Abstract
Goals
To evaluate differences in metrics of quality and site performance in academic and community sites participating in a multicenter study.
Background
In the Individualized Dosing Efficacy Versus Flat Dosing to Assess Optimal Pegylated Interferon Therapy study, the participation of 76 academic-based and 42 community-based US centers provided an opportunity to evaluate various metrics of quality and site performance.
Study
A secondary data analysis of the Individualized Dosing Efficacy Versus Flat Dosing to Assess Optimal Pegylated Interferon Therapy study was performed. There were 3070 treatment-naive, hepatitis C virus genotype 1 infected patients were included. We retrospectively evaluated rates of screen failure, completion, and discontinuation of treatment and follow-up, treatment adherence, and virologic response by site type.
Results
Of the patients screened, 63% and 37% were in academic and community centers, respectively. Screen failure rates were similar (30% to 32%). End-of-treatment response, relapse, and sustained virologic response (SVR) rates in academic and community centers did not differ. SVR was achieved in 40% of patients at academic sites and 39% at community sites. Adherence to ≥80% of peginterferon-α and ribavirin dosing for ≥80% assigned duration was also similar (46% in academic and 47% in community centers). In both academic and community centers, 54% of patients completed treatment; there were similar discontinuation rates for treatment failure and adverse events.
Conclusions
There were no significant differences in adherence, adverse events, rates of discontinuation, on-treatment virologic response, and SVR when comparing academic and community sites. The performance of academic-based and experienced community-based sites in clinical trials is largely similar for the treatment of chronic hepatitis C.
Keywords: peginterferon-α, ribavirin, hepatitis C, site
Clinical trials assess the efficacy of interventions on a specific disease or state. However, the caveat is that these results may not be universally applicable to all treatment settings, thus calling into question the efficacy of the intervention of interest. Typically, phase 3 trials are conducted in closely supervised settings in academic-based and community-based centers. Community-based trials are then performed to establish the “real-life” effectiveness of treatments in a less controlled setting.1 Although academic centers are often targeted as clinical trial sites, clinical trials can include patients enrolled from different practice settings including community-based private practices and managed care settings (Health Maintenance Organizations, Veterans Affairs). There is a perception that patients undergoing treatment for chronic hepatitis C at academic centers have greater access to resources as compared with patients treated at community-based sites and thus, the outcomes are superior to those at nonacademic sites.
The extent to which differences between community and academic sites may influence treatment outcomes among patients receiving peginterferon-α (PEG-IFN) plus ribavirin (RBV) for chronic hepatitis C infection in the context of clinical trials is not well defined. In the Weight-Based Dosing of Peginterferon-α-2b plus Ribavirin (WIN-R) study,2 the influence of weight-based RBV on treatment of chronic hepatitis C in combination with PEG-IFN-α-2b was examined. This prospective, randomized study included predominantly community-based sites as well as some academic centers. For hepatitis C virus (HCV) genotype 1, a low sustained virologic response (SVR) rate of 29.8% was seen in the WIN-R study compared with 42% in the initial registration study. This difference was partially attributed to site issues and a larger spectrum of patients enrolled.3 The influence of the involvement of community-based sites on these results was not directly assessed.
Like the WIN-R study, the Individualized Dosing Efficacy Versus flat dosing to Assess optimal pegylated interferon therapy (IDEAL) study was conducted in the United States among 118 centers which were both academic institutions and community-based sites (including managed care and Veterans Affairs sites). The purpose of this analysis was to evaluate various metrics of quality and site performance in academic and community sites participating in the multicenter IDEAL study to determine whether difference in these parameters and outcomes exist.4
MATERIALS AND METHODS
Study Design
The IDEAL study has been described in detail elsewhere.2 Briefly, this was a phase 3B, randomized, parallel group, US multicenter study of PEG-IFN-α-2b 1.5 or 1.0 μg/kg/wk or PEG-IFN-α-2a combined with RBV for the treatment of chronic HCV infection. PEG-IFN-α-2b dose was double-blinded, and PEG-IFN-α-2a and RBV were administered as open-label treatments. The primary endpoint was SVR, defined as undetectable HCV-RNA at 24 weeks after completing therapy. If the 24-week posttreatment HCV-RNA was missing, the 12-week posttreatment level was used. The study was approved by each center's institutional review board and was conducted in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient Population
Patients were enrolled from 118 academic and hepatitis C treatment experienced community centers in the United States. Persons aged 18 to 70 years of age were eligible if they had compensated liver disease due to HCV genotype 1 infection and were treatment-naive. In addition, patients had to meet the following eligibility criteria: white blood cell count of ≥3000/mm3; absolute neutrophil count of ≥1500/mm3; platelet count of ≥80,000/mm3; hemoglobin ≥12 g/d for women and ≥13 g/dL for men; normal serum creatinine and thyroid-stimulating hormone; controlled diabetes mellitus (HbA1c≤8.5%); no known human immunodeficiency virus or hepatitis B virus infections; absence of moderate and severe psychiatric disorders and/or active substance abuse as well as uncontrolled medical conditions such as obesity (weight >125 kg), and unstable heart disease.
Assessments
The outcomes of interest in this analysis were rates of screen failure, completion and discontinuation of treatment and follow-up, treatment adherence, and virologic response by site type (academic vs. community centers). Virologic response included rapid virologic response (undetectable HCV-RNA at treatment week 4), complete early virologic response (undetectable HCV-RNA at treatment week 12), and SVR (undetectable HCV-RNA at the end of the 24-wk posttreatment follow-up period). Relapse rates, defined as undetectable HCV-RNA at the end-of-treatment (EOT) with detectable HCV-RNA level at the end of follow-up, were also evaluated. HCV-RNA levels were measured using the COBAS TaqMan assay (Roche Diagnostics), with a lower limit of quantitation at 27 IU/mL, undetectable. Adherence was assessed with 80:80:80 adherence representing patients who received ≥80% of PEG-IFN and RBV doses for ≥80% of the assigned duration.
Statistical Analysis
As the primary results demonstrated no statistical difference between any of the 3 treatment arms, data from the 3 treatment arms of the study were combined for all analyses. Categorical variables were summarized using proportions, and continuous variables were summarized using means and SDs. P-values for comparison were presented on the basis of χ2 test for categorical variables. All P-values reported are nominal P-values, and have not been adjusted for multiple comparisons. All statistical analyses were done using SAS software, Cary, NC.
RESULTS
Patients
In the 76 (64%) academic-based and 42 (36%) community-based sites, 4469 patients were screened for participation in the study. Of those patients, 2799 (63%) and 1670 (37%) patients were screened in academic and community centers, respectively. A similar percentage of patients in academic-based and community-based centers failed the screening period [32% (884/2799) and 30% (502/1670), respectively]. Although the reasons for failing screening were similar among the center types with protocol ineligibility being the most common (Table 1), the demographics differed. The proportion of patients failing screening who were African American (35% vs. 16%, P < 0.001) or aged older than 40 years (87% vs. 81%, P = 0.004) was higher at academic than community centers. The mean rate of enrollment at both academic and community sites was approximately 1 case/site/month.
TABLE 1.
Patients Who Failed Protocol Screening in Academic and Community Centers [n (%)]
| Academic Centers |
Community Centers |
|
|---|---|---|
| n = 884 | n = 502 | |
| Reasons for failing screening period | ||
| Because of protocol ineligibility | 660 (75) | 378 (75) |
| Because of patient did not wish to continue | 137 (15) | 78 (16) |
| Because of lost to follow-up | 66 (7) | 26 (5) |
| Because of noncompliance with protocol | 19 (2) | 19 (4) |
| Because of adverse events | 2 (0.2) | 1 (0.2) |
| Males | 537 (61) | 306 (61) |
| Age [mean (SD)] (y) | 48.5 (7.8) | 47.1 (7.8) |
| > 40 | 769 (87) | 408 (81) |
| Weight [mean (SD)] (kg) | 85.1 (18.6) | 84.6 (18.7) |
| Race | ||
| White | 490 (55) | 348 (69) |
| Black | 309 (35) | 78 (16) |
| Hispanic | 60 (7) | 58 (12) |
| Asian | 17 (2) | 4 (1) |
Of the 4469 patients screened, 3070 patients were treated: 1905 (62%) patients in academic institutions and 1165 (38%) patients in community-based centers (Table 2), and 13 patients were randomized and not treated (10 in academic-based and 3 in community-based centers). The mean number of patients treated in academic centers compared with community centers were 25.7 (± 22.8) and 27.7 (± 25.7) patients. The baseline demographics and disease characteristics of the patients treated were comparable between center types, although there were racial differences in the populations. More African American patients were treated in academic centers (21% vs. 15%) than community sites, whereas more Hispanic patients were treated at community sites (10% vs. 5%).
TABLE 2.
Patient Demographics and Disease Characteristics of Treated Patients
| Academic Centers |
Community Centers |
|
|---|---|---|
| (n = 1905) | (n = 1165) | |
| Male | 1125 (59) | 708 (61) |
| Age [mean (SD)] (y) | 47.6 (8.1) | 47.4 (7.8) |
| > 40 | 1623 (85) | 990 (85) |
| Weight [mean (SD)] (kg) | 83.4 (16.3) | 83.5 (16.3) |
| Race | ||
| White | 1348 (71) | 841 (72) |
| Black | 400 (21) | 170 (15) |
| Hispanic | 96 (5) | 117 (10) |
| Asian | 28 (1) | 23 (2) |
| Baseline HCV-RNA | ||
| > 600,000 IU/mL | 1561 (82) | 957 (82) |
| Genotype 1 subtype | ||
| 1a | 1172 (62) | 747 (64) |
| 1b | 695 (36) | 390 (33) |
| 1a1b | 34 (2) | 24 (2) |
| ALT | ||
| Abnormal (>ULN) | 645 (73) | 944 (81) |
| Steatosis* | ||
| Absent | 694 (36) | 423 (36) |
| Present | 1107 (58) | 699 (60) |
| Metavir fibrosis score* | ||
| F0/1/2 | 1603 (84) | 992 (85) |
| F3/4 | 198 (10) | 130 (11) |
Data missing for 147 patients (104 in academic-based and 43 in community-based centers).
ALT indicates alanine aminotransferase; HCV, hepatitis C virus; ULN, upper limit of normal.
Virologic Response
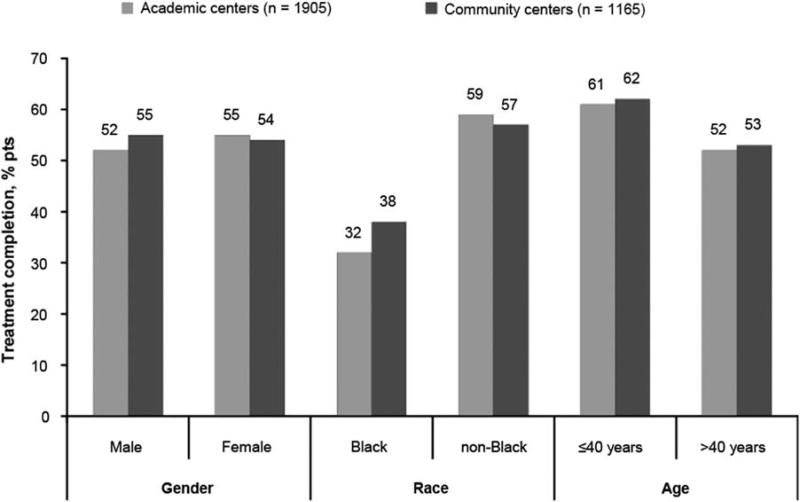
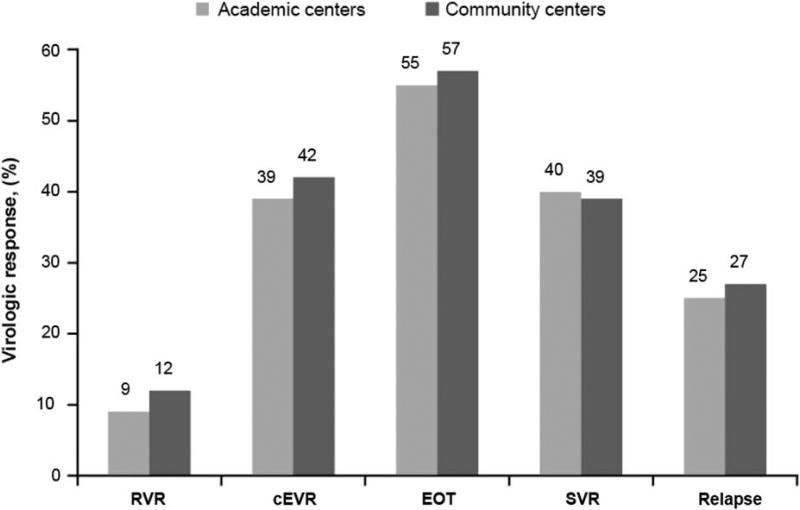
In both academic and community centers, 54% of patients completed treatment (Table 3). There were similar discontinuation rates for treatment failure and lost to follow-up at each center type. In addition, treatment completion rates were similar across various demographic characteristics as well as regions in the United States (Fig. 1). In terms of virologic response, SVR, EOT response, and relapse rates were similar in patients enrolled at academic and community centers (P = 0.64 for SVR and P = 0.39 for EOT). The proportions of patients with rapid virologic response in community centers was higher than in academic centers (12% vs. 9%, P = 0.02); whereas rates of complete early virologic response (P = 0.20) were similar at community and academic sites (Fig. 2).
TABLE 3.
Study Participation [n (%)]
| Academic Centers |
Community Centers |
||
|---|---|---|---|
| (n = 1905) | (n = 1165) | P | |
| Treatment phase | |||
| Completed | 1020 (54) | 634 (54) | 0.64 |
| Discontinued | 885 (46) | 531 (46) | 0.64 |
| Because of treatment failure | 520 (27) | 310 (27) | 0.68 |
| Because of adverse events | 234 (12) | 128 (11) | 0.28 |
| Because the patient did not wish to continue | 63 (3) | 45 (4) | 0.42 |
| Because of lost to follow-up | 44 (2) | 34 (3) | 0.30 |
| Because of noncompliance with protocol | 18 (1) | 13 (1) | 0.65 |
| Because of protocol ineligibility | 4 (<1) | 1 (0.1) | 0.41 |
| Administrative | 2 (<1) | 0 | 0.27 |
| 24-wk follow-up phase | |||
| Completed | 1507 (79) | 910 (78) | 0.51 |
| Discontinued | 166 (9) | 103 (9) | 0.90 |
| Because of lost to follow-up | 101 (5) | 62 (5) | 0.98 |
| Because the patient did not wish to continue | 54 (3) | 34 (3) | 0.89 |
| Because of noncompliance with protocol | 5 (<1) | 4 (<1) | 0.69 |
| Because of adverse events | 6 (<1) | 3 (<1) | 0.78 |
| Never entered follow-up phase | 232 (12) | 151 (13) | 0.52 |
FIGURE 1.
Treatment completion rates by demographic characteristics. P > 0.05 for all comparisons (nominal P-values, unadjusted for multiple comparisons).
FIGURE 2.
Virologic response rates in community and academic sites. cEVR indicates complete early virologic response; EOT, end-of-treatment response; RVR, rapid virologic response; SVR, sustained virologic response.
There was no significant difference in SVR rates between community and academic centers within most selected patient subgroups (Table 4). In addition, rates of adherence in the groups were similar with 80:80:80 rates of 46% (874/1905) in the academic centers and (47%) 552/1165 in the community centers.
TABLE 4.
Sustained Virologic Response Rates by Demographic Characteristics [% (n/N)]
| Academic Centers |
Community Centers |
|
|---|---|---|
| (n = 1905) | (n = 1165) | |
| Sex | ||
| Male | 38 (428/1125) | 40 (283/708) |
| Female | 43 (332/780) | 38 (172/457) |
| Race | ||
| Black | 22 (88/400) | 22 (37/170) |
| Nonblack | 45 (672/1505) | 42 (418/995) |
| Age (y) | ||
| ≤ 40 | 54 (153/282) | 48 (84/175) |
| > 40 | 37 (607/1623) | 37 (371/990) |
Safety
The percentage of serious adverse events (11% vs. 8%, P = 0.03) (Table 5) was slightly higher in academic centers compared with community centers. Other safety parameters including dose modifications and discontinuations of treatment occurred in similar percentages of patients in academic and community centers. The number of deaths (8/1905 vs. 4/1165, P = 0.74) was similar as well.
TABLE 5.
Adverse Events, Discontinuations, and Dose Modifications by Type of Center
| Academic Center | Community Center | P | |
|---|---|---|---|
| Deaths (n) | 8 | 4 | 0.75 |
| Treatment-emergent AE [n (%)] | 1886 (99) | 1146 (98) | 0.12 |
| SAE [n (%)] | 206 (11) | 97 (8) | 0.025 |
| Dose modifications due to AE* [n (%)] | 744 (39) | 479 (41) | 0.26 |
| Discontinuation due to AE [n (%)] | 234 (12) | 128 (11) | 0.28 |
Excludes those who discontinued treatment due to AE.
AE indicates adverse event; SAE, significant adverse event.
DISCUSSION
This analysis demonstrates that there were no significant differences in the outcomes seen in the treatment of chronic hepatitis C when comparing academic-based and community-based site performance. There were minimal differences in efficacy, safety, and adherence between academic-based and community-based sites. Our findings are supportive of recent data from oncology literature suggests that there was little difference in survival in clinical trials when comparing enrollment settings (academic, community, Veterans Hospital Administration sites).5
Although these findings are encouraging for the continued inclusion of a broad variety of sites in phase 3 clinical trials, these results may not be generalizable to all community sites enrolling patients in clinical trials or to the performance of interferon-based therapy for HCV outside of the clinical trial setting. The practices in community centers outside of clinical trials for HCV treatment have been previously reviewed. In an observational study of patients in Canada, SVR rates were 62% compared to 32% in academic and community clinics, respectively, although there was no difference observed in terms of dose reductions or treatment discontinuations.6 Jensen et al7 studied academic, private, and Veterans’ Affairs treatment sites in regard to interferon therapy for HCV. In their retrospective analysis, there were lower rates of EOT response in nonacademic centers. Thus, the oversight and infrastructure incumbent upon community centers participating in clinical trials may not have been in place in these sites and studies.
The community sites chosen to enroll patients in the IDEAL study were selected from a list of community sites after a process that included evaluation of staff and resources, previous experience in clinical trials, and history of successful enrollment in clinical trials. Therefore, these sites were possibly more experienced and invested in the treatment of hepatitis C patients and may have increased infrastructure to support participation in large clinical trials. A potential criterion for inclusion of community and even academic sites in large clinical trials should be the level of commitment and participation to the area or disease of study.
The inclusion of patients screened and enrolled for clinical trials in community settings is important for assessing treatment efficacy. These data allow for confidence in the utilization of these selected community-based sites in large clinical trials and the conclusions derived from those data.
Acknowledgments
Supported by Schering-Plough Corporation, now Merck & Co. Inc., Whitehouse Station, NJ.
S.N. and L.D.P. are former employees of Merck Research Laboratories. S.N. is a former employee of Schering-Plough Corporation and has received consultant fees from Schering-Plough Corporation. Currently, she is employed by Bristol-Myers Squibb and owns stock in Merck & Co., Schering-Plough Corporation, Bristol-Myers Squibb, and Johnson and Johnson. J.L. is an employee and stock holder in Merck & Co. M.S.S. reports receiving institutional grant funding from Merck, consulting fees from Vertex, Abbott Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim Pharmaceuticals, Gilead, Janssen, Merck, Novartis, and Roche/Genentech. L.D.P. is a stock holder and former employee of Merck Research Laboratories. J.G.M. has received grant support and consulting fees from Merck. A.J.M. reports having received institutional grant funding from Achillion, Bristol-Myers Squibb, Gilead, Abbott Laboratories, Pfizer, Medtronic, Merck and Co., Scynexis and Vertex, and consulting fees from Achillion, Bristol-Myers Squibb, Merck, and Vertex. J.H.J. was funded by NIDDK T32 Training Grant #3020023 and the American Association for the Study of Liver Diseases Advanced/Transplant Hepatology Fellowship Award.
Dr Jou was funded by NIDDK T32 Training Grant #3020023 and the American Association for the Study of Liver Diseases Advanced/ Transplant Hepatology Fellowship Award.
REFERENCES
- 1.Marotta P, Hueppe D, Zehnter E, et al. Efficacy of chronic hepatitis C therapy in community-based trials. Clin Gastroenterol Hepatol. 2009;7:1028–1036. doi: 10.1016/j.cgh.2009.05.003. quiz 1022. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson IM, Brown RS, Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 5.Lamont EB, Landrum MB, Keating NL, et al. Differences in clinical trial patient attributes and outcomes according to enrollment setting. J Clin Oncol. 2010;28:215–221. doi: 10.1200/JCO.2008.21.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers RP, Cooper C, Sherman M, et al. Outcomes of chronic hepatitis C therapy in patients treated in community versus academic centres in Canada: final results of APPROACH (a prospective study of peginterferon alfa-2a and ribavirin at academic and community centres in Canada). Can J Gastroenterol. 2011;25:503–510. doi: 10.1155/2011/698780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen DM, Cotler SJ, Lam H, et al. A comparison of hepatitis C treatment and outcomes at academic, private and Veterans’ Affairs treatment centres. Aliment Pharmacol Ther. 2004;19:69–77. doi: 10.1046/j.1365-2036.2003.01817.x. [DOI] [PubMed] [Google Scholar]