Abstract
All 16 stereoisomers of the sex pheromone of pine sawfly (3,7,11-trimethylundecanol propanoate ester) have been synthesized on a 10- to 20-mg scale by a split-parallel fluorous mixture-synthesis approach. Spectral data obtained for all 32 compounds (16 alcohols and the corresponding propionates) matched well with published data, thereby validating the fluorous-tag encoding of diastereoisomers. This fluorous-tag encoding method is recommended for the efficient synthesis of multiple stereoisomers for spectroscopic studies, biological tests, or other structure–function relationships.
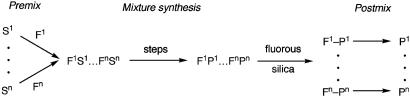
Fluorous mixture synthesis (FMS) is a solution-phase technique that captures the efficiency inherent in mixing compounds yet still allows for an orderly separation of the mixtures to provide individual pure target products in the end (1–6). In a “premix” stage (Fig. 1) individual substrates are prepared and tagged (protected) with homologous fluorous groups, and the tagged compounds are mixed. The molecules are elaborated during the “mixture-synthesis” stage. Here, the benefit of the tags is reaped, because the number of reactions and separations required is divided by the number of tagged compounds in the mixture. Well planned splits can further increase compound numbers while minimizing effort (4). The mixture-synthesis stage ends with demixing, which is a chromatographic separation over fluorous silica gel of each mixture into its individual tagged components. In the final “postmix” stage, the tags are removed in parallel to produce the target products. The problems of analysis, separation, and identification inherent in mixture synthesis (7, 8) are all addressed by this tagging and demixing strategy.
Fig. 1.
Stages of FMS. Premix: synthesize substrates S, encode with homologous fluorous tags F, and mix. Mixture synthesis: elaborate the target molecules by solution-phase reactions and demix the final mixture by fluorous chromatography. Postmix: remove the tags in parallel to reveal the target products P.
The initial tagging step introduces a simple code between the fluorous tag and the substrate that is tagged. This code is “decoded” in the demixing stage by order of elution of the tagged products during chromatography because fluorous silica gel separates primarily based on fluorine content (9). When enantiomers are tagged in the technique of quasiracemic synthesis (5), the decoding cannot fail, because the tagged domains of the molecules are identical and only the tags are different. When nonisomeric tagged analogs are demixed, the decoding can be verified readily and reliably by HPLC-MS (3, 4). But when diastereomers are tagged, there is no independent method to verify that the mixtures have been decoded properly by the demixing. In principle, spectra can be recorded to assign stereostructures to the final products, but the exercise then becomes circular, because the synthesis is typically undertaken to assign spectra from the stereostructures (8).
We sought to show in principle that demixing could successfully decode mixtures to provide all 16 stereoisomers of a natural product bearing four stereocenters. To avoid the above assignment by circular logic, we required for comparison 16 known stereoisomers of a natural product that have been prepared by unambiguous asymmetric synthesis routes. Outside of the familiar sugars (aldohexoses, for example, contain four stereocenters), there are precious few examples of asymmetric syntheses of all stereoisomers of natural products with four stereocenters. This dearth surely is not due to a lack of interest, because stereostructure is a prime target for variation in any type of structure–function study. Also, methods for stereoselective synthesis abound, so this cannot be the reason either. Clearly it is simply too much work to make 16 isomers of even relatively simple natural products by traditional means.
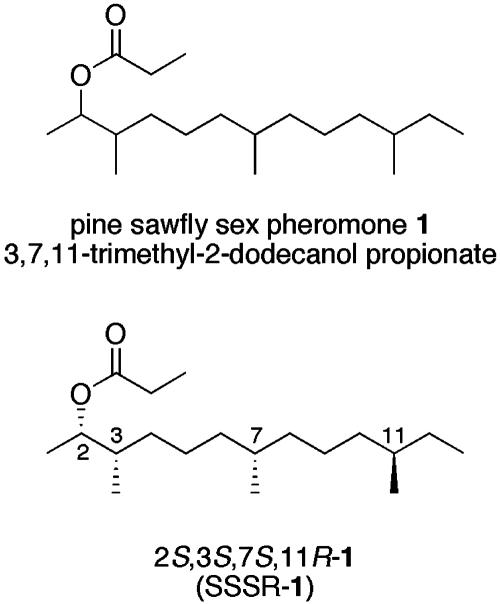
Hedenström and coworkers (10) rose to the challenge and made all 16 isomers of the sex pheromone 1 of the pine sawfly Microdiprion pallipes (3,7,11-trimethyl-2-tridecyl propionate) both as a mixture and in pure form in a Herculean effort. At least two of these isomers [2S,3S,7S,11R (SSSR-1) and 2S,3S,7S,11S (SSSS-1)] are present in females of this pine sawfly (11, 12). Before that, Nakamura and Mori (13) reported synthesis of the 2S,3S,7S,11R and 2S,3R,7R,11R isomers of this pheromone. By way of example, the former isomer is shown below. SSSR-1 and all pheromone structures in this article are presented with the backbone in the plane of the page such that when any chain substituent (Me or OH) is above the plane, the absolute configuration of its stereocenter is R, and when any chain substituent is below the plane, the absolute configuration of its stereocenter is S. We report herein the asymmetric syntheses of all 16 stereoisomers of 1 by a strategy that combines FMS with splitting. Successful decoding of all 16 isomers was validated by comparison with the published data of Hedenström and coworkers (10). Substantial amounts (10–20 mg) of each of the final products have been produced.
Materials and Methods
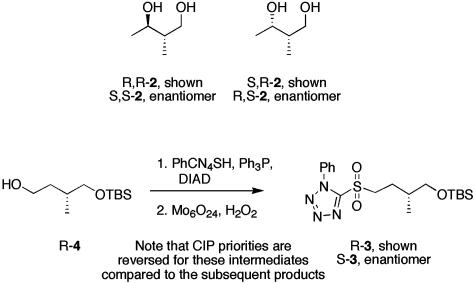
The building blocks needed for the synthesis are shown in Scheme 1. Diols R,R-2 and S,S-2 are known compounds that were prepared by standard procedures (14, 15). R,S-2 and S,R-2 were prepared by Mitsunobu reactions of the appropriate β-hydroxybutyrate esters, followed by reduction. N-phenyltetrazole sulfones R-3 and S-3 were prepared from known alcohol 4 by Mitsunobu reaction with N-phenyltetrazolyl thiol (PhCN4SH) and diisopropylazodicarboxylate followed by oxidation.
Scheme 1.
Note that Cahn–Ingold–Prelog priorities are reversed for these intermediates compared with the subsequent products.
Experimental details for the representative key intermediates M-7 and M-12 involved in the FMS are described here. Experimental details for other intermediates are described in ref. 16.
(3R)-5-[4-(tert-Butyldimethylsilanyloxy)-3-methylbutane-1-sulfonyl]-1-phenyl-1H-tetrazole (R-3). Diisopropylazodicarboxylate (3.8 ml) was added via a syringe to a solution of (3R)-4-(tert-butyldimethylsilanyloxy)-3-methylbutan-1-ol (R-4) (3.47 g, 15.9 mmol), triphenylphosphine (5.0 g, 19.1 mmol), and 1-phenyl-1H-tetrazole-5-thiol (PhCN4SH, 3.4 g, 19.1 mmol) in tetrahydrofuran (THF) (120 ml). After stirring at room temperature for 3 h, the reaction mixture was diluted with ethanol (120 ml) and cooled to 0°C. (NH4)6Mo7O24·4H2O (2.97 g, 2.4 mmol) and 30% hydrogen peroxide (24.2 ml, 237 mmol) were added, and the reaction mixture was warmed to room temperature. After stirring at room temperature for 14 h, water (200 ml) was added, and the reaction mixture was extracted with dichloromethane (5 × 70 ml). The organic layers were combined, washed with brine, dried with magnesium sulfate, and concentrated. After flash column chromatography on silica gel (5:1 hexane/ethyl acetate), (3R)-5-[4-(tert-butyldimethylsilanyloxy)-3-methylbutane-1-sulfonyl]-1-phenyl-1H-tetrazole (R-3) was isolated as a colorless oil (5.28 g, 81%).
(4R,5S)-(4,5-Dimethyl-2-[4-(4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluorononyloxy)phenyl]-[1,3]dioxane (RS-11b). (2R,3S)-2-Methylbutane-1,3-diol (1.1 g, 10.6 mmol) was dissolved in THF (100 ml) and cooled to 0°C by using an ice bath. Triethylamine (11.8 ml, 84.6 mmol) was added. Chlorotrimethylsilane (10.7 ml, 84.6 mmol) was added slowly, and the reaction mixture was warmed to room temperature. After 16 h at room temperature, the reaction was quenched with saturated sodium bicarbonate solution (50 ml), and the layers were separated. The aqueous layer was extracted with ether (2 × 50 ml). The organic layers were combined, washed with brine, and concentrated. Fast flash column chromatography on silica gel (20:1:0.1 hexane/ethyl acetate/triethylamine) gave (2R,3S)-2-methyl-1,3-bis(trimethylsilanyloxy)butane (2.20 g, 84%), which was used in the next step without characterization.
Trimethylsilyltriflate (60 μl, 0.33 mmol) was added to dichloromethane (75 ml) and cooled to -78°C. A solution of (2R,3S)-2-methyl-1,3-bis(trimethylsilanyloxy)butane (1.98 g, 7.97 mmol) in dichloromethane (15 ml) was added. Then a solution of 4-(4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluorononyloxy)benzaldehyde (3.2 g, 6.64 mmol) in dichloromethane (30 ml) was added slowly. After stirring at -78°C for 12 h, the reaction was quenched by adding pyridine (0.3 ml) and warmed to room temperature. Ethyl acetate (100 ml) and saturated sodium bicarbonate (100 ml) were added and stirred for 10 min. The phases were separated, and the aqueous layer was extracted with ether (3 × 25 ml). The organic layers were combined, washed with brine, dried with magnesium sulfate, and concentrated. Flash column chromatography on silica gel (20:1 hexane/ethyl acetate) gave (4R,5S)-(4,5-dimethyl-2-[4-(4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluorononyloxy)phenyl]-[1,3]dioxane (RS-11b) as a white solid (3.06 g, 81%).
3-(Fluorous-4-methoxybenzyloxy)-2-methylbutyraldehyde M-7. A solution of diisobutyl aluminum hydride in hexanes (1 M, 33.4 ml, 33.4 mmol) was added slowly to a solution of M-11 (6.47 g, 11.14 mmol; calculated based on average molecular weight of the mixture) in dichloromethane (340 ml) at 0°C. After stirring at 0°C for 3 h, the reaction mixture was quenched by the addition of saturated ammonium chloride solution (50 ml) and saturated sodium potassium tartrate solution (300 ml), and the resulting suspension was stirred vigorously for 1 h. The crude reaction mixture was extracted with ether (4 × 100 ml). The organic layers were combined, washed with brine, dried with magnesium sulfate, and concentrated. Flash column chromatography on silica gel (3:1 hexane/ethyl acetate) gave the primary alcohol mixture 3-(fluorous-4-methoxybenzyloxy)-2-methylbutan-1-ol (6.45 g, 99%).
Dess–Martin periodinane (5.6 g, 13.2 mmol) was added to a solution of the mixture of primary alcohols 3-(fluorous-4-methoxybenzyloxy)-2-methylbutan-1-ol (6.42 g, 11.0 mmol) in dichloromethane (300 ml). After stirring at room temperature for 3 h, the reaction mixture was quenched by the addition of saturated sodium bicarbonate solution (200 ml) and sodium thiosulfate solution (200 ml) and stirred for 30 min. The phases were separated, and the aqueous layer was extracted with ether (3 × 100 ml). The organic layers were combined, washed with brine, dried with magnesium sulfate, and concentrated. Flash column chromatography on silica gel (7:3 hexane/ethyl acetate) gave aldehyde M-7 (5.98 g, 94%).
tert-Butyl-[7-(fluorous-4-methoxybenzyloxy)-2,6-dimethyloct-4-enyloxy]dimethyl-silane M-12R. A solution of sodium hexamethyldisilazide in THF (1 M, 6.1 ml, 6.1 mmol) was added slowly to a solution of sulfone S-3 (2.71 g, 6.62 mmol) in THF (100 ml) at -78°C. After stirring at -78°C for 30 min, a solution of M-7 (2.83 g, 4.87 mmol) in THF (20 ml) was added slowly via a syringe. After stirring at -78°C for 2 h, the reaction mixture was quenched with saturated ammonium chloride (100 ml) and water (100 ml) and warmed to room temperature. The reaction mixture was extracted with ether (5 × 100 ml). The organic layers were combined, washed with brine, dried with magnesium sulfate, and concentrated. Flash column chromatography on silica gel (10:1 hexane/ethyl acetate) gave alkene M-12R (3.51 g, 94%).
Results and Discussion
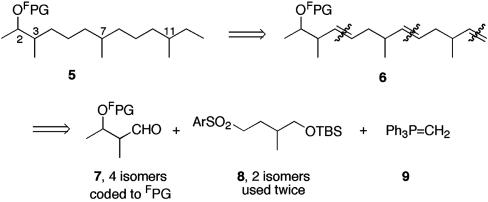
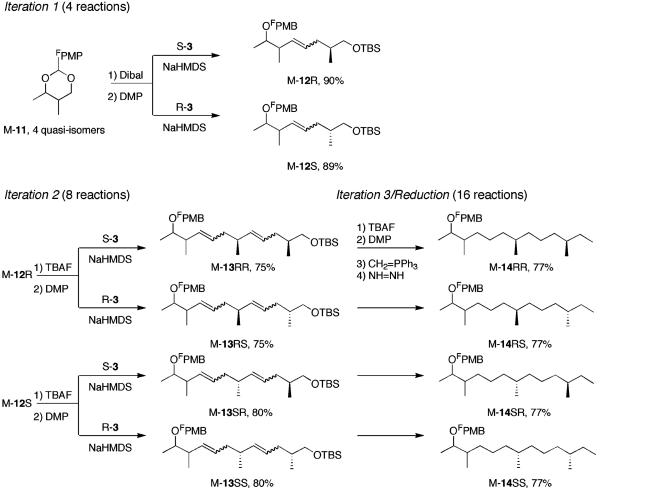
Retrosynthetic Analysis. Protected alcohol 5 was targeted as the final intermediate before demixing, and the plan for its synthesis is summarized in Fig. 2. Formal dehydrogenation of 5 produced triene 6, which in turn could be prepared from the three building blocks, 7–9, by sequential olefination reactions. The plan calls for synthesis of all four stereoisomers of 7 coded to fluorous tags in the protecting group (FPG), followed by mixing. This mixture of “quasidiastereomers” (“quasi” because the tags differ, so they are not isomers) is split twice for successive coupling with each enantiomer of 8, and then each of the four mixtures is endcapped with 9, followed by hydrogenation. This process results in four mixtures of four compounds 5 that should fully encode the configurations of the 16 pheromones. The configurations at C7 and C11 are encoded spatially by the mixture that a given quasiisomer is in, and the configurations at C2 and C3 are encoded by the fluorous tags within each mixture. Four demixings should give 16 pure isomers of 5.
Fig. 2.
Retrosynthetic analysis. FPG is a fluorous protecting group (tag).
This strategy calls for introducing all stereocenters through the building blocks, so there is no need for diverging stereoselective reactions during the mixture synthesis. Also, the inherent efficiency in mixture synthesis is nicely illustrated by the tactic of using building block 8 twice, which allows for an iterative approach in which a simple sequence of deprotection, oxidation, and olefination is repeated three times. In traditional parallel synthesis, the last sequence alone would require 48 reactions (3 steps times 16 substrates), so it would almost always be easier to make another building block to avoid this effort. The total plan in Fig. 2 calls for only 32 synthetic reactions, including all three iterations of the olefination and the hydrogenation.
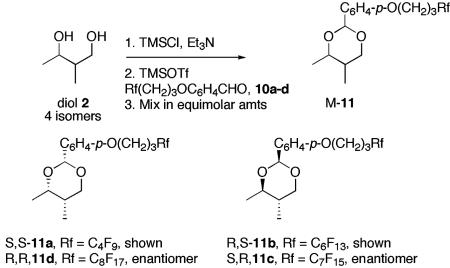
The Premix Stage. The syntheses of the six building blocks (all four isomers of 2-methyl-1,3-butane diol 2 and the two enantiomers of phenylsulfonyl tetrazole 3) were straightforward and are summarized in Materials and Methods. In the last step of the premix stage, each diol 2 was reacted individually with a different fluorous p-methoxybenzaldehyde derivative 10a–10d by the method of Noyori and coworkers (17) to code its configuration to the fluoroalkyl group in the tagged acetals 11a–11d (Fig. 3). These acetals then were mixed to start the core of the synthesis.
Fig. 3.
(Upper) Encoding stereocenters with fluorous tags. (Lower) Coding of configuration to perfluoroalkyl.
The Mixture Stage. Mixture synthesis. The mixture stage of the synthesis is summarized in Fig. 4. This stage consisted of three iterative sequences of deprotection of a 1°-alcohol, oxidation to an aldehyde, and olefination. The mixture was split before the first and second olefinations, but not the third (end capping). The last iteration was followed by hydrogenation to remove all the double bonds. Mixture M-11 (6.4 g) was reduced with diisobutyl aluminum hydride to open the acetal (18), and the alcohol was oxidized with Dess–Martin periodinane to give an aldehyde, which was split into two portions. One portion (2.8 g) was coupled in a one-pot Julia reaction (19) with sulfone S-3 to give alkene M-12R in 94% yield, whereas the other portion (3.1 g) was coupled with enantiomeric sulfone R-3 to give alkene M-12S in 93% yield.
Fig. 4.
The FMS stage. FPMP, fluorous p-methoxyphenyl group; FPMB, fluorous p-methoxybenzyl group; Dibal, diisobutyl aluminum hydride; DMP, Dess–Martin periodinane; NaHMDS, sodium hexamethyl disilazide; TBS, t-butyldimethlysilyl; TBAF, tetrabutylammonium fluoride.
Mixtures M-12R and M-12S were subjected to a series of t-butyldimethylsilyl deprotection (100% yield for both) and oxidation (81% and 87% yield, respectively) to give aldehydes (2.3 and 2.6 g, respectively), which were split into two portions and coupled with sulfones S-3 or R-3 to give four alkenes: M-13RR (1.4 g), M-13RS (1.2 g), M-13SR (1.6 g), and M-13SS (1.4 g). Once again, the Julia olefinations proceeded in high yields. Alkene mixtures M-13 were subjected to last iteration of deprotection (100% yield), oxidation (85% yield), and Wittig reaction with methyl triphenylphosphonium iodide (97% yield). Diimide reduction (94% yield) gave protected pheromone precursors as four mixtures: M-14RR, -RS, -SR, and -SS.
Mixture characterization. All the mixtures shown in Fig. 4 were characterized by HPLC-MS over a Fluofix column with atmospheric-pressure chemical ionization. In all the cases, tag-based separations were observed. The identity of the products could be readily ascertained by the mass spectrum of each peak. Just as in regular solution-phase syntheses of single compounds, the relative retention factors of the starting and product mixtures on standard silica gel were routinely used to evaluate the progress of reactions. Although each mixture consisted of four compounds (neglecting the geometric isomers around double bonds), each behaved like a single compound during silica TLC analysis. The compounds with the stereoisomeric organic fragments but different fluorous tags also did not separate during flash chromatography on normal silica gel, so this was routinely used for mixture purification without demixing. Liquid chromatography NMR spectroscopy was also used to characterize seven of the intermediate mixtures. In the cases of intermediates containing mixtures of geometric isomers around the internal double bond, the mixture was analyzed after a diimide reduction.
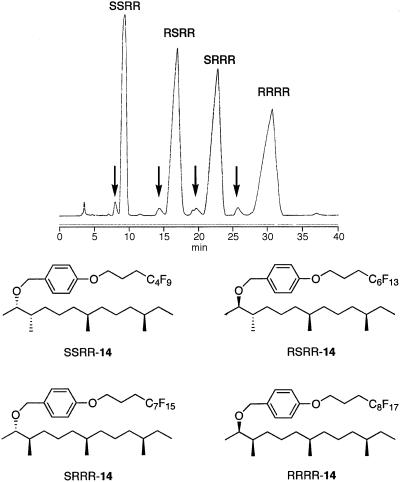
Demixing. Mixture M-14RR (476 mg) was demixed by using FluoroFlash semiprep HPLC column† (20 × 250 mm) under a gradient elution from 95:5 acetonitrile/water to 100% acetonitrile in 30 min and isocratic acetonitrile until 50 min to give four fluorous p-methoxybenzyl (PMB) ethers: SSRR-14, RSRR-14, SRRR-14, and RRRR-14. A maximum of 86 mg of the mixture was demixed in a single run, and a typical chromatogram is shown in Fig. 5.
Fig. 5.
A typical preparative demixing of 14. Arrows indicate minor unsaturated impurities removed in demixing.
During demixing of M-14RR, four major peaks and four minor peaks were collected individually. The products from each of the major peaks were the fluorous PMB ethers 14 that eluted in order of tag from SSRR-14 carrying C4F9 tag up to RRRR-14 carrying C8F17 tag. Proton NMR analysis of the fractions from the minor peaks revealed the presence of olefinic resonances; hence, these must be mixtures of partially reduced alkenes resulting from incomplete diimide reduction.
The ability to separate the underreduced by-products from the desired fully reduced products demonstrates that demixing is more than simple separation of tagged compounds. Ancillary separations observed during demixing enable purification of each of the compounds.
The overall mass recovery during the demixing of M-14RR was 98%. The four desired fluorous PMB ethers accounted for 96% of the recovered mass, whereas the by-products accounted for 2%. The mole ratio of the tagged compounds (C4F9/C6F13/C7F15/C8F17) isolated from demix ing of M-14RR was 1:1:1:1, which is the same as the composition of the four acetals in the first mixture M-11, indicating that all the fluorous compounds showed similar reactivities in all the steps involved in the FMS.
The other three mixtures, M-14RS, -SR, and -SS, were demixed similarly to provide the other 12 isomers of 14 (data not shown). The overall recovery of the desired products from all three mixtures was close to 90%. Mixture M-14RS had the highest amount of by-products (5%), whereas the other two mixtures had 2–3% by-products. The molar composition of isolated fluorous PMB ethers was close to 1:1:1:1 in all the cases.
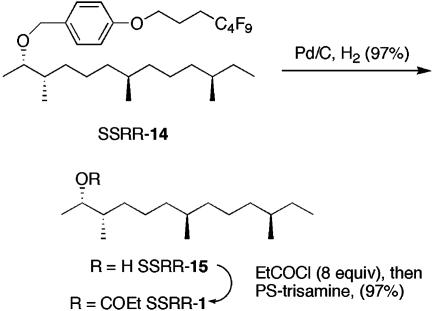
The Postmix Stage. The postmix stage comprised 32 easy reactions, and a representative postmix process is shown in Fig. 6. Fluorous PMB ether SSRR-14 was detagged by hydrogenolysis to give the corresponding alcohol SSRR-15 (20 mg). After filtration to remove the catalyst and tag followed by evaporation, approximately half of the alcohol was acylated with excess propionyl chloride to give the corresponding propionate ester SSRR-1. Excess propionyl chloride was quenched with polymer-supported trisamine (20), and the reaction mixture then was purified by filtration. The purity of the resulting propionate SSRR-1 obtained directly after filtration was excellent as assayed by 1H and 13C NMR spectroscopy. Detagging and subsequent propionylation of the rest of the 15 fluorous PMB ethers were carried out in a similar manner to give ≈10 mg of every alcohol and propanoate ester.
Fig. 6.
The postmix stage.
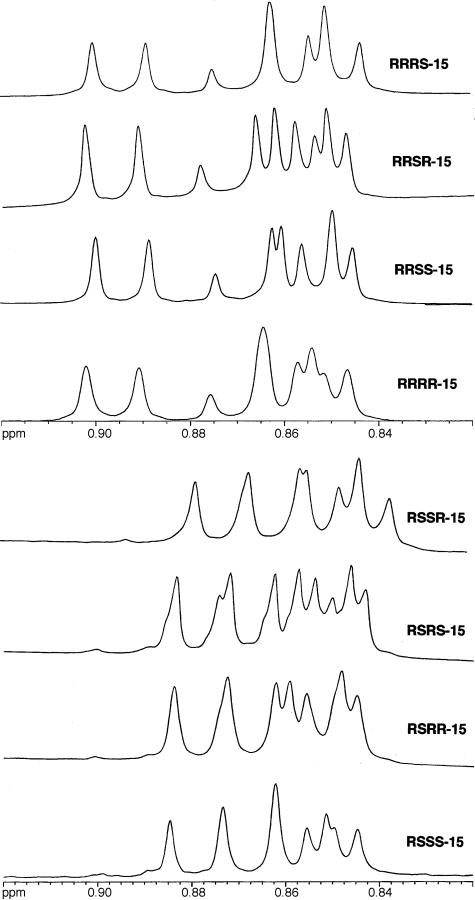
The NMR spectral data (1H and 13C) for all 16 isomers of alcohols 15 and propionates 1 matched well with the data of Hedenström and coworkers (10), which then confirms that the encoding/decoding strategy worked as planned. We also were able to identify unique fingerprint resonances in the 1H NMR spectra (600 MHz) of the eight diastereomeric alcohols of 15. Fig. 7 shows the upfield region of the spectra of 15 with the resonances for four of the five methyl groups (the three methyl groups attached to C3, C7, C11, and the C13 methyl). A total of nine lines are expected from these four methyl groups (one triplet and three doublets). The differential overlap of these resonances from the four methyl groups generates a unique pattern in the spectrum of each of the diastereomer. As a result, isomer identification by 1H NMR spectroscopy is much quicker and more reliable than by 13C NMR spectroscopy.
Fig. 7.
A portion of the 1H NMR spectra of isomers 15.
Conclusions
All 16 stereoisomers of the sex pheromone of pine sawfly have been synthesized on a 10- to 20-mg scale by a split-parallel FMS approach. Spectral data (1H and 13C NMR) obtained for all 32 compounds (16 alcohols 15 and the corresponding propionates 1) matched well with the data of Hedenström and coworkers (10), thereby validating the fluorous-tag encoding of diastereoisomers. This method is recommended for the efficient synthesis of multiple stereoisomers for spectroscopic studies, biological tests, or other structure–function relationships.
Figure 2.
Acknowledgments
We dedicate this article to Professor Julius Rebek on the occasion of his 60th birthday. We thank F.-M. Lin for obtaining HPLC-NMR spectra and the National Institutes of Health for funding this work.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FMS, fluorous mixture synthesis; THF, tetrahydrofuran; PMB, p-methoxybenzyl.
References
- 1.Luo, Z. Y., Zhang, Q. S., Oderaotoshi, Y. & Curran, D. P. (2001) Science 291, 1766-1769. [DOI] [PubMed] [Google Scholar]
- 2.Curran, D. P. & Oderaotoshi, Y. (2001) Tetrahedron 57, 5243-5253. [Google Scholar]
- 3.Curran, D. P. & Furukawa, T. (2002) Org. Lett. 4, 2233-2235. [DOI] [PubMed] [Google Scholar]
- 4.Zhang, W., Luo, Z., Chen, C. H. T. & Curran, D. P. (2002) J. Am. Chem. Soc. 124, 10443-10450. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, Q. S., Rivkin, A. & Curran, D. P. (2002) J. Am. Chem. Soc. 124, 5774-5781. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, Q. S., Lu, H. J., Richard, C. & Curran, D. P. (2004) J. Am. Chem. Soc. 126, 36-37. [DOI] [PubMed] [Google Scholar]
- 7.Houghten, R. A., Pinilla, C., Appel, J. R., Blondelle, S. E., Dooley, C. T., Eichler, J., Nefzi, A. & Ostresh, J. M. (1999) J. Med. Chem. 42, 3743-3778. [DOI] [PubMed] [Google Scholar]
- 8.An, H. Y. & Cook, P. D. (2000) Chem. Rev. (Washington, D.C.) 100, 3311-3340. [DOI] [PubMed] [Google Scholar]
- 9.Curran, D. P. (2001) Synlett, 1488-1496.
- 10.Larsson, M., Nguyen, B.-V., Högberg, H.-E. & Hedenström, E. (2001) Eur. J. Org. Chem., 353-363.
- 11.Bergström, G., Wassgren, A.-B., Anderbrandt, O., Ochieng, S. A., Östrand, F., Hansson, B. S., Hedenström, E. & Höberg, H.-E. (1998) Naturwissenschaften 85, 244-248. [Google Scholar]
- 12.Hedenström, E. & Andersson, F. (2002) J. Chem. Ecol. 28, 1237-1254. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura, Y. & Mori, K. (1999) Eur. J. Org. Chem. 2175-2182.
- 14.White, J. D., Theramongkol, P., Kuroda, C. & Engebrecht, J. R. (1988) J. Org. Chem. 53, 5909-5913. [Google Scholar]
- 15.Oppolzer, W., Blagg, J., Rodriguez, I. & Walther, E. (1990) J. Am. Chem. Soc. 112, 2767-2769. [Google Scholar]
- 16.Dandapani, S. (2004) Ph.D. thesis (University of Pittsburgh, Pittsburgh).
- 17.Tsunoda, T., Suzuki, M. & Noyori, R. (1980) Tetrahedron Lett. 21, 1357-1360. [Google Scholar]
- 18.Ishihara, K., Mori, A. & Yamamoto, H. (1990) Tetrahedron 46, 4595-4599. [Google Scholar]
- 19.Blakemore, P. R. (2002) J. Chem. Soc. Perkin Trans. 1, 2563-2579.
- 20.Booth, R. J. & Hodges, J. C. (1997) J. Am. Chem. Soc. 119, 4882-4887. [Google Scholar]