Abstract
Two conceptually different and novel radical-mediated cascade reactions leading to a total synthesis of the steroid (±)-estrone 1 and to a synthesis of 14-epiestrone 40 are described. Treatment of the iododienynone 23 with Bu3SnH/2,2′-azobis(isobutyronitrile) (AIBN) triggers a 13-endo-dig radical macrocyclization followed by two sequential radical transannulation reactions leading to the crystalline estrane 24 in 50% yield. The x-ray crystal structure of 24 established its trans, syn, stereochemistry. Transposition of the enone functionality in 24 next led to 38, which was then converted into 39 by reductive methylation. Deprotection of the methyl ether 39 finally gave 14-epiestone 40. When the substituted iodovinylcyclopropane 55 was treated similarly with Bu3SnH/AIBN, the resulting radical center underwent a different sequence of cascade macrocyclization-transannulation reactions producing the trans, anti, trans estrane 56 in 12% overall yield. Oxidation of 56, using CrO3-H2SO4 next led to the cyclopentanone 57, which, on deprotection using BBr3 gave (±)-estrone 1. A number of alternative substituted iodopolyenynones and iodovinylcyclopropanes, i.e., 8a, 8b, 33, 49a, and 49b, underwent similar radical-mediated cascade cyclizations leading to other estranes, i.e., 21a, 21b, 35, and 50, and, in one case, to the 6,6,5,6-tetracycle 51, in variable overall yields. The structures and stereochemistries of several estranes were established by using x-ray crystal structure measurements in combination with analysis of their NMR spectroscopic data and correlation with literature precedent.
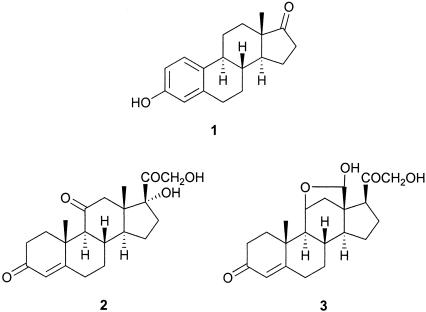
Since the synthesis of the female sex hormone estrone 1 by Anner and Miescher in 1948 (1), and the syntheses of nonaromatic steroids, such as cortisone 2 and aldosterone 3 during the 1950s, a plethora of ingenious designs have been explored to synthesize members of the steroid family of natural products. Prominent among the methods that have been developed are those based on Diels–Alder reactions (2–12), transition metal-catalyzed cyclizations of enynes and triynes (13–16), and biogenetic–type electrophilic cyclizations of polyene precursors (17–21).
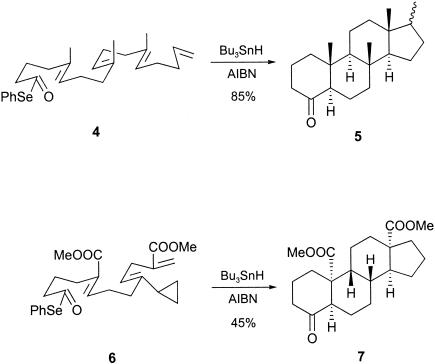
Over the past 10 years, we (22–26) and others (27–38) have examined the scope of a variety of cascade radical-mediated processes to elaborate steroids and other polycyclic ring systems. Thus, we have already described an approach to steroids based on consecutive 6-endo-trig (ref. 39 and references therein) and sequential transannular (40) radical cyclizations from appropriate polyene selenyl ester precursors, illustrated in the conversions 4 → 5 and 6 → 7, respectively.
We have now extended our studies and examined a different approach to estrone 1 and estranes, whereby the nonaromatic tricyclic B,C,D ring system is produced in a single step by radical-mediated macrocyclizations in tandem with consecutive transannulations from an appropriate ortho-disubstituted arylpolyene precursor.
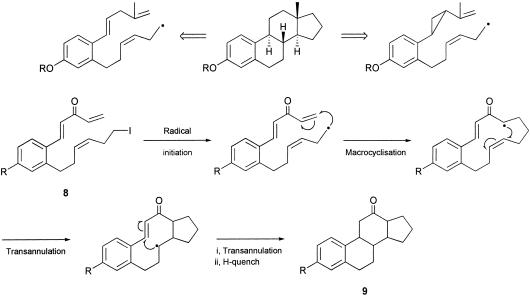
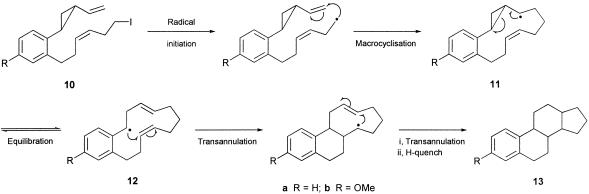
These approaches to estranes are captured in a retrosynthetic manner in Scheme 1. Thus, in one approach, we have studied the sequential 13-endo-trig macrocyclization/transannulation radical cascade from the iodopolyene precursor 8, and in the second approach, we have explored the alternative radical macrocyclization/transannulation cascade from the iodovinylcyclopropane 10 (Scheme 2). These investigations are brought together in this article and have culminated in a conceptually distinct total synthesis of estrone 1.
Scheme 1.
Retrosynthesis analysis.
Scheme 2.
Radical cascade reactions leading to estranes.
It is well established that electron-deficient alkenes (and alkynes), e.g., 8, act as efficient radical acceptor groups, or electrophores, in macrocyclizations involving nucleophilic carbon-centered radicals (41–45). There are also many illustrations of the applications of radical-mediated transannulations in the current literature (46–48). The similarity in the chemistries of alkenes and cyclopropanes is also well known, as is the propensity for cyclopropylmethyl radicals to undergo irreversible conversions into but-3-enyl radicals, i.e., 11 → 12. Clearly, the pathways followed by the planned radical cascades 8 → 9 and 10 → 13 will depend on a range of features, including the stereochemistries of the alkenes and cyclopropanes, the conformational preferences of the large and medium ring intermediates and, of course, competing side reactions involving radical quenching and hydrogen abstraction processes.
Experimental Methods
For general details, see ref. 49. Specific details of the synthesis and characterizations of all of the compounds researched in this article can be found in Supporting Methods, which is published as supporting information on the PNAS web site.
Results and Discussion
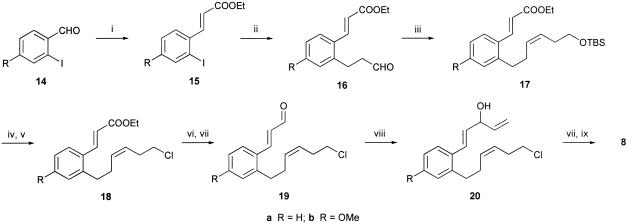
We began our investigation of the radical macrocyclization/transannulation cascade 8 → 9 by synthesizing the appropriate iodopolyenone starting materials 8a and 8b. These syntheses were achieved in rapid fashion from known o-iodobenzaldehydes 14 after (i) Wadsworth–Emmons olefination reactions to the corresponding cinnamate esters 15; (ii) Heck reactions (50) between the iodoaromatics 15 and propen-1-ol, producing the corresponding arylpropanals 16 (Scheme 3); (iii) Z-selective Wittig reactions of 16 with triphenyl(t-butylsilyloxy)propyltriphenylphosphonium iodide leading to 17; (iv) functional group interconversions via 18 ultimately producing 19; (v) Grignard reactions between 19 and the appropriate organomagnesium reagent, and oxidation of the resulting secondary alcohol 20 to the corresponding dienone; and finally, (vi) chloride–iodide interchange producing the iodopolyenones 8.
Scheme 3.
(i) EtO2CCH2PO(OEt)2, BuLi, tetrahydrofuran (THF), 72% yield; (ii) CH2 CHCH2OH, Pd(OAc)2, n-Bu4NCl, dimethylformamide (DMF), 62–53% yield; (iii) IPh3P(CH2)3OTBS (TBS, t-butyldimethylsilyl), potassium hexamethyldisilazane (KHMDS), THF, 80% yield; (iv) tetrabutylammonium fluoride (TBAF), THF, 90% yield; (v) N-chlorosuccinimide (NCS), PPh3,Cl2CH2, 84–69% yield; (vi) iBu2AlH, Cl2CH2, 81–78% yield; (vii) pyridinium dichromate (PDC), Cl2CH2, 92–62% yield; (viii) H2C
CHCH2OH, Pd(OAc)2, n-Bu4NCl, dimethylformamide (DMF), 62–53% yield; (iii) IPh3P(CH2)3OTBS (TBS, t-butyldimethylsilyl), potassium hexamethyldisilazane (KHMDS), THF, 80% yield; (iv) tetrabutylammonium fluoride (TBAF), THF, 90% yield; (v) N-chlorosuccinimide (NCS), PPh3,Cl2CH2, 84–69% yield; (vi) iBu2AlH, Cl2CH2, 81–78% yield; (vii) pyridinium dichromate (PDC), Cl2CH2, 92–62% yield; (viii) H2C CHMgBr, THF, 88–62% yield; (ix) NaI, MeCOCH2Me, 92–87% yield.
CHMgBr, THF, 88–62% yield; (ix) NaI, MeCOCH2Me, 92–87% yield.
When solutions of the iodotrienones 8a and 8b in benzene were treated separately with Bu3SnH and catalytic 2,2′-azobis(isobutyronitrile) (AIBN) over 6 h under high-dilution conditions, work-up and purification by chromatography led to the isolation of approximately equal amounts of two crystalline 12-ketosteroid products, from each reaction, in combined yields of ≈50% (for preliminary studies, see ref. 51). Crystals of one of the products resulting from cyclization of 8a were suitable for x-ray analysis, which showed that the 12-ketosteroid 21a had the cis, anti, trans stereochemistry drawn.
A close correlation between the NMR spectroscopic data recorded for 21a and the data measured for the major tetracyclic product resulting from the methoxy derivative 8b was obtained, thereby demonstrating that this steroid, likewise, had the same cis, anti, trans geometry, i.e., 21b. Attempts to grow crystals of the other diastereoisomers of the 12-ketosteroids produced from 8a/b, for x-ray studies proved to be fruitless. Therefore, their relative stereochemistries remain unresolved.
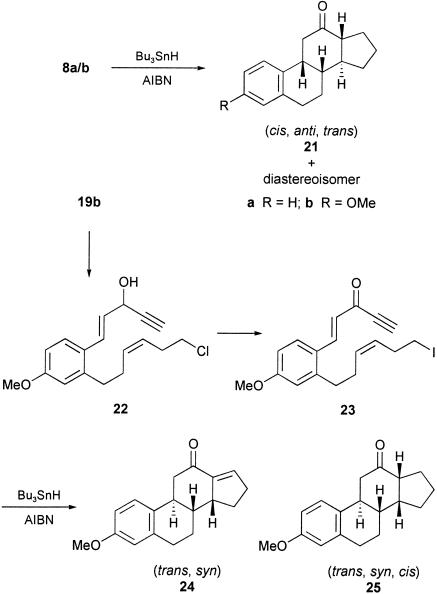
With the objective of incorporating unsaturation in the five-membered D ring, we next examined the cascade radical cyclization of the corresponding terminal acetylene analogue of 8b, i.e., 23, in the presence of Bu3SnH/AIBN. Two crystalline tetracyclic products were produced in this cyclization in a combined yield of 63%. The structure and stereochemistry of the major product (≈40%) was established as the trans, syn diastereoisomer of the cyclopentaphenanthren-12-one (i.e., 24) by single-crystal x-ray analysis, and the minor product was shown to be the trans, syn, cis diastereoisomer 25 of the 12-ketosteroid, 21b. The structure and stereochemistry of the estrane 25, which results from reduction of the enone 24 under the Bu3SnH/AIBN reducing conditions followed from 1D NMR, correlated spectroscopy (COSY), heteronuclear multiple quantum correlation (HMQC), and nuclear Overhauser effect (NOE) difference experiments. Indeed, under better-controlled conditions, i.e., use of only 1.2 eq of Bu3SnH, it was possible to isolate only the steroidal enone 24 in ≈50% yield from the reaction.
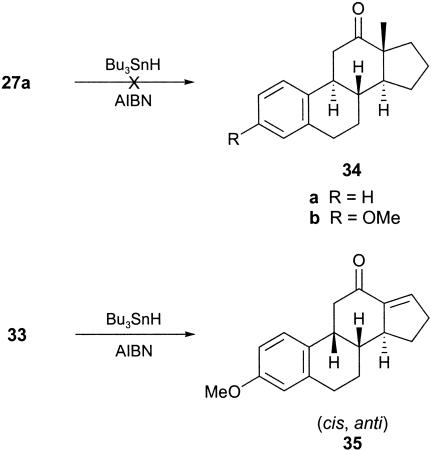
In addition to lacking methyl and carbonyl group substitution at C-18 and C-17,† respectively, neither of the synthesized tetracycles 21b and 24 had the required trans, anti, trans B/C/D ring fused geometry for ultimate elaboration to estrone 1. Therefore, we attempted to overcome some of these issues by studying the outcomes of the cascade radical cyclizations of the methyl-substituted iodotriene 27a and the E-geometrical isomer 33 corresponding to the dienynone 23.
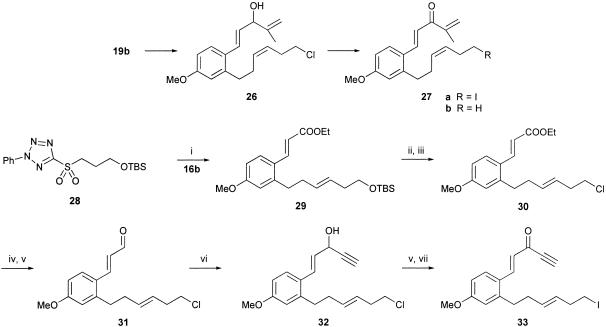
The methyl-substituted iodotrienone 27a was prepared from the corresponding substituted cinnamaldehyde 19b by using the same sequence of reactions that had been used earlier to elaborate 8b from 19b (Scheme 4). Likewise, the E-alkene 33 was prepared in a straightforward manner and used an E-selective Julia olefination reaction between the aldehyde 16b and the sulfone 28, leading to the alkene 29, which was then converted into 33 by using a sequence of reactions identical to those used to prepare 23 from 17.
Scheme 4.
(i) TBSO(CH2)3SO2-2Ph-2H-tetrazole 28, potassium hexamethyldisilazane, 77% yield; (ii) tetrabutylammonium fluoride, THF, 84% yield; (iii) N-chlorosuccinimide, PPh3, Cl2CH2, 95% yield; (iv) iBu2AlH, Cl2CH2, 80% yield; (v) MnO2, Cl2CH2, 86–79% yield; (vi) HC CMgBr, THF, 62% yield; and (vii) NaI, MeCOEt, 82% yield.
CMgBr, THF, 62% yield; and (vii) NaI, MeCOEt, 82% yield.
To our surprise, when the iododienone 27a was treated with Bu3SnH/AIBN, instead of the corresponding estrane (i.e., 34b) the only product produced was that resulting from H-quench of the precursor radical, i.e., 27b, in 62% yield. Whether this outcome was due to the additional steric bulk of the methyl group substituent on the enone electrophore in 27a or occurred by an intramolecular H-quench from the same methyl group, producing a stabilized allylic radical, or both, is not known. However, when the E-iododienynone 33 was treated with Bu3SnH/AIBN, it underwent the anticipated sequence of radical cyclizations and produced the estrane 35, albeit in a disappointing 13% overall yield. No other products resulting from this reaction could be characterized. The cis, anti stereochemistry of the 12-ketosteroid 35 followed from single-crystal x-ray analysis. In summary, the polyenynone 23 with Z-geometry in the iodohexenyl side chain undergoes a cascade of radical cyclizations leading to the trans, syn estrane 24, whereas the corresponding E-isomer 33 undergoes the same series of cyclizations producing the diastereoisomeric cis, anti estrane 35.
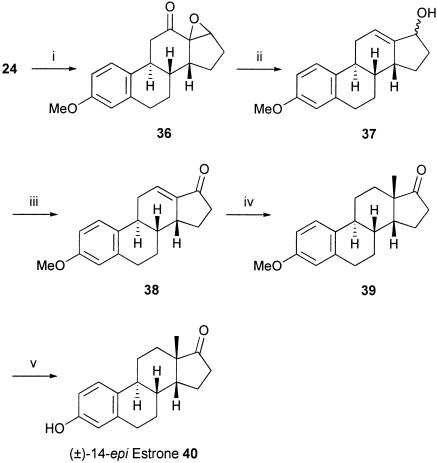
Because of the difficulty that we anticipated in attempting to epimerize selectively either the C-9 center in 35 or the C-14 center in 24 or any of the intermediates between them and trans, anti, trans estrone itself, we decided at this point to establish chemistry to convert the anti, syn tetracyclic enone 24 into 14-epiestrone 40. After exploring a number of approaches (52), we carried out an enone transposition on 24, using the Wharton procedure (53–55) which, in three steps, i.e., epoxidation (to 36), hydrazone formation and fragmentation (to 37), and oxidation, first gave the corresponding C12,C13 enone 38. Reductive methylation of 38, after conjugate addition of iBu2AlH in THF/hexamethylphosphoramide (HMPA) at -50°C with methylcopper catalysis, and trapping the intermediate “ate” complex with MeI (56), was diastereoselective and next gave 14-epiestrone methyl ether 39 (Scheme 5). Finally, demethylation of 39 by using BBr3 in THF gave 14-epiestrone 40, as colorless crystals, identical to that described in the literature (57, 58).
Scheme 5.
(i)H2O2, NaOH, THF, t-BuOH, 40% yield; (ii)NH2NH2·H2O, HOAc, 30% yield; (iii) tetrapropylammonium perruthenate (TPAP), 4-methylmorpholine N-oxide (NMMO), 54% yield; (iv) hexamethylphosphoramide (HMPA), i-BuAIH, CuIMeLi; then MeI, 66% yield; and (v) BBr3, THF, 79% yeild.
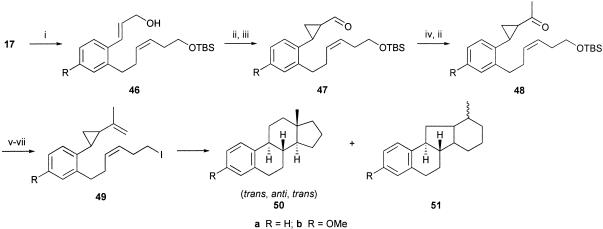
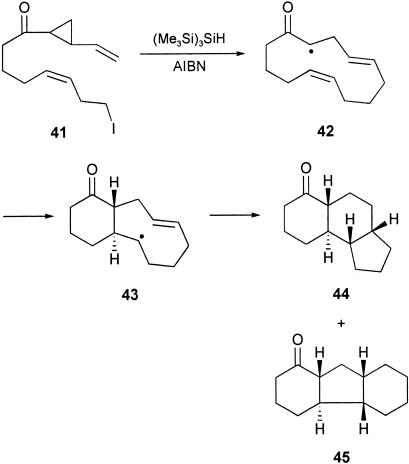
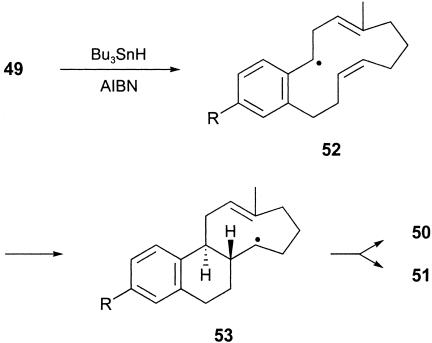
After the success in elaborating the estra-1,3,5(10)-triene ring system, based on the cascade of radical cyclizations 8 → 9 (Scheme 1), we examined the alternative radical cascade 10 → 11 → 12 → 13 to estranes by using a vinylcyclopropane electrophore in the first radical macrocyclization step (Scheme 2). In an earlier investigation (59), we showed that the iodovinylcyclopropyl ketone 41 underwent a macrocyclization (to 42) followed by successive transannulation reactions via 43 in the presence of (Me3Si)3SiH/AIBN, leading to a 1:2 mixture of the isomeric tricycles 44 and 45 in a combined yield of 65%. Therefore, we were optimistic that the analogous benzene-substituted vinylcyclopropane system 49 would take part in a similar sequence of cascade cyclizations leading to the corresponding estrane 50 (59). The iodovinylcyclopropanes 49a and 49b were synthesized in a straightforward manner from the earlier prepared cinnamates 17a and 17b, respectively, as outlined in Scheme 6. To our satisfaction, when the methoxy derivative 49b was treated with Bu3SnH/AIBN in refluxing benzene, work-up and chromatography separated the known trans, anti, trans diastereoisomer of the estrane 50b, whose 13C NMR spectroscopic data were identical with those reported in the literature (60). The estrane 50b was produced alongside an unidentified tetracycle and also the product of reduction of the carbon-to-iodide bond in the starting material, in a combined yield of 45%. When the iodovinylcyclopropane 49a was treated similarly with Bu3SnH/AIBN, it too underwent a cascade of cyclizations but, instead of producing the corresponding estrane 50a, it gave, in 25% yield, an isomeric compound whose spectroscopic data were comparable with those of the unidentified tetracyclic product produced alongside 50b from 49b. The new tetracycle produced from 49a showed six methylene, five methine, and one methyl carbon signal in its 13C NMR spectrum, which, with other data, supported assignment of the alternative 6,6,5,6-tetracyclic structure 51a for this compound. However, we were not able to assign a relative stereochemistry for this interesting structure. Reflecting on the outcome of the triple radical cyclization from the related system 41, leading to both 44 and 45, it is perhaps not surprising that the corresponding radical cascade from the benzene-substituted vinylcyclopropane 49 would similarly produce mixtures of the tetracyclic products 50 and 51. The radical cascade from 49 proceeds via 52 and then the 9-membered radical intermediate 53 (compare 43), which, by competing 5-exo/6-endo-trig transannulations could lead to either the 6,6,6,5- or the 6,6,5,6-tetracycle, i.e., 50 and 51, as observed.
Scheme 6.
(i) iBu2AlH, Cl2CH2, 82% yield; (ii) Et2Zn, CH2I2, 70% yield; (iii) pyridinium dichromate, 84% yield; (iv) MePPh3Br, n-BuLi, 86% yield; (v) AcOH, THF, 95% yield; and (vi) I2, imidazole, PPh3, 73% yield.
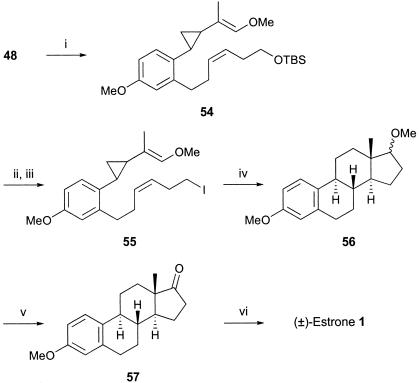
With the aforementioned model studies complete, but mindful of some limitations, we decided to synthesize the iodovinyl ether cyclopropane 55 and study its cascade radical cyclization to the estrane 56 en route to estrone 1. Although imposing additional steric congestion at the vinylcyclopropane electrophore, the presence of the terminal vinyl methyl ether group in 55 was also expected to make this center more electrophilic to the incoming nucleophilic radical in the initial macrocyclization step in the overall radical cascade sequence.
The precursor iodovinyl ether cyclopropane 55 was synthesized from the previously prepared vinyl methyl ketone 48 by using a Horner–Wittig reaction with methoxymethyldiphenylphosphine oxide in the presence of lithium diisopropylamide, which first gave a 1:1 mixture of Z and E isomers of the intermediate 54. Deprotection of 54 followed by treatment of the resulting alcohol with I2, imidazole, and PPh3, using procedures developed earlier in our studies, then gave the iodovinylcyclopropane 55 (Scheme 7).
Scheme 7.
(i) MeOCH2POPh2, lithium diisopropylamide, THF, then NaH, THF, 90% yield; (ii) tetrabutylammonium fluoride, THF, 98% yield; (iii)I2, imidazole, PPh3, 80% yield; (iv) Bu3SnH, AIBN, toluene, 12% yield; (v) CrO3, catalytic H2SO4, Me2CO, 94% yield; and (vi) BBr3, THF, 79% yield.
To our satisfaction, when a solution of the iodide 55 in toluene at reflux was treated with Bu3SnH/AIBN, work-up and chromatography gave the known 3,7-dimethoxyestrane 56 (61) as a crystalline solid, in 12–15% overall yield. The major product isolated, in 52% yield, was that corresponding to reduction of the carbon-to-iodide bond in the starting material. Bearing this in mind, and with a cascade of four C—C bond-forming reactions involved in the conversion 55 → 56, each of the radical reactions proceeds with an average yield of ≈65%. Oxidation of the ring D methyl ether in 56 by using Jones' procedure next gave estrone methyl ether 57 which was then demethylated by using BBr3, leading to (±)-estrone 1, which had physical and spectroscopic properties identical with those described in the literature (62, 63).
In summary, two conceptually different radical-mediated cascade reactions from ortho-disubstituted aryl polyene and arylvinylcycloprane precursors, leading to the tricyclic B, C, and D rings of estranes in a single step, have been developed. The investigations culminated in a total synthesis of (±)-estrone 1, and, separately, of 14-epiestrone 40. Unfortunately, not all of the stereochemistries of the estranes prepared in model studies could be established unambiguously, and rationalization of the mechanisms of key events taking place during these cascade processes will remain a topic of conjecture until some of these issues are resolved. Nevertheless, a novel concept in the elaboration of ring A aromatic steroids from simple starting materials, in a single step, has been established, which could have wider applications in the synthesis of other polycyclic natural (and nonnatural) products.
Supplementary Material
Figure 1.
Figure 2.
Figure 6.
Figure 8.
Figure 10.
Figure 12.
Acknowledgments
We thank Drs. A. J. Blake and C. Wilson for x-ray crystal structure determinations. This work was supported by an Engineering and Physical Sciences Research Council studentship (to S.M.) and AstraZeneca.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AIBN, 2,2′-azobis(isobutyronitrile); THF, tetrahydrofuran; TBS, t-butyldimethylsilyl.
Footnotes
Steroid ring numbering is used throughout this paper.
References
- 1.Anner, G. & Miescher, K. (1948) Helv. Chim. Acta 31, 2173-2183. [DOI] [PubMed] [Google Scholar]
- 2.Grieco, P. A., Takigawa, T. & Schillinger, W. J. (1980) J. Org. Chem. 45, 2247-2251. [Google Scholar]
- 3.Funk, R. L. & Vollhardt, K. P. C. (1980) J. Am. Chem. Soc. 102, 5253-5261. [Google Scholar]
- 4.Nicolaou, K. C., Barnette, W. E. & Ma, P. (1980) J. Org. Chem. 45, 1463-1470. [Google Scholar]
- 5.Michellys, P.-Y., Pellissier, H. & Santelli, M. (1993) Tetrahedron Lett. 34, 1931-1934. [Google Scholar]
- 6.Couturier, M. & Deslongchamps, P. (1996) Synlett, 1140-1142.
- 7.Ouellet, L., Langlois, P. & Deslongchamps, P. (1997) Synlett, 689-690.
- 8.Sakamoto, Y., Yasuharu, H. & Takahashi, T. (1995) Synlett, 231-232.
- 9.Pellissier, H. & Santelli, M. (1996) Tetrahedron 52, 9093-9100. [Google Scholar]
- 10.Lavoie, R., Toró, A. & Deslongchamps, P. (1999) Tetrahedron 55, 13037-13050. [Google Scholar]
- 11.Quinkert, G., Del Grosso, M., Döring, A., Döring, W., Schenkel, R. I., Bauch, M., Dambacher, G. T., Bats, J. W., Zimmermann, G. & Dürner, G. (1995) Helv. Chim. Acta 78, 1345-1391. [Google Scholar]
- 12.Sugahara, T. & Ogasawara, K. (1996) Tetrahedron Lett. 37, 7403-7406. [Google Scholar]
- 13.Vollhardt, K. P. C. (1985) Pure Appl. Chem. 57, 1819-1826. [Google Scholar]
- 14.Lecker, S. H., Nguyen, N. H. & Vollhardt, K. P. C. (1986) J. Am. Chem. Soc. 108, 856-858. [Google Scholar]
- 15.Sugihara, T., Coperet, C., Owczarczyk, Z., Harding, L. S. & Negishi, E. (1994) J. Am. Chem. Soc. 116, 7923-7924. [Google Scholar]
- 16.Trost, B. M. & Shi, Y. (1993) J. Am. Chem. Soc. 115, 9421-9438. [Google Scholar]
- 17.Corey, E. J. & Lin, S. (1996) J. Am. Chem. Soc. 118, 8765-8766. [Google Scholar]
- 18.Fish, P. V., Johnson, W. S., Jones, G. S., Tham, F. S. & Kullnig, R. K. (1994) J. Org. Chem. 59, 6150-6152. [Google Scholar]
- 19.Johnson, W. S., Wiedhaup, K., Brady, S. F. & Olson, G. L. (1974) J. Am. Chem. Soc. 96, 3979-3984. [Google Scholar]
- 20.van Tamelen, E. E & Anderson, R. J. (1972) J. Am. Chem. Soc. 94, 8225-8228. [DOI] [PubMed] [Google Scholar]
- 21.van Tamelen, E. E., Holton, R. A., Hopla, R. E. & Konz, W. E. (1972) J. Am. Chem. Soc. 94, 8228-8229. [Google Scholar]
- 22.Boehm, H. M., Handa, S., Pattenden, G., Roberts, L., Blake, A. J. & Li, W. S. (2000) J. Chem. Soc. Perkin Trans. 1, 3522-3538.
- 23.Double, P. & Pattenden G. (1998) J. Chem. Soc. Perkin Trans. 1, 2005-2008.
- 24.Pattenden, G., Roberts, L. & Blake, A. J. (1998) J. Chem. Soc. Perkin Trans. 1, 863-868.
- 25.Handa, S. Nair, P. S. & Pattenden, G. (2000) Helv. Chim. Acta 83, 2629-2643. [Google Scholar]
- 26.Handa, S. & Pattenden, G. (1997) Contemp. Org. Synth. 4, 196-215. [Google Scholar]
- 27.Curran, D. P. & Rakiewicz, D. M. (1985) Tetrahedron 41, 3943-3958. [Google Scholar]
- 28.Caspar, M. L., Davis, D. G., Weng, X. & Zoretic, P. A. (1991) Tetrahedron Lett. 32, 4819-4822. [Google Scholar]
- 29.Katouda, W., Sakamoto, Y., Takahashi, T., Tomida, S. & Yamada, H. (1995) Tetrahedron Lett. 36, 2273-2276. [Google Scholar]
- 30.Ribeiro, A. A., Shen, Z., Wang, M. & Zoretic, P. A. (1995) Tetrahedron Lett. 36, 2925-2928. [Google Scholar]
- 31.Ribeiro, A. A., Zhang, Y. & Zoretic, P. A. (1995) Tetrahedron Lett. 36, 2929-2932. [Google Scholar]
- 32.Curran, D. P. & Jahn, U. (1995) Tetrahedron Lett. 36, 8921-8924. [Google Scholar]
- 33.Chen, Z., Ribeiro, A. A., Zhang, Y. & Zoretic, P. A. (1996) Tetrahedron Lett. 37, 7909-7912. [Google Scholar]
- 34.Sakamoto, Y., Takahashi, T., Tomida, S. & Yamada, H. (1997) J. Org. Chem. 62, 1912-1913. [DOI] [PubMed] [Google Scholar]
- 35.Fang, H., Ribeiro, A. A. & Zoretic, P. A. (1998) J. Org. Chem. 63, 7213-7217. [DOI] [PubMed] [Google Scholar]
- 36.Doi, T., Takahashi, T. & Tomida, S. (1999) Tetrahedron Lett. 40, 2363-2366. [Google Scholar]
- 37.Ihara, M., Katsumata, A., Kuroyanagi, J. & Takasu, K. (1999) Tetrahedron Lett. 40, 6277-6280. [Google Scholar]
- 38.Wu, S., Journet, M. & Malacria, M. (1994) Tetrahedron Lett. 35, 8601-8604. [Google Scholar]
- 39.Batsanov, A., Chen, L., Gill, G. B. & Pattenden, G. (1996) J. Chem. Soc. Perkin Trans. 1, 45-55.
- 40.Handa, S., Pattenden, G. & Li, W.-S. (1998) J. Chem. Soc. Chem. Commun., 311-312.
- 41.Porter, N. A., Magnin, D. R. & Wright, B. T. (1986) J. Am. Chem. Soc. 108, 2787-2788. [Google Scholar]
- 42.Cox, N. J. G., Mills, S. D. & Pattenden, G. (1992) J. Chem. Soc. Perkin Trans. 1, 1313-1321.
- 43.Hitchcock, S. A. & Pattenden, G. (1990) Tetrahedron Lett. 31, 3641-3644. [Google Scholar]
- 44.Hitchcock, S. A. & Pattenden, G. (1992) J. Chem. Soc. Perkin Trans. 1, 1323-1328.
- 45.Baldwin, J. E., Adlington, R. M. & Ramcharitar, S. (1992) Tetrahedron 48, 3413-3428. [Google Scholar]
- 46.Porter, N. A., Chang, V. H.-T., Magnin, D. R. & Wright, B. T. (1988) J. Am. Chem. Soc. 110, 3554-3560. [Google Scholar]
- 47.Myers, A. G. & Condroski, K. R. (1993) J. Am. Chem. Soc. 115, 7926-7927. [Google Scholar]
- 48.Houldsworth, S. J., Pattenden, G., Pryde, D. C. & Thomson, N. M. (1997) J. Chem. Soc. Perkin Trans. 1, 1091-1093.
- 49.Pattenden, G., González, M. A., Little, P. B., Millan, D. S., Plowright, A. T., Tornos, J. A. & Ye, T. (2003) Org. Biomol. Chem. 1, 4173-4208. [DOI] [PubMed] [Google Scholar]
- 50.Jeffery, T. (1984) J. Chem. Soc. Chem. Commun., 1287-1289.
- 51.Pattenden, G., McCulloch, S. & Cardente, M. A. G. (2001) C. R. Acad. Sci. Ser. II, 571-574.
- 52.Affo, W. (2003) Ph.D. thesis (Univ. of Nottingham, Nottingham, U.K.).
- 53.Wharton, P. S. (1961) J. Org. Chem. 26, 4781-4782. [Google Scholar]
- 54.Barton, D. H. R., Motherwell, R. S. H. & Motherwell, W. B. (1981) J. Chem. Soc. Perkin Trans. 1, 2363-2367.
- 55.Filippo, M., Fezza, F., Izzo, I., De Riccardis, F. & Sodano, G. (2000) Eur. J. Org. Chem., 3247-3252.
- 56.Satomi, H., Hayashi, T., Tsuda, T. & Saegusa, T. (1987) J. Org. Chem. 52, 439-443. [Google Scholar]
- 57.Johns, W. F. & Johnson, W. S. (1957) J. Am. Chem. Soc. 79, 2005-2009. [Google Scholar]
- 58.Prousa, R., Schonecker, B., Tresselt, D. & Ponsold, K. (1986) J. Prakt. Chem. 328, 55-70. [Google Scholar]
- 59.Pattenden, G. & Wiedenau, P. (1997) Tetrahedron Lett. 38, 3647-3650. [Google Scholar]
- 60.Zeigan, D., Radeglia, R. & Engelhardt, G. (1983) J. Prakt. Chem. 325, 651-656. [Google Scholar]
- 61.Node, M., Hori, H. & Fujita, E. (1976) J. Chem. Soc. Perkin Trans. 1, 2237-2240. [DOI] [PubMed]
- 62.Johnson, W. S., Christiansen, R. G. & Ireland, R. E. (1957) J. Am. Chem. Soc. 79, 1995-2005. [Google Scholar]
- 63.Ciuffreda, P., Ferraboschi, P., Verza, E. & Manzocchi, A. (2001) Magn. Reson. Chem. 39, 648-650. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.