Abstract
The convergent synthesis of a benzofuran analog of the carbacyclic ansa compound kendomycin has been achieved by assembling three major fragments by means of epoxide opening and directed ortho lithiation. The crucial tetrahydropyran ring was formed by a highly stereoselective cationic cyclization. Analysis of all synthesized tetrahydropyran-arene compounds reveals a hindered sp2-sp3 rotation, which results in rotational isomers or atropisomers affecting macrocyclization reactions. The latter could only be achieved by means of Horner–Wadsworth–Emmons olefination.
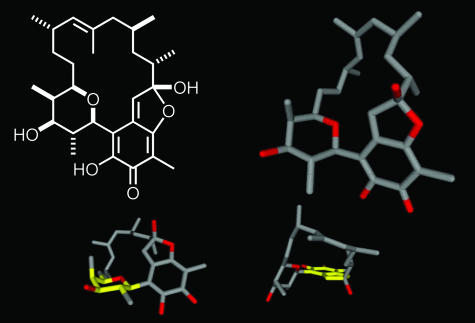
Organic compounds derived from nature have been an abundant source of drugs and drug leads and have provided a rich array of interesting synthetic targets. Among various selection criteria, novel and complex structural features combined with interesting biological activities might be the most important ones to promote a molecule to a target for natural product synthesis. Kendomycin (1), a novel streptomyces metabolite, which was presented at a meeting in 1999, consummately meets all the requirements and thus was selected as a target for our research one year later. Originally, 1 had been published as (-)-TAN 2162 in the patent literature as a potent endothelin receptor antagonist and antiosteoporotic compound (1–3). With its reisolation from a different streptomyces strain, the absolute configuration of 1 has been determined. Additionally, the biosynthetic pathway and further antibiotic and cytotoxic activities have been reported (4, 5). The structure of 1 (Fig. 1) features an aliphatic ansa chain that is attached by carbons C5 and C18 to a quinone methide core. Thus, 1 is one of the very few ansa-carbon natural products that have been isolated so far. Likewise, the quinone methide structure, which can be regarded as a 1,6-oxidation product of the corresponding benzofuran, has no precedence in natural product chemistry.
Fig. 1.
Structure of kendomycin (1) with different views of the crystal structure.
Particular attention should be devoted to the fully substituted tetrahydropyran ring, which is, combined with the quinone methide, a sterically encumbered region in the molecule, possibly prone to atropisomeric phenomena. In this contribution we give a progress report on our total synthesis of kendomycin (6–8).
First-Generation Approach
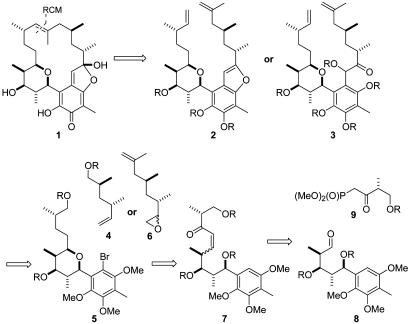
Our initial synthetic plan (Fig. 2) focused on a ring-closing metathesis (RCM) macrocyclization of the seco intermediates 2 or 3. It was obvious that the formation of the quinone methide chromophore should be deferred to the end of the synthesis, envisaging either an oxidation of the corresponding benzofuran or a 1,6-elimination. Key intermediates 2 and 3 were to be assembled either by a Heck coupling of alkene 4 and arylbromide 5 or an epoxide opening reaction of 5 with epoxide 6. The tetrahydropyran ring should be formed by an intramolecular hetero-Michael addition of enone 7, which should be derived from a Horner–Wadsworth–Emmons (HWE) olefination of aldehyde 8 with β-ketophosphonate 9.
Fig. 2.
Retrosynthetic scheme of the first generation approach. R, suitable protecting group.
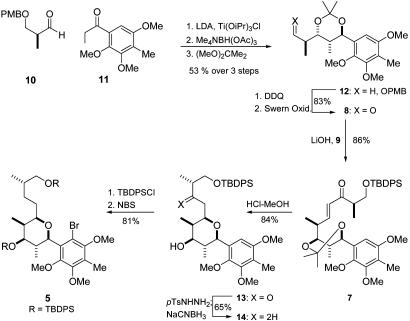
As outlined in Fig. 3, the stereotetrad of the tetrahydropyran ring was constructed by aldol addition methodology and diastereoselective reduction of β-hydroxy ketones. The first key step in this approach was the reaction of the known aldehyde 10 (9) with the titanium enolate of phenyl ketone 11 (10). In this aldol addition the desired syn-adduct was formed as the main isomer (58%) but with only moderate diastereoselectivity (≈2:1). On diastereoselective reduction with Me4NBH(OAc)3 (11) and subsequent treatment with 2,2-dimethoxypropane, acetonide 12 was obtained in high yield and diastereoselectivity (91%, 93:7).
Fig. 3.
Synthesis of the stereotetrad and tetrahydropyran formation. PMB, p-methoxybenzyl; LDA, lithium diisopropylamide; DDQ, 2,3-dichloro-5,6-dicyanobenzoquinone; TBDPS, tert-butyldiphenylsilyl; pTs, para-toluenesulfonyl.
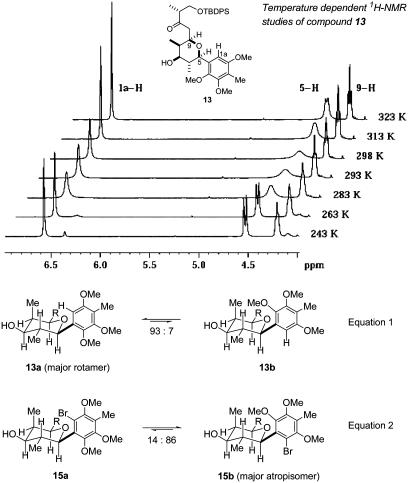
The construction of the tetrahydropyran ring commenced with a deprotection-oxidation sequence of 12 to furnish aldehyde 8, which then was coupled with phosphonate 9 (12) in a HWE reaction to afford pure (E)-enone 7. On removal of the acetonide with diluted HCl in methanol, the free diol underwent the desired Michael cyclization in situ to afford tetrahydropyran 13 in excellent yield and diastereoselectivity (84%, 97:3). Subsequent carbonyl reduction by the tosylhydrazone was performed with NaCNBH3 to give 14, which was transformed into the fully substituted arene 5 by protection (TBDPSCl) and electrophilic bromination with N-bromosuccinimide (NBS). In the course of the NMR analysis of the novel aryl-tetrahydropyran compounds some interesting dynamic phenomena were observed and were attributed to a restricted sp2-sp3 rotation at the C(4a)–C(5) bond (for review on sp2-sp3 atropisomerism, see ref. 13). A temperature-dependent 1H NMR study of compound 13 (Fig. 4) revealed, at low temperatures, the presence of a second set of signals (≈7%), in which 5-H appeared now as the expected doublet with a trans-diaxial coupling constant. From additional low-temperature 2D NMR experiments (-40°C) and comparison of the hyperfine splittings it was concluded that, at low temperatures, compound 13 exists as a 93:7 equilibrium of rotamers 13a and 13b (Eq. 1 in Fig. 4). The rotation barrier is considerably higher in aryl bromide 15. Analysis of 2D NMR experiments revealed 15 to be a 1:5 mixture of the isomers 15a and 15b (Eq. 2 in Fig. 4). In this case the 1H NMR spectra of 15 were invariant with the temperature (up to 100°C), indicating that compound 15 exists as a mixture of atropisomers. In fact, HPLC allowed a baseline separation of 15a and 15b. After 30 min the atropisomers were fully equilibrated again.
Fig. 4.
Dynamic aspects of tetrahydropyran-arene compounds 13 and 15.
Although the above-mentioned syntheses and analyses provided an interesting insight into the dynamics of our compounds, which would prove quite useful later (see below), there were also some drawbacks to this first approach. In particular, the number of synthetic steps and the low diastereoselectivity in the aldol step did not meet the requirements of modern organic synthesis. Thus, we strived for an alternative approach that aimed for a more stereocontrolled and shorter synthesis of the tetrahydropyran moiety.
Second-Generation Approach
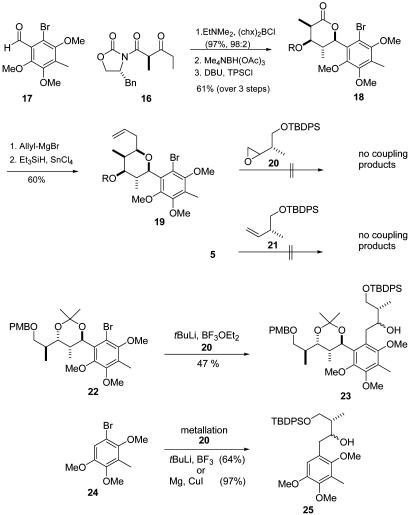
Whereas in our first approach an achiral ethyl phenyl ketone and a chiral aldehyde had been used as the aldol donor and acceptor, respectively, the roles of acceptor and donor were now exchanged (Fig. 5). This meant that a chiral aldol donor, such as Evans' keto imide 16 (14), could potentially be used in conjunction with aldehyde 17. In fact, conversion of β-keto imide 16 to the (E)-enol borinate (c-C6H11)2BCl/NEt3 (15, 16), and reaction with aldehyde 17 afforded the desired anti-aldol adduct almost quantitatively with high diastereoselectivity (>98:2). Subsequent anti-selective reduction with Me4NBH(OAc)3, treatment with a catalytic amount of 1,8-diaza-bicyclo-[5.4.0]-undec-7-ene, and in situ protection with triphenylsilyl chloride furnished lactone 18. The formation of the tetrahydropyran ring was achieved by addition of allylmagnesium bromide to form the corresponding lactol, which was without any further purification reduced to tetrahydropyran 19 with Et3SiH in the presence of SnCl4.
Fig. 5.
Modified synthesis of the tetrahydropyran ring and attachment of the eastern side chain. chx, cyclo-hexyl; DBU, 1,8-diaza-bicyclo-[5.4.0]-undec-7-ene; TPS, triphenylsilyl; PMB, para-methoxybenzyl; TBDPS, tert-butyldiphenylsilyl.
Next, the right-hand carbon chain had to be attached to the aromatic core. Unfortunately, the opening of epoxide 20 (17) with aryl bromide 19 failed completely, as did the Pd(0)-catalyzed coupling of aryl bromide 5 with alkene 21 and the corresponding alkyne, respectively. Eventually, a moderate yield could be obtained in the opening of epoxide 20 with aryl bromide 22 to give coupling product 23. Pleasingly, the efficiency of this coupling could be significantly enhanced by using the ortho-unsubstituted aryl bromide 24 to furnish homobenzylic alcohol 25.
Third-Generation Approach
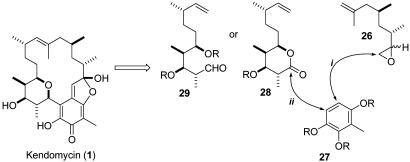
Because the presence of the tetrahydropyran ring was detrimental to the introduction of the second carbon chain, it seemed mandatory that the order of the coupling reactions be reversed. Thus, the right-hand fragment 26 should be attached first by an epoxide opening reaction with arene 27 and then the left-hand fragments 28 or 29 containing the tetrahydropyran precursor should be introduced (Fig. 6).
Fig. 6.
Revised retrosynthesis of 1 with reversed order of coupling reactions. R, suitable protecting group.
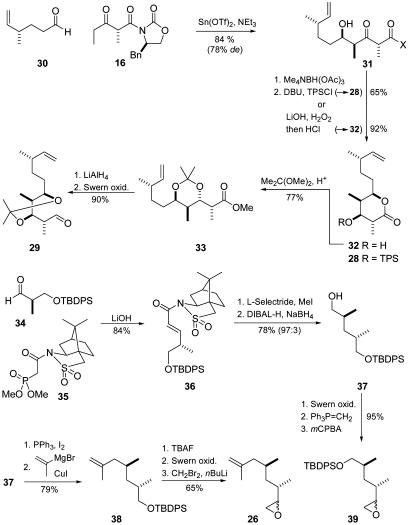
The synthesis of the western fragments (Fig. 7) started from citronellene, which was converted into aldehyde 30 (18). Subsequent Evans aldol reaction with keto imide 16 (see above) was performed under the appropriate conditions (tin triflate, triethylamine) to generate the desired syn-aldol adduct 31 in good yield and selectivity. Subsequent anti-selective reduction and treatment with 1,8-diaza-bicyclo-[5.4.0]-undec-7-ene/triphenylsilyl chloride or LiOH/H2O2 furnished the corresponding γ-lactones 28 and 32, respectively. The latter was then transferred into the protected methyl ester 33 on treatment with dimethoxypropane. Finally, a reduction–oxidation sequence completed the synthesis of the desired aldehyde 29.
Fig. 7.
Synthesis of the left-side fragments 31 and 28 and the right-side fragments 26 and 38. Tf, trifluoromethanesufonyl; L-Selectride, lithium tri-sec-butylborohydride; Superhydride, lithium triethylborohydride; TBAF, tetrabutylammonium fluoride; DIBAL-H, diisobutylaluminum hydride; mCPBA, meta-cloroperoxybenzoic acid.
For the synthesis of the eastern fragment, TBDPS-protected Roche aldehyde 34 was subjected to a HWE olefination with phosphonate 35 (19) to afford the corresponding N-enoyl sultam 36. Subsequent to α-alkylation with methyl iodide by using Oppolzer's 1,4-addition/enolate-trapping protocol (20), reductive removal of the auxiliary afforded primary alcohol 37 in good yield and diastereoselectivity (78%, 97:3). After conversion into the iodide (81%) this compound was coupled with isopropenyl-magnesium bromide under Schlosser–Fouquet conditions (21, 22) to furnish 38 in excellent yield (97%). Deprotection of 38 and subsequent Swern oxidation furnished the corresponding aldehyde, which was transformed directly into epoxide 26 by addition of lithiated dibromomethane (23). To achieve higher flexibility in the macrocyclization step a further epoxide (39) was synthesized from 37 by Swern oxidation, Wittig methylenation, and treatment with meta-cloroperoxybenzoic acid.
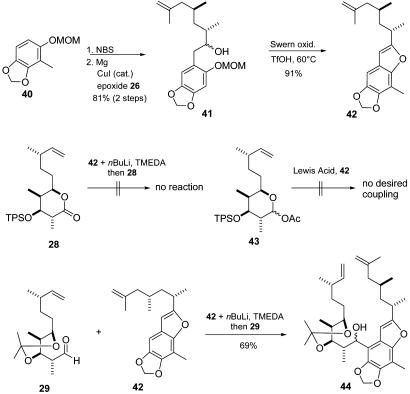
Regarding the crucial coupling reactions, the known substituted benzene derivative 40 (24) appeared to be a suitable candidate (Fig. 8). In fact, 40 could be readily converted into the corresponding Grignard compound and was successfully coupled with epoxide 26 to give alcohol 41. Oxidation of the diastereomeric mixture of alcohols 41 gave the corresponding ketone, which was condensed with the phenolic oxygen by treatment with triflic acid to furnish benzofuran 42 in good yield (91%). Since bromination reactions on a related benzofuran were not completely regioselective, we investigated the direct ortho-metallation on 42. Thus, treatment of 42 with nBuLi/tetramethylethylenediamine gave ortho-lithiation position, as proven by D2O quench of the resulting carbanionic species.
Fig. 8.
Synthesis of benzofuran 44 and attachment of the western side chain. TMEDA, tetramethylethylenediamine; TfOH, trifluoromethanesulfonic acid.
Relying on our previous results in the second approach, lithiated benzofuran 42 was first treated with lactone 28, but unfortunately no reaction could be detected. As a possible alternative coupling, we investigated C-glycosidation type reactions of the corresponding acetal 43 under Lewis acid conditions. Again none of the desired coupling product was formed, but we found small amounts of a product mixture resulting from an initial reaction of the oxonium intermediate with the terminal double bond of 42. Both of these results revealed again the problems involved with the coupling of our sterically encumbered fragments. Finally, we subjected lithiated benzofuran 42 to the more reactive and less hindered aldehyde 29. To our delight, the desired coupling proceeded smoothly and furnished benzylic alcohol 44 as an ≈5:1 mixture of diastereomers. Compound 44 contained all the carbons of 1; however, the problem of tetrahydropyran ring formation still remained.
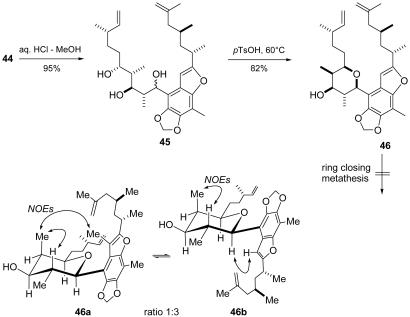
Our first attempt to achieve the cyclization of 44 by oxidation and hemiketal formation (compare 18→19) failed. In consideration of the stabilization of benzylic carbenium species we focused next on a SN1-type cyclization (Fig. 9) (25, 26). To our delight, after removal of the acetonide-protecting group (0.01 M HCl in MeOH) the resulting triol 45 cyclized readily on treatment with a catalytic amount of p-TsOH in toluene at 60°C. The cyclization could also be carried out in a single step but suffered from a somewhat lower yield. With tetrahydropyran-arene compound 46 in hand, we had solved our hitherto most sophisticated problem, namely to connect the synthetically demanding tetrahydropyran ring with the fully substituted aromatic core. Additionally, we had achieved a short and convergent synthesis of the full carbon skeleton with all independent stereogenic centers in place.
Fig. 9.
Modified synthesis of the tetrahydropyran ring, and macrocyclization attempts by RCM. pTsOH, para-toluenesulfonic acid; NOEs, nuclear Overhauser effects.
With confidence we turned toward the macrocyclization that we had planned to perform using a RCM reaction. Unfortunately our optimism had been premature, because neither 46 nor its OH-protected analogs underwent the desired cyclization. At this point of the synthesis a review of the analysis of our substrate was appropriate. The 5,9-cis-configuration of the tetrahydropyran ring in 46 had been assigned by 2D NMR experiments and, as anticipated, compound 46 revealed the now well known dynamic behavior. In particular, HH-NOESY experiments indicated the presence of individual rotamers 46a and 46b in a ratio of 1:3 at room temperature. At that time, it was not yet clear to which extent the restricted rotation in 46 interfered with the envisaged RCM reaction. However, it was likely that the restricted rotation and the preference of rotamer 46b over the more kendomycin-like rotamer 46a might be disadvantageous for our purposes.
Fourth-Generation Approach
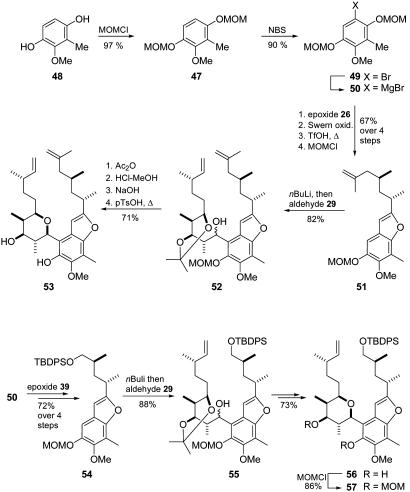
In the course of the previous approach we had observed some additional drawbacks. First, as preliminary experiments had shown, the methylendioxy protection group was not appropriate for secure removal at the late stage of the synthesis. Second, the ortho-lithiation step required immoderate excess of benzofuran 42 (3 eq) to obtain satisfying yields. Because we had also observed a distinct influence of the substitution pattern of the aromatic core in terms of the preferred rotamer in tetrahydropyran-arene compounds (see above), a more flexible aromatic template was considered vitally important. Keeping all this in mind, we identified compound 47 as the central aromatic core, which was easily available from known p-hydroquinone 48 (27) on treatment with methoxymethyl chloride (MOMCl) (Fig. 10). Subsequent bromination with NBS gave bromide 49, which was then converted to Grignard compound 50. Subsequent treatment with epoxide 26 and application of the previously established ox idation–condensation procedure furnished the corresponding benzofuran with a free OH at carbon C4. After reprotection with MOMCl we obtained compound 51, as a particularly suitable candidate for the planned ortho-directed lithiation. In fact, subsequent coupling with aldehyde 29 provided benzylic alcohol 52 without the above-mentioned drawbacks as an ≈9:1 mixture of diastereomers. Since the configuration of the benzylic position was irrelevant in the ensuing reaction sequence, the diastereomeric mixture was carried through the next steps. Thus, subsequent treatment with acetic anhydride, diluted HCl, and NaOH gave the diastereomeric mixture of the corresponding triol, which was cyclized in the above-mentioned manner to furnish compound 53 in good yield as a single diastereomer. The modified removal of the acetonide-protecting group had become necessary since we had observed acetonide migration in the original procedure (diluted aqueous HCl in MeOH). To achieve more flexibility in the macrocyclization, Grignard compound 50 was also coupled with epoxide 39 and was then subjected to the same sequence to afford tetrahydropyran-arene compound 56 (by intermediates 54 and 55), which was then protected with MOMCl to give compound 57 (Fig. 10).
Fig. 10.
Synthesis of key compounds 53 and 57. TfOH, trifluoromethanesulfonic acid; pTsOH, para-toluenesulfonic acid.
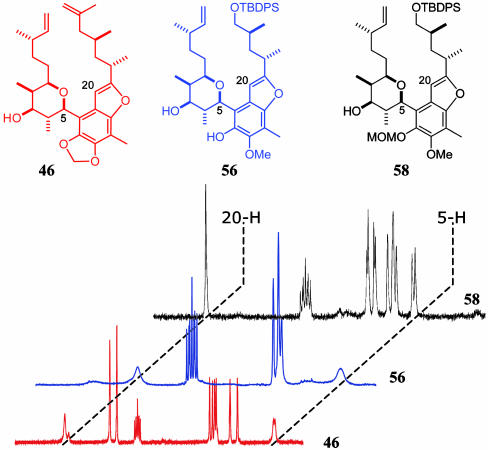
During the course of the synthesis of the various tetrahydropyran-arene compounds, three different types of rotational isomerism had been observed. This is further illustrated by considering compounds 46, 56, and 58 and the corresponding 1H NMR spectra as representative examples (Fig. 11). It is remarkable that, if we neglect the differences in the eastern side chain, the apparently small differences of the 4-O substituents affect the dynamic behavior to such an extent. As mentioned above, compound 46 exists as a 3:1 mixture of atropisomers, revealing two singlets for the 20-H in the proton NMR spectrum. On the other hand, phenol 56 is a mixture of rotamers with the coalescence temperature at about room temperature (broadened signals for 5-H and 20-H), whereas the corresponding MOM protected compound 58 exists as a single atropisomer with a kendomycin-like orientation (sharp singlet for 20-H and sharp doublet for 5-H).
Fig. 11.
1H NMR spectra of tetrahydropyran-arene compounds 46, 56, and 58 at 27°C.
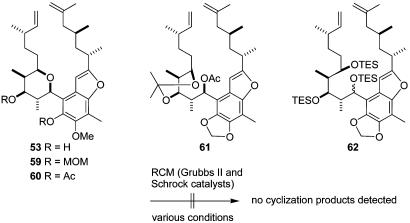
Compound 53 and its protected analogs 59 and 60 were subjected to various RCM conditions (Grubbs II catalyst or Schrock catalyst using standard procedures and increased temperatures), but unfortunately none of them underwent the desired cyclization (Fig. 12). Since RCM reactions with substrates that did not even bear the tetrahydropyran ring were also unsuccessful (e.g., 61 and 62), we concluded that the presence of the planar benzofuran unit might also be problematic for the ring closure. We temporarily gave up the RCM strategy, assuming that the less restricted rotation might be necessary but not the only requirement for a successful RCM reaction in our systems.
Fig. 12.
Further attempts of macrocyclization by using various RCM conditions on different substrates. TES, triethylsilyl.
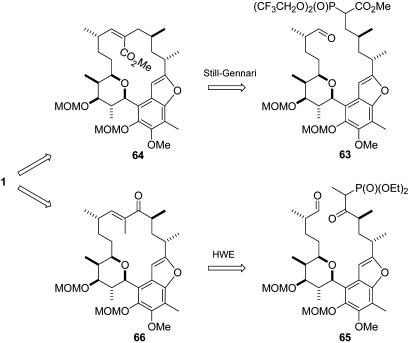
Intramolecular HWE reactions have been successfully used in macrocyclization reactions (28–31). The increased driving force of the HWE reaction compared with the metathesis reaction would make the former the method of choice for the aimed macrocyclization. Compared with the RCM strategy, a significant increase of synthetic operations would be unavoidable in our case, but, if successful, the HWE reaction should also provide the desired E-geometry of the trisubstituted olefin, an issue known to be problematic with RCM reactions. With regard to our system, we had either the option to perform the Z-selective Still–Gennari variant of the HWE reaction (63→64) with a β-phosphono ester or to use the common E-selective HWE olefination (65→66) with a β-keto phosphonate (Fig. 13).
Fig. 13.
Alternative macrocyclization approach by means of HWE reactions.
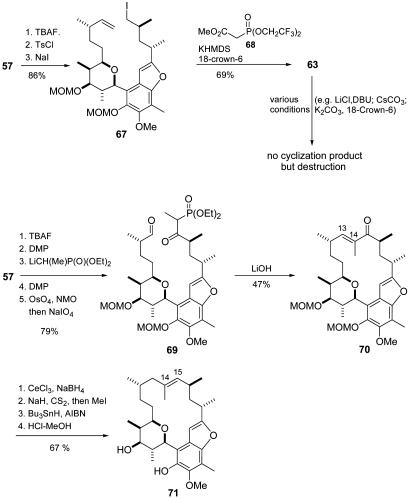
For the Still–Gennari approach, key compound 57 was deprotected with tetrabutylammonium fluoride to afford the corresponding primary alcohol, which was tosylated and then converted to iodide 67 by treatment with NaI in acetone. After alkylation of phosphono ester 68, the terminal alkene was cleaved by successive treatments with OsO4/N-methylmorpholine-N-oxide and NaIO4 to provide seco compound 63. Unfortunately, we could not find any reaction conditions to get the desired macrocyclization to work.
To set up the HWE substrate (Fig. 14), 57 was deprotected and oxidized to give the corresponding aldehyde, which was treated with deprotonated diethyl ethanephosphonate to furnish the desired β-keto phosphonate 69 after oxidation (for more details, see Supporting Text, which is published as supporting information on the PNAS web site). Dihydroxylation and subsequent glycol cleavage gave the corresponding aldehyde, which on moderate heating with finely dispersed LiOH for 18 h, smoothly cyclized to afford the macrocyclic enone 70 in acceptable yield. The unwanted carbonyl function proved resistant toward direct reduction. But after numerous experiments, a two-step procedure using Luche conditions and Barton McCombie xanthate reduction delivered a deoxygenated compound. Because of the pseudo-C2-symmetry of the upper part of the molecule, it was complicated to assign the position of the tri-substituted double bond. Finally, after deprotection and careful removal of all traces of stannane residues, it could be proven with several 2D NMR techniques (COSY, NOESY, and total correlated spectroscopy) that the double bond had migrated to give the undesired regioisomer 71. Because no other olefinic isomer was detected, we concluded that the migration presumably occurred on formation of the xanthate intermediate by a hetero-Claisen rearrangement (32). To prove this assumption, work should be done on a modified HWE approach (with reversed reactive sites) which then should lead to the desired regioisomer.
Fig. 14.
Still–Gennari–HWE and HWE macrocyclization approach. TBAF, tetrabutylammonium fluoride; TsCl, toluenesulfonyl chloride; KHMDS, potassium hexamethyldisilazane; DMP, Dess–Martin periodinane = 1,1,1-triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one; NMO, N-methylmorpholine-N-oxide; AIBN, azobisisobutyronitrile.
Supplementary Material
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HWE, Horner–Wadsworth–Emmons; RCM, ring-closing metathesis; MOM, methoxymethyl chloride; NBS, N-bromosuccinimide.
References
- 1.Funahashi, Y., Kawamura, N. & Ishimaru, T. (1996) Jap. Patent 08231551 [A2960910]; (1996) Chem. Abstr. 126, 6553. [Google Scholar]
- 2.Funahashi, N. & Kawamura, N. (1996) Jap. Patent 08231552; (1996) Chem. Abstr. 126, 326518. [Google Scholar]
- 3.Su, M. H., Hosken, M. I., Hotovec, B. J. & Johnston, T. L. (1998) U.S. Patent 5728727; (1998) Chem. Abstr. 128, 239489. [Google Scholar]
- 4.Bode, H. B. & Zeeck, A. J. (2000) J. Chem. Soc. Perkin Trans. 1 , 323-328.
- 5.Bode, H. B. & Zeeck, A. J. (2000) J. Chem. Soc. Perkin Trans. 1 , 2665-2670.
- 6.Martin, H. J., Drescher, M., Kählig, H., Schneider, S. & Mulzer, J. (2001) Angew. Chem. Int. Ed. Engl. 40, 3186-3188. [DOI] [PubMed] [Google Scholar]
- 7.Marques, M. M. B., Pichlmair, S., Martin, H. J. & Mulzer, J. (2002) Synthesis 18, 2766-2770. [Google Scholar]
- 8.Pichlmair, S., Marques, M. M. B., Green, M. P., Martin, H. J. & Mulzer, J. (2003) Org. Lett. 5, 4657-4659. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou, K. C., Patron, A. P., Ajito, K., Richter, P. K., Khatuya, H., Bertinato, P., Miller, R. A. & Tomaszewski, M. J. (1996) Chem. Eur. J. 2, 847-868. [Google Scholar]
- 10.Knoelker, H.-J. & Froehner, W. (1997) Tetrahedron Lett. 38, 4051-4054. [Google Scholar]
- 11.Evans, D. A., Chapman, K. T. & Carreira, E. M. (1988) J. Am. Chem. Soc. 110, 3560-3578. [Google Scholar]
- 12.Mulzer, J. & Berger, M. (1998) Tetrahedron Lett. 39, 803-806. [Google Scholar]
- 13.Eliel, E. L., Wilen, S. H. & Mander, L. N. (1994) Stereochemistry of Organic Compounds (Wiley, New York), pp. 1150-1153.
- 14.Evans, D. A., Clark, J. S., Metternich, R., Novack, V. J. & Sheppard, G. S. (1990) J. Am. Chem. Soc. 112, 866-868. [Google Scholar]
- 15.Cowden, C. J. & Paterson, I. (1997) Org. React. (N. Y.) 51, 1-200. [Google Scholar]
- 16.Evans, D. A., Ng, H. P., Clark, J. S. & Rieger, D. L. (1992) Tetrahedron 48, 2127-2142. [Google Scholar]
- 17.Konno, K., Fujishima, T., Maki, S., Liu, Z., Miura, D., Chokki, M., Ishizuka, S., Yamagucchi, K., Kan, Y., Kurihara, M., et al. (2000) J. Med. Chem. 43, 4247-4265. [DOI] [PubMed] [Google Scholar]
- 18.Ireland, R. E., Thaisrivongs, S. & Dussault, P. H. (1988) J. Am. Chem. Soc. 110, 5768-5779. [Google Scholar]
- 19.Oppolzer, W., Dupuis, D., Poli, G., Raynham, T. M. & Bernardinelli, G. 1988) Tetrahedron Lett. 29, 5885-5889. [Google Scholar]
- 20.Oppolzer, W. & Poli, G. (1986) Tetrahedron Lett. 27, 4717-4720. [Google Scholar]
- 21.Lipshutz, B. H. (2002) in Organometallics in Synthesis, ed. Schlosser, M. (Wiley, Chichester, U.K.), pp. 740-763.
- 22.Tamura, M. & Kochi, J. K. (1972) J. Organomet. Chem. 42, 205-228. [Google Scholar]
- 23.Michnick, T. J. & Matteson, D. S. (1991) Synlett 9, 631-632. [Google Scholar]
- 24.Corey, E. J., Gin, D. Y. & Kania, R. S. (1996) J. Am. Chem. Soc. 118, 9202-9203. [Google Scholar]
- 25.Grassberger, M. A., Fehr, T., Horvath, A. & Schulz, G. (1992) Tetrahedron 48, 413-430. [Google Scholar]
- 26.Williams, D. R., Kiryanov, A. A., Emde, U., Clark, M. P., Berliner, M. A. & Reeves, J. T. (2003) Angew. Chem. Int. Ed. Engl. 42, 1258-1262. [DOI] [PubMed] [Google Scholar]
- 27.Heckrodt, T. J. & Mulzer, J. (2003) J. Am. Chem. Soc. 125, 4680-4681. [DOI] [PubMed] [Google Scholar]
- 28.Williams, D. R., Cortez, G. S., Bogen, S. L. & Rojas, C. M. (2000) Angew. Chem. Int. Ed. Engl. 39, 4612-4615. [DOI] [PubMed] [Google Scholar]
- 29.Tatsuta, K., Masuda, N. & Nishida, H. (1998) Tetrahedron Lett. 39, 83-86. [Google Scholar]
- 30.Richardson, T. I. & Rychnovsky, S. D. (1997) J. Am. Chem. Soc. 119, 12360-12361. [Google Scholar]
- 31.Marshall, J. A. & DeHoff, B. S. (1986) Tetrahedron Lett. 27, 4873-4876. [Google Scholar]
- 32.Kadota, I., Takamura, H. & Yamamto, Y. (2001) Tetrahedron Lett. 42, 3469-3651. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.