.Stocking, E. M. & Williams, R. M. (2003) Angew. Chem. Int. Ed. Engl. 42, 3078-3115. [DOI] [PubMed] [Google Scholar]
- 13.Marsault, E., Toró, A., Nowak, P. & Deslongchamps, P. (2001) Tetrahedron 57, 4243-4260. [Google Scholar]
- 14.Evans, D. A., Chapman, K. T. & Bisaha, J. (1988) J. Am. Chem. Soc. 110, 1238-1256. [Google Scholar]
- 15.Boeckman, R. K., Jr., & Barta, T. E. (1985) J. Org. Chem. 50, 3421-3423. [Google Scholar]
- 16.Roush, W. R. & Kageyama, M. (1985) Tetrahedron Lett. 26, 4327-4330. [Google Scholar]
- 17.Roush, W. R., Kageyama, M., Riva, R., Brown, B. B., Warmus, J. S. & Moriarty, K. J. (1991) J. Org. Chem. 56, 1192-1210. [Google Scholar]
- 18.Mergott, D. J., Frank, S. A. & Roush, W. R. (2002) Org. Lett. 4, 3157-3160. [DOI] [PubMed] [Google Scholar]
- 19.Still, W. C. & Galynker, I. (1981) Tetrahedron 37, 3981-3996. [Google Scholar]
- 20.Vedejs, E. & Gapinski, D. M. (1983) J. Am. Chem. Soc. 105, 5058-5061. [Google Scholar]
- 21.Still, W. C. & Novack, V. J. (1984) J. Am. Chem. Soc. 106, 1148-1149. [Google Scholar]
- 22.Schreiber, S. L., Sammakia, T., Hulin, B. & Schulte, G. (1986) J. Am. Chem. Soc. 108, 2106-2108. [Google Scholar]
- 23.Porco, J. A., Jr., Schoenen, F. J., Stout, T. J., Clardy, J. & Schreiber, S. L. (1990) J. Am. Chem. Soc. 112, 7410-7411. [Google Scholar]
- 24.Roush, W. R., Koyama, K., Curtin, M. L. & Moriarty, K. J. (1996) J. Am. Chem. Soc. 118, 7502-7512. [Google Scholar]
- 25.Vanderwal, C. D., Vosburg, D. A., Weiler, S. & Sorensen, E. J. (2003) J. Am. Chem. Soc. 125, 5393-5407. [DOI] [PubMed] [Google Scholar]
- 26.Evans, D. A. & Starr, J. T. (2003) J. Am. Chem. Soc. 125, 13531-13540. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann, R. W. (1989) Chem. Rev. (Washington, D.C.) 89, 1841-1860. [Google Scholar]
- 28.Smith, A. B., III., Safonov, I. G. & Corbett, R. M. (2002) J. Am. Chem. Soc. 124, 11102-11113. [DOI] [PubMed] [Google Scholar]
- 29.Bols, M., Binderup, L., Hansen, J. & Rasmussen, P. (1992) J. Med. Chem. 35, 2768-2771. [DOI] [PubMed] [Google Scholar]
- 30.Dess, D. B. & Martin, J. C. (1983) J. Org. Chem. 48, 4155-4156. [Google Scholar]
- 31.Meyer, S. D. & Schreiber, S. L. (1994) J. Org. Chem. 59, 7549-7552. [Google Scholar]
- 32.Corey, E. J. & Fuchs, P. L. (1972) Tetrahedron Lett. 13, 3769-3772. [Google Scholar]
- 33.Pryor, W. A., Giamalva, D. & Church, D. F. (1983) J. Am. Chem. Soc. 105, 6858-6861. [Google Scholar]
- 34.Parikh, J. R. & Doering, W. v. E. (1967) J. Am. Chem. Soc. 89, 5505-5507. [Google Scholar]
- 35.Soai, K., Yokoyama, S., Ebihara, K. & Hayasaka, T. (1987) J. Chem. Soc. Chem. Commun., 1690-1691.
- 36.Soai, K., Yokoyama, S. & Hayasaka, T. (1991) J. Org. Chem. 56, 4264-4268. [Google Scholar]
- 37.Pu, L. & Yu, H.-B. (2001) Chem. Rev. (Washington, D.C.) 101, 757-824. [DOI] [PubMed] [Google Scholar]
- 38.Theisen, P. D. & Heathcock, C. H. (1988) J. Org. Chem. 53, 2374-2378. [Google Scholar]
- 39.Paterson, I., Yeung, K.-S. & Smaill, J. B. (1993) Synlett, 774-776.
- 40.Roush, W. R., Champoux, J. A. & Peterson, B. C. (1996) Tetrahedron Lett. 37, 8989-8992. [Google Scholar]
- 41.Burri, K. F., Cardone, R. A., Chen, W. Y. & Rosen, P. (1978) J. Am. Chem. Soc. 100, 7069-7071. [Google Scholar]
- 42.Stork, G. & Nakamura, E. (1979) J. Org. Chem. 44, 4010-4011. [Google Scholar]
- 43.Nicolaou, K. C., Seitz, S. P., Pavia, M. R. & Petasis, N. A. (1979) J. Org. Chem. 44, 4011-4013. [Google Scholar]
- 44.Blanchette, M. A., Choy, W., Davis, J. T., Essenfeld, A. P., Masamune, S., Roush, W. R. & Sakai, T. (1984) Tetrahedron Lett. 25, 2183-2186. [Google Scholar]
- 45.Wang, L. C., Luis, A. L., Agaplou, K., Jang, H. Y. & Krische, M. J. (2002) J. Am. Chem. Soc. 124, 2402-2403. [DOI] [PubMed] [Google Scholar]
- 46.Frank, S. A., Mergott, D. J. & Roush, W. R. (2002) J. Am. Chem. Soc. 124, 2404-2405. [DOI] [PubMed] [Google Scholar]
- 47.Ballestri, M., Chatgilialoglu, C., Clark, K. B., Griller, D., Giese, B. & Kopping, B. (1991) J. Org. Chem. 56, 678-683. [Google Scholar]
- 48.Chatgilialoglu, C. (1992) Acc. Chem. Res. 25, 188-194. [Google Scholar]
- 49.Roush, W. R. & Bennett, C. E. (1999) J. Am. Chem. Soc. 121, 3541-3542. [Google Scholar]
- 50.Roush, W. R., Gung, B. W. & Bennett, C. E. (1999) Org. Lett. 1, 891-893. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard, N. & Roush, W. R. (2003) Org. Lett. 5, 81-84. [DOI] [PubMed] [Google Scholar]
- 52.Durham, T. B. & Roush, W. R. (2003) Org. Lett. 5, 1871-1874. [DOI] [PubMed] [Google Scholar]
- 53.Chong, P. Y. & Roush, W. R. (2002) Org. Lett. 4, 4523-4526. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt, R. R. & Jung, K.-H. (2000) in Carbohydrates in Chemistry and Biology, eds. Ernst, B., Hart, G. W. & Sinaÿ, P. (Wiley–VCH, Weinheim, Germany), Vol. 1, pp. 5-59. [Google Scholar]
- 55.Schmidt, R. R. (1994) Adv. Carbohydr. Chem. Biochem. 50, 21-123. [DOI] [PubMed] [Google Scholar]
- 56.Trumtel, M., Tavecchia, P., Veyrières, A. & Sinaÿ, P. (1989) Carbohydr. Res. 191, 29-52. [DOI] [PubMed] [Google Scholar]
- 57.Veyrières, A. (2000) in Carbohydrates in Chemistry and Biology, eds. Ernst, B., Hart, G. W. & Sinaÿ, P. (Wiley–VCH, Weinheim, Germany), Vol. 1, pp. 368-405. [Google Scholar]
- 58.Marzabadi, C. H. & Franck, R. W. (2000) Tetrahedron 56, 8385-8417. [Google Scholar]
- 59.Toshima, K. & Tatsuta, K. (1993) Chem. Rev. (Washington, D.C.) 93, 1503-1531. [Google Scholar]
- 60.Thiem, J. & Klaffke, W. (1990) Top. Curr. Chem. 154, 285-332. [Google Scholar]
- 61.Scriven, E. F. & Turnbull, K. (1988) Chem. Rev. (Washington, D.C.) 88, 297-368. [Google Scholar]
- 62.Roush, W. R., Bennett, C. E. & Roberts, S. E. (2001) J. Org. Chem. 66, 6389-6393. [DOI] [PubMed] [Google Scholar]
- 63.Lal, B., Pramanik, B. N., Manhas, M. S. & Bose, A. K. (1977) Tetrahedron Lett. 18, 1977-1980. [Google Scholar]
- 64.Mitsunobu, O. (1981) Synthesis, 1-28.
- 65.Kolb, H. C., VanNieuwenhze, M. S. & Sharpless, K. B. (1994) Chem. Rev. (Washington, D.C.) 94, 2483-2547. [Google Scholar]
- 66.Zhang, J. & Kováč, P. (1999) J. Carbohydr. Chem. 18, 461-469. [Google Scholar]
- 67.Ellervik, U. & Magnusson, G. (1997) Tetrahedron Lett. 38, 1627-1628. [Google Scholar]
- 68.Hartwig, W. (1983) Tetrahedron 39, 2609-2645. [Google Scholar]
- 69.Staudinger, H. & Meyer, J. (1919) Helv. Chim. Acta 2, 635-646. [Google Scholar]
- 70.Gololobov, Y. G. & Kasukhin, L. F. (1992) Tetrahedron 48, 1353-1406. [Google Scholar]
- 71.Knapp, S., Jaramillo, C. & Freeman, B. (1994) J. Org. Chem. 59, 4800-4804. [Google Scholar]
- 72.Bartra, M., Urpí, F. & Vilarrasa, J. (1987) Tetrahedron Lett. 28, 5941-5944. [Google Scholar]
- 73.Bartra, M., Romea, P., Urpí, F. & Vilarrasa, J. (1990) Tetrahedron 46, 587-594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.