Abstract
Reidispongiolide A is a representative member of the sphinxolide/reidispongiolide group of cytotoxic 26-membered macrolides of marine origin. By interacting with actin in the cell cytoskeleton, the reidispongiolides and sphinxolides are potent microfilament destabilizing agents that represent a promising mechanism of action for developing novel anticancer drugs. An aldol-based synthesis of a library of diastereomers of C8–C16 and C5–C16 fragments and detailed NMR comparison with a reported degradation fragment enabled a configurational assignment for a major part of the reidispongiolide macrocyclic core, thus setting a solid foundation for ongoing synthetic efforts.
Marine macrolides provide a fascinating range of structural and functional diversity, which may find application inter alia as specific molecular probes for the investigation of the cytoskeleton and cell cycle events. Unique to eukaryotic cells, the actin cytoskeleton plays a critical role in the determination of cell shape, and in a variety of cellular processes, including cell motility, division, adhesion, and intracellular transportation (1). In comparison with microtubule-interacting agents (2), such as the taxanes and vinca alkaloids, the development of anticancer drugs based on selective binding to actin and disruption of microfilament assembly has attracted relatively little attention. However, recent insight into the role played by microfilaments in cell division and metastasis has highlighted the potential of certain structural classes of actin-binding natural products as useful leads for cancer chemotherapy (3).
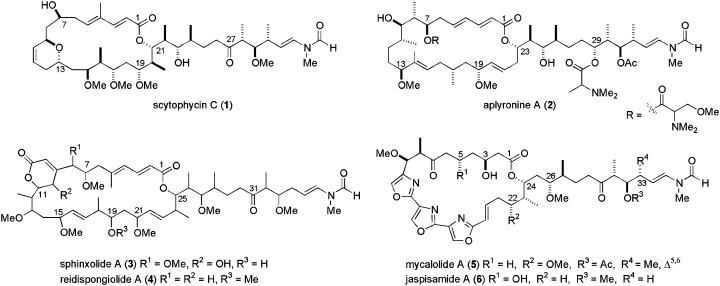
A growing family of actin-binding macrolides that disrupt microfilament organization includes the scytophycins (e.g., 1, Fig. 1), isolated from the blue-green algae Scytonema pseudohofmanni (4), aplyronines (e.g., 2), isolated from the sea hare Aplysia kurodai (5), and sphinxolides (e.g., 3, planar structure), first reported from an unidentified Pacific nudibranch (6) and later reisolated from the New Caledonian marine sponges Neosiphonia superstes and Reidispongia coreulea, along with the congeneric reidispongiolides (e.g., 4, planar structure) (7, 8). A related family of trisoxazole-containing marine macrolides, typified by mycalolide A (5) and jaspisamide A (6), are also antimicrofilament agents (9). Recently, an x-ray crystal structure of the latter compound bound to actin (10) highlighted the importance of the characteristic N-vinyl formamide-containing side chain, common to all these macrolides, in determining their biological activity. Moreover, all these actin-binding macrolides inhibit the proliferation of human cancer cell lines; for example, the scytophycins and sphinxolides exhibit potent cytotoxicity against L1210 and KB cells, with IC50 values ranging from ng/ml to pg/ml (9). In addition, aplyronine A shows potent antitumor activity in vivo and has potential as a clinical candidate (9). Notably, both the scytophycins and sphinxolides circumvent multidrug resistance mediated by the overexpression of P-glycoprotein or multidrug resistance protein (11). Although these preliminary studies demonstrate the potential of these macrolides as leads for the development of novel anticancer drugs, the scarcity of material available from the natural source has generally hampered extensive preclinical development.
Fig. 1.
Structurally related actin-binding macrolides.
As part of ongoing studies into the stereochemical determination and synthesis of cytotoxic macrolides of marine origin and designed analogues (12–15), we initiated a project directed toward the total synthesis of the reidispongiolides and sphinxolides, linked intimately with the elucidation of their relative and absolute stereochemistry. Herein, we propose a configurational assignment of reidispongiolide A based on an accumulation of synthetic studies, performed in association with detailed NMR analysis.
Materials and Methods
Experimental details along with physical and spectral data for all new compounds are published as supporting information on the PNAS web site.
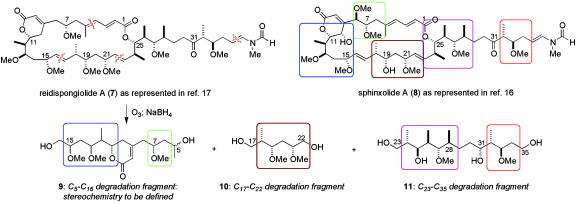
Previous Structural Studies on the Sphinxolides and Reidispongiolides
The planar structure 3 for sphinxolide A, a 26-membered macrocyclic lactone, was first reported in 1989 by Pietra and coworkers (6). However, only recently has a partial configurational assignment for this highly oxygenated and stereochemically elaborate macrolide (17 stereocenters and 6 alkenes) been proposed (16). Extensive analysis of sphinxolide by NMR methods, by Gomez-Paloma and coworkers (16), based largely on the interpretation of proton-carbon 2,3J couplings, enabled the assignment of the relative configurations in the five isolated stereoclusters, as indicated in the boxed regions of structure 8 in Scheme 1. Additionally, controlled ozonolysis of the closely related macrolide reidispongiolide A (having the proposed stereostructure 7), followed by reductive workup, provided the three distinct polyol fragments 9 (mixture of C5 epimers), 10 and 11 (mixture of C31 epimers) (17).
Scheme 1.
Proposed stereostructures of reidispongiolide A and sphinxolide A and chemical degradation fragments of reidispongiolide A.
As a prelude to a planned total synthesis of the sphinxolide/reidispongiolide macrolides, the preparation in our group of these three degradation fragments (or stereoisomers thereof) was required to simplify the stereochemical quandary and establish rigorously the interconnections between the isolated stereoclusters. To this end, we and the D'Auria group independently reported the synthesis of the degradation fragments 10 and 11 (17–19), and established unequivocally the relationship between the three corresponding stereoclusters in structure 7. We now focused our efforts on attaining a reliable configurational assignment for the remaining C5–C16 portion of reidispongiolide A, through the synthesis of the third degradation fragment 9, thus enabling the full stereostructure of these cytotoxic macrolides to be determined.
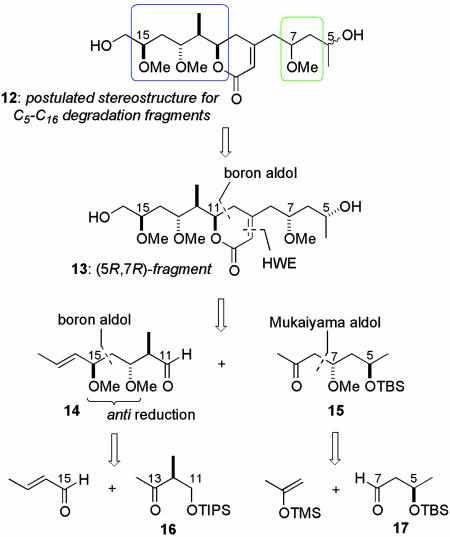
Initial Synthesis of the Postulated C5–C16 Degradation Fragment
Based on the uncertainty regarding the configurational relationship between the C11–C15 stereotetrad and the isolated methoxy-bearing stereocenter at C7, we designed a flexible and modular route to the C5–C16 degradation fragment, having the stereochemistry proposed as shown in Scheme 2 based inter alia on the findings of Gomez-Paloma and coworkers (16) and D'Auria and coworkers (17). Initially, the (5R,7R)-configured stereoisomer 13 was targeted, incorporating the C7 and C13 stereochemistry of the related macrolide scytophycin C (4) (1, Fig. 1). It was anticipated that the construction of the dihydropyranone ring could be accomplished by using an intramolecular Horner–Wadsworth–Emmons reaction. Further, it was envisaged that a substrate-controlled 1,5-anti-boron aldol coupling would provide the necessary stereoinduction from C7 to install the requisite C11 center, and 14 and 15 could be accessed by means of boron (20) and Mukaiyama (21) aldol-coupling strategies, respectively.
Scheme 2.
Retrosynthesis for the postulated C5–C16 degradation fragment.
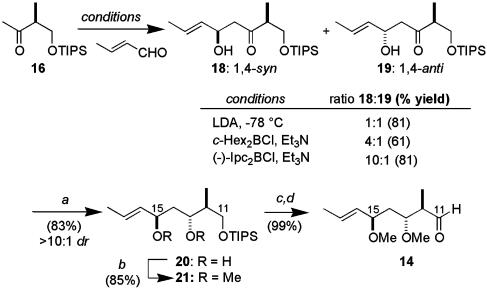
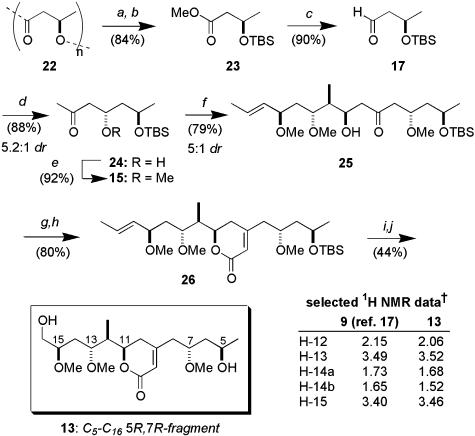
The convergent assembly of (5R,7R)-13 began with the aldol coupling of the methyl ketone 16 [prepared in 3 steps (51%) from methyl (S)-2-methyl-3-hydroxypropionate (22)] and crotonaldehyde (Scheme 3). Although reaction of the lithium enolate provided a 1:1 mixture of epimeric alcohols, which would find use in our later studies (see below), useful levels of diastereoselectivity could be realized for the 1,4-syn-adduct 18 when a boron aldol coupling was performed with c-Hex2BCl/Et3N (23, 24). The induction was further enhanced by using (-)-Ipc2BCl (20) to provide 18 in 10:1 dr.† This aldol adduct proved to be a suitable substrate for hydroxyl-directed reduction, securing a 1,3-anti-relationship between C13 and C15. By using Me4NBH(OAc)3 (26), diol 20 was obtained cleanly (83%, >10:1 dr). The stereochemical relationship between the hydroxyl-bearing centers was determined by nuclear Overhauser effect analysis of the corresponding p-methoxybenzylidene acetal (see supporting information for details). Conversion into the bis-methyl ether (NaH and MeI), was followed by cleavage of the triisopropylsilyl (TIPS) ether [tetra-n-butylammonium fluoride (TBAF)] and Dess–Martin oxidation, providing the aldehyde 14 (84%).
Scheme 3.
Stereocontrolled synthesis of aldehyde 14. Conditions: a, Me4NBH(OAc)3, MeCN-HOAc (3:1), -20°C; b, NaH, MeI, THF; c, TBAF, THF; d, Dess–Martin periodinane, NaHCO3, CH2Cl2.
Next, we turned to the preparation of the methyl ketone 15. Methanolysis of polyhydroxybutyrate 22 (MeOH, H2SO4) was followed by t-butyldimethylsilyl (TBS) protection. Controlled reduction of 23 (diisobutylaluminium hydride, -90°C) gave the corresponding enantiopure aldehyde (76%). Treatment with 2-(trimethylsiloxy)propene and Me2AlCl effected a 1,3-anti-selective (27) Mukaiyama aldol coupling with 5.2:1 dr.† After methylation of 24 with Meerwein's salt, we next explored the critical 1,5-anti-boron aldol coupling of the resulting ketone 15 and 14. By using our standard conditions (c-Hex2BCl, Et3N) (28), the desired adduct 25 was obtained in 70% yield (5:1 dr). With the six stereocenters of the postulated (5R,7R)-degradation fragment 13 secured, construction of the dihydropyranone moiety was sought. Yamaguchi esterification (29) of the β-hydroxyketone 25 with diethylphosphonoacetic acid provided the desired ester. Because of the inherent instability of this substance, it was treated directly with Ba(OH)2 in wet tetrahydrofuran (THF) (30, 31) to effect the Horner–Wadsworth–Emmons cyclization, thus generating the dihydropyranone 26 (80%), along with minor amounts (< 10%) of the corresponding elimination product. Finally, acidic removal of the TBS ether was followed by brief exposure to ozone (5–10 s) and reductive workup (NaBH4, MeOH) to selectively cleave the side chain alkene, generating the postulated C5–C16 degradation fragment 13 (44%).
Comparison of both the 1H and 13C NMR spectra for 13 with the data reported by D'Auria and colleagues (17) indicated it was clearly a diastereomer of the C5–C16 degradation fragment obtained from reidispongiolide A. The three methoxy resonances, reported (17) at δ 3.37, 3.40, and 3.45 ppm appear at δ 3.42, 3.43, and 3.48 ppm, respectively, for (7R,5R)-13. Although these inconsistencies may reflect an incorrect relationship between the methoxy-bearing stereocenter at C7 and the C11–C15 stereocluster, a number of more pronounced discrepancies (see comparison NMR data listed in Scheme 4) suggested the reported configurational assignment of the C5–C16 degradation fragment as 12 was erroneous. Understandably, the Naples group reported difficulties in assigning the relative configuration in the C13–C15 region of sphinxolide A because of the “rapid interconversion of different conformers with comparable populations” (16); in fact, eventual assignment of the relative stereochemistry at both C13 and C15 required reacquisition of some spectral data at lower temperatures. Based on these reported difficulties and the observed spectroscopic inconsistencies between 9 and 13, the stereostructure of this region of the parent sphinxolide/reidispongiolide macrolides, as depicted in the blue box of 8 (Scheme 1), now came under question.
Scheme 4.
Synthesis of the postulated C5–C16 degradation fragment 13. Conditions: a, MeOH, H2SO4; b, TBSCl, imidazole, dimethylformamide; c, diisobutylaluminium hydride, CH2Cl2, -90°C; d, 2-trimethylsiloxypropene, Me2AlCl, CH2Cl2, -90°C; e,Me3OBF4,CH2Cl2; f,(i) c-Hex2BCl, Et3N, Et2O, -78°C; (ii) 14,Et2O; g, diethylphosphonoacetic acid, DMAP, Et3N, 2,4,6-trichlorobenzoyl chloride, CH2Cl2, 0°C; h, Ba(OH)2, THF, 0°C; i, p-TsOH, MeOH, 0°C; j, O3, MeOH, -78°C; NaBH4, -78°C to rt. †, 500 MHz, CD3OD, δ (ppm).
Synthesis of Fragment Library for NMR Analysis
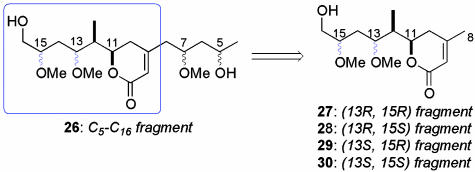
After this initial setback, we initiated a synthetic program to elucidate the relative stereochemistry within the C11–C15 region of the reidispongiolides/sphinxolides. As we expected, the remote C5 and C7 stereocenters would have little effect on the chemical shifts observed in the C11–C16 region of the degradation fragment, it was anticipated that access to a library of diastereomeric C8–C16 subunits would allow a confident configurational analysis. Although Kishi and coworkers (32, 33) have elegantly demonstrated the predictive ability of universal NMR databases for stereochemical assignment of acyclic compounds, this did not help the present situation. Our concerns regarding the assignment of C13 and C15 directed our initial efforts toward construction of the four C8–C16 diastereomers 27–30 detailed in Scheme 5. It was expected that the synthesis of this compound library would follow an route analogous to that disclosed above and, hence, discussion of this work is limited to the preparation of the (13R,15R)- and (13S,15R)-diastereomers 27 and 29, respectively.
Scheme 5.
Library of synthetic C8–C16 diastereomers.
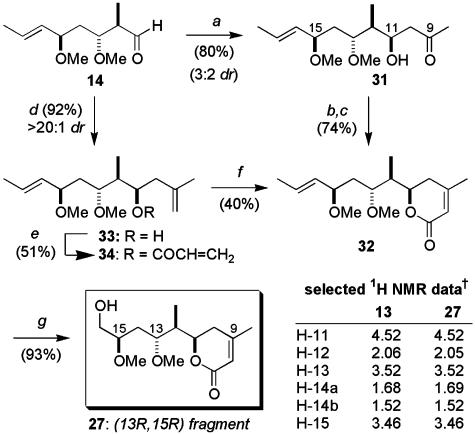
Having already prepared the aldehyde 14, the synthesis of the (13R,15R)-configured library member 27 was first investigated. Based on earlier work (27), we anticipated a useful level of induction for the Mukaiyama aldol coupling of 2-trimethylsiloxypropene and 14. In the event, only modest selectivity was observed for the BF3·OEt2-promoted reaction (3:2 dr, Scheme 6). Although other Lewis acids failed to offer any improvement, the diastereomeric aldol adducts were readily separable by flash chromatography and the major product, incorporating the (R)-configuration at C11,† was converted into the corresponding dihydropyranone 32 by using the Horner–Wadsworth–Emmons approach detailed above. Fortunately, the disappointing substrate control could be circumvented by relying instead on Brown's methodology (34) and ring-closing metathesis (35) to introduce the required dihydropyran moiety. Thus, treatment of 14 with the chiral methallylborane reagent derived from 2-methylpropene and (+)-Ipc2BOMe gave homoallylic alcohol 33 exclusively (Scheme 6). Esterification with acrylic acid [4-dimethylaminopyridine (DMAP) and N,N′-dicyclohexylcarbodiimide] provided 34, which underwent ring-closing metathesis mediated by Grubbs' second-generation ruthenium catalyst to give the dihydropyranone 32. Finally, brief exposure to ozone, followed by reductive workup (NaBH4), provided the (13R,15R)-fragment 27. Notably, its spectral data were in close agreement to those observed for the analogous region of the full synthetic C5–C16 degradation fragment 13 (Scheme 4), supporting our assertion that the isolated C5–C7 stereocluster would have minimal effect on the C11–C15 1H NMR chemical shifts.
Scheme 6.
Synthesis of the (13R,15R) fragment 27. Conditions: a, 2-(trimethylsiloxy)propene, BF3·OEt2,CH2Cl2, -90°C; b, diethylphosphonoacetic acid, DMAP, Et3N, 2,4,6-trichlorobenzoyl chloride, CH2Cl2,0°C; c, Ba(OH)2, THF, 0°C; d, (i) 2-methylpropene, n-BuLi, TMEDA, Et2O, -78°C to rt; (ii) (+)-Ipc2BOMe, Et2O; (iii) 14, Et2O, -78°C; e, acrylic acid, N,N′-dicyclohexyldicarbodiimide, DMAP, CH2Cl2; f, Grubbs' II, CH2Cl2,reflux; g,O3, MeOH, -78°C; NaBH4, -78°C to rt. †, 500 MHz, CD3OD, δ (ppm).
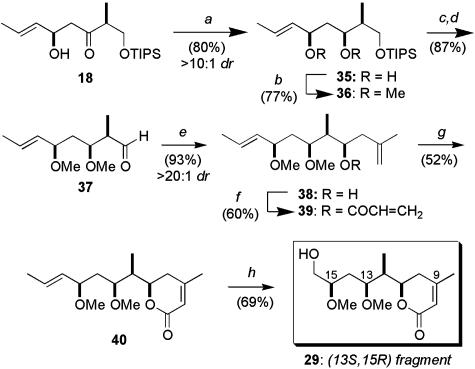
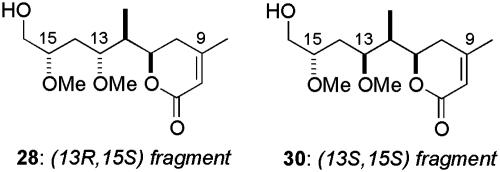
We next turned to the synthesis of the (13S,15R)-diastereomer 29, which now used a 1,3-syn-selective reduction (24) of the β-hydroxy ketone 18 (c-Hex2BCl and LiBH4), providing the diol 35 in good yield (80%, >10:1 dr, Scheme 7). The relationship between the hydroxyl-bearing stereocenters was determined by 13C NMR analysis of the corresponding acetonide using the method reported by Rychnovsky and Skalitzky (36). After our disappointment in the Mukaiyama aldol coupling of the related aldehyde 14 (Scheme 6), synthesis of the dihydropyranone again relied on the Brown methallylboration–ring-closing metathesis protocol.† Subsequent chemoselective ozonolysis, followed by reductive workup, generated the (13S,15R)-fragment 29. Synthesis of fragments 28 and 30 (Fig. 2), and hence completion of the library of C8–C16 diastereomers, involved treatment of the ketone 19, obtained previously from the lithium aldol reaction between methyl ketone 16 and crotonaldehyde (Scheme 3), in a manner identical with that presented in Schemes 6 and 7.
Scheme 7.
Synthesis of the (13S,15R) fragment 29. Conditions: a, (i) c-Hex2BCl, Et3N, -78°C; (ii) LiBH4; b, NaH, MeI, THF; c, TBAF, THF; d, Dess–Martin periodinane, NaHCO3,CH2Cl2; e,(i) 2-methylpropene, n-BuLi, TMEDA, Et2O, -78°C to rt; (ii) (+)-Ipc2BOMe, Et2O; (iii) 37, -78°C; f, acrylic acid, N,N′-dicyclohexyldicarbodiimide, DMAP, CH2Cl2; g, Grubbs' II, CH2Cl2, reflux; h, O3, MeOH, -78°C; NaBH4, -78°C to rt.
Fig. 2.
Additional synthetic C8–C16 diastereomers.
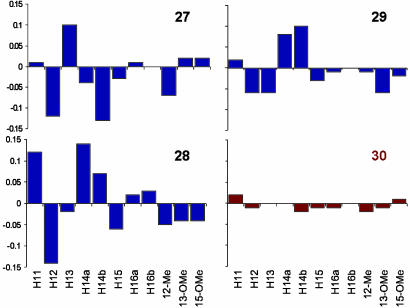
Examination of the 1H and 13C NMR spectra of the fragment library in CD3OD revealed distinctly different chemical shifts for each of the diastereomers 27–30. Comparative 1H NMR data are presented in Fig. 3 as the difference in ppm between each individual proton resonance in the fragments 27–30 and those reported for the analogous protons of the natural C5–C16 degradation fragment 9 (17). This analysis convincingly defines the relative stereochemistry within this region of the degradation fragment, and consequently reidispongiolide A, as that represented by the (13S,15S)-diastereomer 30.
Fig. 3.
Comparative 1H NMR analysis of the fragment library. Bars represent deviation in ppm between individual proton chemical shifts observed for the diastereomers 27–30 and those reported for the natural C5–C16 degradation fragments 917 (500 MHz, CD3OD).
Configurational Assignment of the C5–C16 Degradation Fragment of Reidispongiolide A
Confident in this reassignment of the relative configuration within the isolated C11–C15 stereocluster, the establishment of a relationship between this subunit and the isolated C7 methoxy-bearing stereocenter in the parent macrolide was investigated next. We anticipated that a synthesis of potential C5–C16 degradation fragments, bearing the relative stereochemistry defined by 30 and either (R)- or (S)-configuration at C7, would enable stereochemical determination of the C5–C16 degradation fragment 9. In particular, we expected that subtle differences in the chemical shift of the diastereotopic methylene protons at C8 and C10 would indicate the correct relative configuration. With this in mind, we designed a flexible aldol-based approach, using ketone 45 (or ent-45) and aldehyde 48 (Schemes 8, 9, 10). In addition, as the C5–C16 degradation fragment 9, obtained from the ozonolysis/reduction of reidispongiolide A, was isolated as a 2:1 mixture of C5 epimers (17), our planned approach incorporated a chemoselective ozonolysis-reduction sequence of both a C5 methylene and C15 ethylidene in the presence of the dihydropyranone to access both C5 epimers in each series.
Scheme 8.
Synthesis of the enantiomeric methyl ketones 45 and ent-45. Conditions: a, (i) 2-methylpropene, n-BuLi, TMEDA, Et2O, -78°C to rt; (ii) (+)-Ipc2BOMe, Et2O; (iii) 41, -78°C; b, NaH, MeI, THF; c, TBAF, THF; d, Dess–Martin periodinane, NaHCO3, CH2Cl2; e, MeLi, THF, -78°C.
Scheme 9.
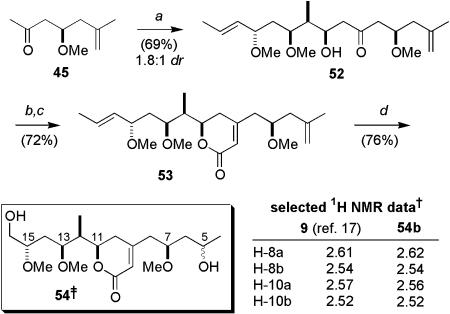
Synthesis of the (7R)-epimer of the revised C5–C16 degradation fragment. Conditions: a, Me4NBH(OAc)3, MeCN-HOAc (3:1), -20°C; b, NaH, MeI, THF; c, TBAF, THF; d, Dess–Martin periodinane, NaHCO3, CH2Cl2; e, (i) ent-45, c-Hex2BCl, Et3N, -78°C, Et2O; (ii) 48; f, diethylphosphonoacetic acid, DMAP, Et3N, 2,4,6-trichlorobenzoyl chloride, CH2Cl2,0°C; g, Ba(OH)2, THF, 0°C; h, O3, MeOH, -78°C; NaBH4, -78°C to rt. †, 500 MHz, CD3OD, δ (ppm). ‡, Isolated as 1:2 mixture of (5R)-51a/(5S)-51b.
Scheme 10.
Synthesis of the (7S)-epimer of the revised C5–C16 degradation fragment. Conditions: a, (i) 45, (-)-Ipc2BCl, Et3N, -78°C, Et2O; (ii) 48; b, diethylphosphonoacetic acid, DMAP, Et3N, 2,4,6-trichlorobenzoyl chloride, CH2Cl2, 0°C; c, Ba(OH)2, THF, 0°C; d, O3, MeOH, -78°C; NaBH4, -78°C to rt. †, 500 MHz, CD3OD, δ (ppm). ‡, Isolated as 1:2 mixture of (5S)-54a/(5R)-54b.
As outlined in Scheme 8, we first prepared the methyl ketones 45 and ent-45, to access both possible relationships between the C7 stereocenter and the C11–C15 stereotetrad. The aldehyde 41 was treated with the methallylborane reagent derived from 2-methylpropene and (+)-Ipc2BOMe (34) to afford the homoallylic alcohol 42 in 85% yield (96% enantiomeric excess).‡ After methyl ether formation, the TBS group was removed to give alcohol 44 in good yield. Oxidation to the aldehyde, addition of MeLi, and subsequent reoxidation provided the volatile methyl ketone 45. By using (-)-Ipc2BOMe, ent-45 was prepared from 41 in an analogous manner. The specific rotation of the secondary alcohols 42 and ent-42 in CHCl3 were +2.9 and -2.8, respectively.
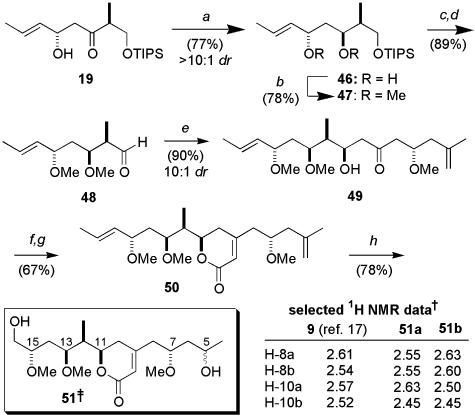
We next examined the coupling of the ketone ent-45 and the aldehyde 48, which had been previously used for the construction of both 28 and 30 (Fig. 2), as would be required for the C5–C16 fragment library. The synthesis of 48 commenced with a 1,3-anti-selective reduction (Me4NBH(OAc)3) of ketone 19 (Scheme 3). Conversion into the bis-methyl ether was then followed by removal of the TIPS group and Dess–Martin oxidation (89%) to afford the desired aldehyde 48. By following our standard protocol (27), the 1,5-anti-boron aldol coupling between 48 and the methyl ketone ent-45 proceeded smoothly, affording the desired aldol adduct in high yield and diastereoselectivity (90%, 10:1 dr).§ Completion of the synthesis of the (7R)-degradation fragments 51 involved formation of the diethylphosphonoacetate, which was directly treated with Ba(OH)2 to provide the Horner–Wadsworth–Emmons product 50 in 67% overall yield. Finally, brief subjection of 50 to ozone and addition of NaBH4 yielded the alcohols 51 as a 2:1 mixture of epimers at C5, which were separated by HPLC. Spectroscopic analysis of alcohols 51 (500 MHz, CD3OD) indicated that they were clearly diastereomers of the C5–C16 degradation fragment 9 (17). Most notably, in the 1H NMR spectra of the purified alcohols, the resonances corresponding to the diastereotopic methylene protons at C8 and C10 were significantly different in chemical shift from those reported for the naturally derived material (Scheme 9) (17). Although this result suggested an incorrect relative configuration at C7 in the alcohols 51, it remained to synthesize the corresponding fragment with the opposite or (S)-configuration at C7 to corroborate this proposal. The synthesis of the (7S) C5–C16 fragment is detailed in Scheme 10. By following our standard conditions (20), the aldol coupling between 45 and 48 with (-)-Ipc2BCl/Et3N afforded the desired β-hydroxyketone 52 as the major adduct.§ In this case, the inherent 1,5-anti-selectivity observed in the coupling of ent-45 and 48 (Scheme 9) was overturned by appropriate choice of Ipc ligand chirality. Completion of the synthesis of (7S)-degradation fragment involved an analogous sequence of reactions to those discussed above, affording the alcohols 54 as a 2:1 mixture of epimers at C5, which were separated by HPLC. Gratifyingly, the spectral data obtained for these alcohols were in good agreement to that reported by D'Auria and coworkers for the C5–C16 fragment obtained in their degradation work (17), thus allowing our assignment of the relationship between the remote stereocenter at C7 and the C11–C15 stereocluster in the parent macrolide reidispongiolide A (7).¶
Conclusion
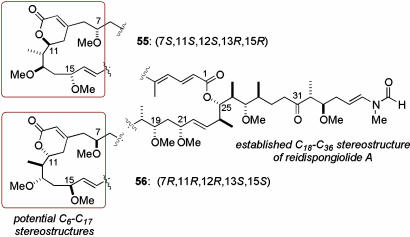
Based on the proposed relative configuration within the C11–C15 subunit of the reidispongiolide/sphinxolide family of cytotoxic marine macrolides, an efficient aldol-based synthesis of the postulated C5–C16 degradation fragment of reidispongiolide A was accomplished. Subsequently, several inconsistencies observed between the spectral data recorded for the synthetic and naturally derived fragments led us to construct a library of C8–C16 and C5–C16 stereoisomers and confidently reassign the relative stereochemistry within this region of reidispongiolide A. At present, the full macrolide may be represented by either stereostructure 55 or 56 (Fig. 4), where the final assignment will rely on a stereocontrolled synthesis of the macrocyclic core and reidispongiolide A itself.
Fig. 4.
The two potential stereostructures of reidispongiolide A, incorporating the revised C5–C16 stereochemistry determined in this work.
Supplementary Material
Acknowledgments
We thank Dr. Ray Finlay (AstraZeneca) for helpful discussions and Professor D'Auria (University of Naples “Federico II,” Naples) for providing copies of their NMR spectra for the natural reidispongiolide degradation fragments and related helpful correspondence. This work was supported by Engineering and Physical Sciences Research Council Grant GR/S19929, a Natural Sciences and Engineering Research Council Canada Fellowship (to R.B.), an Ernst-Schering Research Foundation Fellowship (to H.K.), and AstraZeneca.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DMAP, 4-dimethylaminopyridine; Ipc, isopinocampheyl; TBAF, tetra-n-butylammonium fluoride; TBS, t-butyldimethylsilyl; THF, tetrahydrofuran; TIPS, triisopropylsilyl; rt, room temperature.
Footnotes
Absolute configuration of the generated carbinol stereocenter was determined by spectroscopic analysis of the corresponding α-methoxy-α-trifuoromethylphenylacetic acid esters (25) (see supporting information for details).
Absolute configuration of the generated carbinol stereocenter was determined by spectroscopic analysis of the corresponding α-methoxy-α-trifuoromethylphenylacetic acid esters (25) (see supporting information for details). The enantiomeric excess was determined by analysis of the 19F NMR spectra of the derived α-methoxy-α-trifuoromethylphenylacetic acid esters.
The relative configuration of the generated carbinol center was determined by careful inspection of the 1H NMR chemical shifts (500 MHz, CDCl3) of the diastereotopic methylene protons at C10 with the method reported by Roush et al (37).
Unfortunately, we were unable to obtain an authentic sample of the natural degradation fragment for chiral HPLC comparison with our synthetic sample. Although no separate data have been reported for the individual C5 epimers of the C5–C16 degradation fragment 9, the specific rotation of the 2:1 mixture of alcohols 54 ([α]D = +28.2, MeOH) differed from that reported for the 2:1 mixture of alcohols 9 ([α]D = 0.0, MeOH) (17) obtained from reidispongiolide A.
References
- 1.Kabsch, W. & Vandekerckhove, J. (1992) Annu. Rev. Biophys. Biomol. Struct. 21, 49-76. [DOI] [PubMed] [Google Scholar]
- 2.Hamel, E. (1996) Med. Res. Rev. 16, 207-231. [DOI] [PubMed] [Google Scholar]
- 3.Fenteany, G. & Zhu, S. T. (2003) Curr. Top. Med. Chem. 3, 593-616. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi, M., Moore, R. E., Patterson, G. M. L., Xu, C. F. & Clardy, J. (1986) J. Org. Chem. 51, 5300-5306. [Google Scholar]
- 5.Yamada, K., Ojika, M., Ishigaki, T., Yoshida, Y., Ekimoto, H. & Arakawa, M. (1993) J. Am. Chem. Soc. 115, 11020-11021. [Google Scholar]
- 6.Guella, G., Mancini, I., Chiasera, G. & Pietra, F. (1989) Helv. Chim. Acta 72, 237-246. [Google Scholar]
- 7.D'Auria, M. V., Gomez Paloma, L., Minale, L., Zampella, A., Verbist, J. F., Roussakis, C., Debitus, C. & Patissou, J. (1993) Tetrahedron 49, 8657-8664. [Google Scholar]
- 8.D'Auria, M. V., Gomez Paloma, L., Minale, L., Zampella, A., Verbist, J. F., Roussakis, C., Debitus, C. & Patissou, J. (1994) Tetrahedron 50, 4829-4834. [Google Scholar]
- 9.Yeung, K.-S. & Paterson, I. (2002) Angew. Chem. Int. Ed. Engl. 41, 4632-4653. [DOI] [PubMed] [Google Scholar]
- 10.Klenchin, V. A., Allingham, J. S., King, R., Tanaka, J., Marriott, G. & Rayment, I. (2003) Nat. Struct. Biol. 10, 1058-1063. [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X. Q., Minale, L., Zampella, A. & Smith, C. D. (1997) Cancer Res. 57, 3751-3758. [PubMed] [Google Scholar]
- 12.Paterson, I., Britton, R., Delgado, O. & Wright, A. E. (2004) Chem. Commun., 632-633. [DOI] [PubMed]
- 13.Paterson, I., Chen, D. Y.-K., Coster, M. J., Acena, J. L., Bach, J., Gibson, K. R., Keown, L. E., Oballa, R. M., Trieselmann, T., Wallace, D. J., Hodgson, A. P. & Norcross, R. D. (2001) Angew. Chem. Int. Ed. Engl. 40, 4055-4060. [PubMed] [Google Scholar]
- 14.Paterson, I., De Savi, C. & Tudge, M. (2001) Org. Lett. 3, 3149-3152. [DOI] [PubMed] [Google Scholar]
- 15.Paterson, I., Florence, G. J., Gerlach, K., Scott, J. P. & Sereinig, N. (2001) J. Am. Chem. Soc. 123, 9535-9544. [DOI] [PubMed] [Google Scholar]
- 16.Bassarello, C., Bifulco, G., Zampella, A., D'Auria, M. V., Riccio, R. & Gomez-Paloma, L. (2001) Eur. J. Org. Chem. 2001, 39-44. [Google Scholar]
- 17.Zampella, A., Bassarello, C., Bifulco, G., Gomez-Paloma, L. & D'Auria, M. V. (2002) Eur. J. Org. Chem. 2002, 785-790. [Google Scholar]
- 18.Paterson, I., Ashton, K., Britton, R. & Knust, H. (2003) Org. Lett. 5, 1963-1966. [DOI] [PubMed] [Google Scholar]
- 19.Zampella, A., Sepe, V., D'Orsi, R., Bifulco, G., Bassarello, C. & D'Auria, M. V. (2003) Tetrahedron Asymmetry 14, 1787-1798. [Google Scholar]
- 20.Cowden, C. J. & Paterson, I. (1997) Org. React. 51, 1-200. [Google Scholar]
- 21.Mukaiyama, T. (1982) Org. React. 28, 203-331. [Google Scholar]
- 22.Paterson, I. & Oballa, R. M. (1997) Tetrahedron Lett. 38, 8241-8244. [Google Scholar]
- 23.Paterson, I., Goodman, J. M. & Isaka, M. (1989) Tetrahedron Lett. 30, 7121-7124. [Google Scholar]
- 24.Paterson, I. & Perkins, M. V. (1992) Tetrahedron Lett. 33, 801-804. [Google Scholar]
- 25.Ohtani, I., Kusumi, T., Kashman, Y. & Kakisawa, H. (1991) J. Am. Chem. Soc. 113, 4092-4096. [Google Scholar]
- 26.Evans, D. A., Chapman, K. T. & Carreira, E. M. (1988) J. Am. Chem. Soc. 110, 3560-3578. [Google Scholar]
- 27.Evans, D. A., Dart, M. J., Duffy, J. L. & Yang, M. G. (1996) J. Am. Chem. Soc. 118, 4322-4343. [Google Scholar]
- 28.Paterson, I., Gibson, K. R. & Oballa, R. M. (1996) Tetrahedron Lett. 37, 8585-8588. [Google Scholar]
- 29.Imanaga, J., Hirata, K., Saeki, T., Katsuki, T. & Yamaguchi, M. (1979) Bull. Chem. Soc. Jpn. 52, 1989-1993. [Google Scholar]
- 30.Paterson, I., Yeung, K.-S. & Smaill, J. B. (1993) Synlett, 774-776.
- 31.Alvarez-Ibarra, C., Arias, S., Bañón, G., Fernández, M. J., Rodríguez, M. & Sinisterra, V. (1987) J. Chem. Soc. Chem. Commun., 1509-1511.
- 32.Kobayashi, Y., Lee, J., Tezuka, K. & Kishi, Y. (1999) Org. Lett. 1, 2177-2180. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J., Kobayashi, Y., Tezuka, K. & Kishi, Y. (1999) Org. Lett. 1, 2181-2184. [DOI] [PubMed] [Google Scholar]
- 34.Brown, H. C., Jadhau, P. K. & Perumal, P. T. (1984) Tetrahedron Lett. 25, 5111-5114. [Google Scholar]
- 35.Ramachandran, P. V., Chandra, J. S. & Reddy, M. V. R. (2002) J. Org. Chem. 67, 7547-7550. [DOI] [PubMed] [Google Scholar]
- 36.Rychnovsky, S. D. & Skalitzky, D. J. (1990) Tetrahedron Lett. 31, 945-948. [Google Scholar]
- 37.Roush, W. R., Bannister, T. D., Wendt, M. D., Van Nieuwenhze, M. S., Gustin, D. J., Dilley, G. J., Lane, G. C., Scheidt, K. A. & Smith, W. J. (2002) J. Org. Chem. 67, 4284-4289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.