Abstract
A unified synthetic strategy toward caged Garcinia natural products has been designed and implemented. Central to the strategy is a tandem Claisen/Diels–Alder/Claisen rearrangement of a suitably substituted xanthone precursor to form forbesione (1a). Serving as a template, forbesione is then used to deliver representative members of this family, including desoxygaudichaudione A (4), desoxymorellin (5), and gambogin (10). Studies on the timing of this reaction cascade suggest that the C-ring Claisen/Diels–Alder rearrangement proceeds initially and is followed by the A-ring Claisen reaction. The electronic and steric effects that govern the outcome of this cascade are presented.
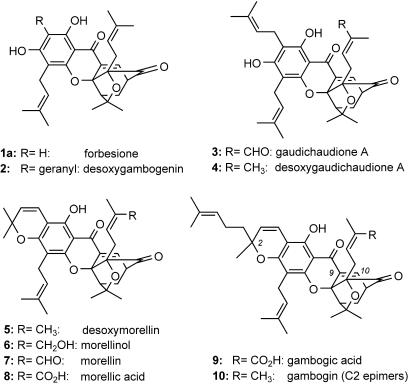
The tropical trees and shrubs of the genus Garcinia (Guttiferae) are widely known for their pigments and use in folk medicines (1, 2). Phytochemically, they are recognized as a rich source of xanthone and xanthonoid natural products with high pharmaceutical potential (3). For example, gamboge, the commercially available exudate of Garcinia hanburyii, has been used in traditional Asian medicine for the treatment of indigestion, inflammation, and ulcers (4). Efforts to identify the bioactive components of these extracts have yielded an ever growing family of natural products, the chemical structures of which feature a unique 4-oxa-tricyclo[4.3.1.03,7]dec-8-en-2-one scaffold built into a common xanthone backbone (5). This motif, present in forbesione (1a) (6) (Fig. 1), is further customized by substitutions on the aromatic residue and peripheral oxidations to produce a variety of structural families such as the morellins (5–8) (7, 8), the gambogins (2, 9, 10) (9, 10), and the gaudichaudiones (3, 4) (11, 12).
Fig. 1.
Representative structures of caged Garcinia natural products.
Aside from their striking chemical architecture, these natural products exhibit interesting biological activity, as exemplified by recent reports indicating that gambogic acid (9) and gaudichaudione A (3) induce apoptosis in certain cancer cells by a novel mechanism of caspase activation (13, 14). Moreover, structure–activity relationship data suggest that the C9–C10 double bond of 9 (gambogic acid numbering) is essential to its biological profile, corroborating previous reports that the caged motif of these natural products is crucial to their biology (11). In fact, whereas gambogic acid (9) tolerates many changes to its hydroxyl and carboxyl functionalities without significant loss to its biological activity, reduction of the C9–C10 double bond with L-Selectride (lithium tri-sec-butylborohydride) breaks down the pharmacophore entirely (13).
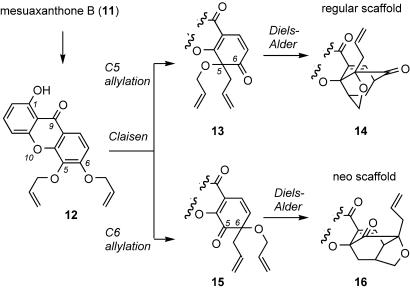
The caged scaffold has been suggested to arise in nature by means of a tandem Claisen/Diels–Alder rearrangement. This scenario, highlighted in Fig. 2, was initially proposed and experimentally tested by Quillinan and Scheinmann in 1971 (15). Using the natural product mesuaxanthone B (11) (Fig. 2) as a starting material, they successfully prepared and tested their hypothesis on 5,6-bis(allyloxy)-1-hydroxy-9H-xanthen-9-one (12). Despite the limited details they provided, they showed that heating 12 in boiling decalin induced the Claisen and subsequently, the Diels–Alder rearrangement, to create the elusive caged structure. Although the site selectivity of the opening Claisen reaction was not addressed, there is a possibility of concomitant allylation at both the C5 and C6 centers, giving intermediates 13 and 15, respectively. These products could then produce an isomeric mixture of 14 and 16, representing the regular caged scaffold and its constitutional isomer, the so-called neo scaffold, respectively. Nicolaou and Li (16) reported similar observations during their application of the Claisen/Diels–Alder reaction cascade to a biomimetic synthesis of 1-O-methylforbesione.
Fig. 2.
Structural motifs arising from the Claisen/Diels–Alder reaction on 12.
Inspired by the unusual 4-oxa-tricyclo[4.3.1.03,7]dec-8-en-2-one scaffold and the biosynthetic issues mentioned above, we sought to design a general synthetic approach to the caged Garcinia natural products that would allow us to tap into their full biological potential (17). Herein we present a biomimetic synthesis of forbesione (1a) by using a site-selective Claisen/Diels–Alder/Claisen reaction cascade (18). Using forbesione as a base structure, we also report a unified synthetic approach to several other members of this family, including deoxygaudichaudione A (4), desoxymorellin (5), and gambogin (10). In addition, we examine the timing of the Claisen/Diels–Alder/Claisen and issues governing the site selectivity of this tandem rearrangement. Finally, we discuss applications of these rearrangements as they relate to the biosynthesis of related caged natural products.
Materials and Methods
General. Phloroglucinol (19) and 2,3,4-trihydroxybenzoic acid (20) were commercially available and used without any additional purification. Compounds 1a, 1b, 1c, 17a, 17b, 17c, 22a, 23c, and 5 were synthesized as described previously (18). Full experimental details and analytical and spectroscopic information for all synthetic compounds are provided in supporting information, which is published on the PNAS web site. Tetrahydrofuran, diethyl ether (Et2O), dichloromethane, toluene, and benzene were purified by passage through a bed of activated alumina. Pyridine, triethylamine, and boron trifluoride etherate (BF3·Et2O) were distilled from calcium hydride before use. Dimethyl sulfoxide (DMSO) and dimethylformamide (DMF) were distilled from calcium hydride under reduced pressure [20 mmHg (1 mmHg = 133 Pa)] and stored over 4-Å molecular sieves until needed. Yields refer to chromatographically and spectroscopically (1H NMR, 13C NMR) homogeneous materials, unless otherwise stated. Reactions were monitored by TLC carried out on 0.25-mm E. Merck silica gel plates (60F-254) and visualized under UV light and/or developed by dipping in solutions of 10% ethanolic phosphomolybdic acid or p-anisaldehyde and applying heat. E. Merck silica gel (60, particle size 0.040–0.063 mm) was used for flash chromatography. Preparative TLC separations were carried out on 0.25- or 0.50-mm E. Merck silica gel plates (60F-254). NMR spectra were recorded on Varian Mercury 300- or 400-MHz and/or Unity 500-MHz instruments and calibrated by using the residual undeuterated solvent as an internal reference. IR spectra were recorded on a Nicolet 320 Avatar Fourier transform IR spectrometer and values are reported in cm-1 units. High-resolution mass spectra were recorded on a VG 7070 HS mass spectrometer under chemical ionization (CI) conditions or on a VG ZABZSE mass spectrometer under fast atom bombardment (FAB) conditions.
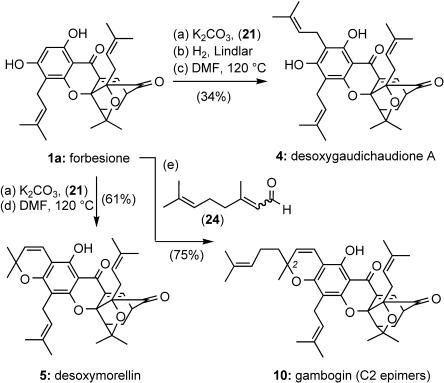
Synthesis of Desoxygaudichaudione A (4). A solution of 40 (13.4 mg, 25.2 μmol) in DMF (1.00 ml) was heated at 125°C and the reaction was continuously monitored by TLC (50% Et2O/hexane). After 2 h, the reaction mixture was diluted with toluene and concentrated several times at 60°C under reduced pressure to remove DMF by azeotropic distillation. The crude material was then purified by preparative TLC (50% Et2O/hexane) to yield desoxygaudichaudione A (4) (10.5 mg, 78%). 4: yellow foam; Rf = 0.41 (50% Et2O/hexane); IR (film) νmax 3,416, 2,968, 2,923, 2,854, 1,738, 1,634, 1,601, 1,441, 1,403, 1,380, 1,334, 1,276, 1,220, 1,181, 1,135, 1,099, 1,065, 1,046, 985, 960, 824 cm-1; 1H NMR (400 MHz, CDCl3) δ 12.92 (s, 1H), 7.44 (d, J = 6.8 Hz, 1H), 6.46 (br s, 1H), 5.23–5.20 (m, 2H), 4.45–4.41 (m, 1H), 3.50 (dd, J = 6.4, 5.2 Hz, 1H), 3.42–3.36 (m, 4H), 2.57–2.55 (m, 2H), 2.47 (d, J = 9.2 Hz, 1H), 2.34 (dd, J = 13.2, 4.8 Hz, 1H), 1.83 (s, 3H), 1.80 (s, 3H), 1.77 (s, 3H), 1.75 (s, 3H), 1.69 (s, 3H), 1.38 (s, 3H), 1.35 (dd, J = 12.4, 6.8 Hz, 1H), 1.29 (s, 3H), 1.03 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 203.3, 179.4, 162.7, 160.0, 156.0, 135.1, 134.8, 134.3, 133.7, 133.6, 121.6, 121.4, 117.7, 107.2, 106.0, 100.7, 90.2, 84.5, 83.1, 49.1, 47.0, 30.2, 29.2, 29.0, 26.0, 25.9, 25.7, 25.6, 22.3, 21.4, 18.2, 18.1, 16.9; high-resolution MS: calculated for C33H41O6 (M + H+) 533.2898, found 533.2910.
Synthesis of Gambogin (10). To a sealed tube containing forbesione (1a) (33.7 mg, 72.4 μmol) in MeOH (2.50 ml) was added freshly prepared citral (24) (33.1 mg, 0.22 mmol), CaCl2·H2O (21.3 mg, 0.14 mmol), and triethylamine (33.3 μl, 0.22 mmol). The reaction mixture was then heated for 3 h. After cooling to 25°C, the reaction mixture was poured into a separatory funnel and partitioned between Et2O and brine. The ethereal extract was dried over MgSO4, filtered, and concentrated. The crude material was then column chromatographed (15% Et2O/hexane) to yield gambogin (10) (27.5 mg, 75%). 10: yellow foam; Rf = 0.40 (30% Et2O/hexane); IR (film) νmax 2,969, 2,925, 1,738, 1,634, 1,595, 1,438, 1,401, 1,380, 1,331, 1,224, 1,174, 1,139, 1,086, 1,048, 984, 960, 871, 840, 758 cm-1; 1H NMR (400 MHz, CDCl3) δ 12.89 (s, 1H), 7.43 (d, J = 7.2 Hz, 1H), 6.69 (d, J = 10.4 Hz, 1H), 5.464/5.456 (d, J = 9.6/10.4 Hz, 1H), 5.24–5.19 (m, 1H), 5.08 (m, 1H), 4.45 (m, 1H), 3.49 (dd, J = 6.4, 4.4 Hz, 1H), 3.38–3.26 (m, 2H), 2.58 (d, J = 7.2 Hz, 2H), 2.49 (d, J = 9.2 Hz, 1H), 2.35 (dd, J = 13.2, 4.4 Hz, 1H), 2.12–2.06 (m, 2H), 1.84–1.64 (m, 2H), 1.77 (s, 3H), 1.72 (s, 3H), 1.68 (s, 6H), 1.58 (s, 3H), 1.422/1.418 (s, 3H), 1.36 (dd, 13.2, 9.6 Hz, 1H), 1.39 (s, 3H), 1.30 (s, 3H), 1.05 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 203.20/203.17, 179.2, 160.7/160.6, 157.6, 157.2, 134.81/134.77, 133.6, 133.53/133.51, 131.8/131.7, 131.50/131.46, 124.62/124.56, 123.7/123.6, 122.1/122.0, 117.7, 115.9/115.8, 107.8/107.7, 102.5, 100.4, 90.4, 84.61/84.58, 83.2, 81.1/81.0, 49.2, 47.0, 42.0/41.9, 30.23/30.20, 29.2, 28.9, 27.5/27.4, 25.82, 25.76, 25.7, 25.6, 22.9/22.8, 21.8, 18.3, 17.7, 16.9/16.8; high-resolution MS: calculated for C38H47O6 (M + H+) 599.3367, found 599.3375.
Spectroscopic Study of the Claisen/Diels–Alder/Claisen Reaction. A solution of 17b (10 mg, 19.7 μmol) in deuterated DMF was heated inside an NMR spectrometer along a temperature gradient from 80°C to 120°C in intervals of 5°C. At each temperature, the reaction was allowed to proceed for 5 min, after which a 1H NMR spectrum of the reaction was taken. After reaching 120°C, the temperature was kept constant until 17b was completely consumed.
Results and Discussion
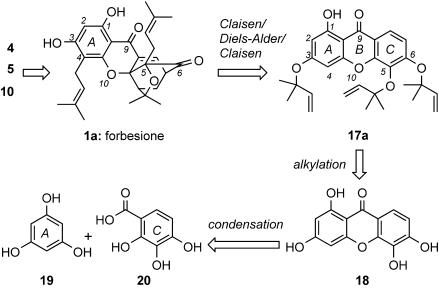
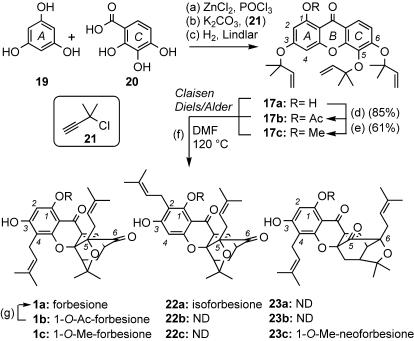
Retrosynthetic Analysis of 1a, 4, 5, and 10. Forbesione (1a), the simplest of the caged Garcinia natural products, is unique in that it contains no substituent at the C2 center of the A ring. As such, it was envisioned to be a template upon which its congeners could be built, a node in a branching synthetic scheme to an array of natural products, such as 4, 5, and 10 (Fig. 3). On the basis of the biosynthetic hypothesis of Quillinan and Scheinmann (15), compound 1a could arise from tris(dimethylallyloxy) precursor 17a, the xanthone motif of which could be traced to 1,3,5,6-tetrahydroxyxanthone 18. It is of interest to mention that 18 is also a natural product found in plants of the genus Hypericum (Guttiferae) (19, 20). Further disconnection across the central ring of xanthone 18 unveils phloroglucinol (19) and 2,3,4-trihydroxybenzoic acid as suitable starting materials. Implementation of this strategy is presented in Schemes 2 and 3.
Fig. 3.
Retrosynthetic analysis of 1a, 4, 5, and 10.
Scheme 2.
Reagents and conditions: reaction a, 3.0 eq of K2CO3,20 eq of 21, 1.5 eq of KI, 10 mol % CuI, Me2CO, 55°C, 4 h, 67% yield; reaction b, 10% Pd/BaSO4, quinoline, EtOAc, 25°C, 15 min, 66% yield; reaction c, DMF, 120°C, 2 h, 78% yield; reaction d, DMF, 120°C, 4 h, 91% yield; and reaction e, 3.0 eq of triethylamine, 3.0 eq of 24, 2.0 eq of CaCl2·H2O, MeOH (sealed tube) 3 h, 75% yield.
Scheme 3.
Timing of the Claisen/Diels–Alder/Claisen rearrangement.
Synthesis of 1a, 4, 5, and 10. Putting our plan into practice, we commenced our study with a ZnCl2-mediated condensation of 19 and 20 in POCl3 to produce xanthone 18 in 46% yield (Scheme 1) (21). Several conditions were tested for the conversion of 18 to the tris(dimethylallyloxy)xanthone 17a (22). The most efficient proved to be a two-step procedure that involved propargylation of the more reactive C3, C5, and C6 phenols with 2-chloro-2-methylbutyne (21) in the presence of KI and K2CO3 under CuI catalysis (25% yield) (23), followed by Lindlar reduction of the pendant alkynes (75% yield) (24). Drawing on previous experience with related structures (25, 26), we heated 17a in DMF at 120°C and obtained a mixture of forbesione (1a) (49% yield) and isoforbesione (22a) (35% yield) (18).
Scheme 1.
Reagents and conditions: reaction a, 1.0 eq of 19, 1.0 eq of 20, 6.5 eq of ZnCl2, POCl3,65°C, 3 h, 46% yield; reaction b, 3.3 eq of K2CO3, 5.1 eq of 21, 3.3 eq of KI, 10 mol % CuI, Me2CO, 45°C, 6 h, 25% yield; reaction c, 10% Pd/BaSO4, quinoline, EtOAc, 25°C, 6 h, 75% yield; reaction d, 25 eq of Ac2O, 25 eq of pyridine, 10 mol % N,N-dimethylaminopyridine, CH2Cl2, 35°C, 24 h, 85% yield; reaction e, 5.0 eq of Cs2CO3, 5.0 eq of MeI, DMF, 25°C, 15 min, 61% yield; reaction f, DMF, 120°C, 1 h, 49% yield of 1a, 35% yield of 22a;, 79% of 1b; 51% of 1c, 24% of 23c; and reaction g, 0.5 M K2CO3 (aq), MeOH, 25°C, 6 h, 91% yield. ND, not detected.
Although these results demonstrated the excellent site selectivity of the C-ring Claisen/Diels–Alder rearrangement for the formation of the desired caged structure, the concomitant formation of isomers 1a and 22a decreased the efficiency of the overall process. However, converting the C1 hydroxyl group of 17a to its corresponding acetate 17b circumvented this issue. Heating 17b in DMF gave rise to 1-O-acetylforbesione (1b) exclusively and, after deprotection, afforded forbesione (1a) in 71% combined yield. Intrigued by the effect of the acetyl group of 1b on the site selectivity of the A-ring Claisen rearrangement, we repeated these studies using 1-O-methyl ether 17c as the starting material. In this case we were able to isolate 1-O-methylforbesione (1c) together with 1-O-methylneoforbesione (23c) in 51% and 24% yield, respectively. The complete experimental procedures and spectroscopic data for all synthesized compounds are included in supporting information.
With forbesione (1a) in hand, attention was turned toward the development of conditions for the functionalization of the C2 center en route to other members of this family. We found that propargylation of the C3 phenol of 1a by using 21 under the previously explored conditions, followed by Lindlar reduction and Claisen rearrangement of resulting alkene, produced desoxygaudichaudione (4) in 34% overall yield (Scheme 2). Along these lines, propargylation of 1a, followed by a Claisen cyclization of the ensuing alkyne, afforded desoxymorellin (5) in 61% overall yield. In a similar manner, condensation of 1a with citral (24) by using triethylamine and CaCl2·H2O in MeOH, afforded gambogin (10) in a 75% overall yield.
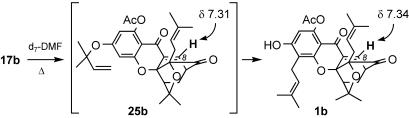
Timing of the Claisen/Diels–Alder/Claisen Reaction Cascade. Having demonstrated the applicability of Claisen/Diels–Alder/Claisen cascade to a unified synthesis of the caged Garcinia natural products, we sought to examine the origins of the observed site selectivity. For this evaluation to be accurate, we needed to know whether the C-ring Claisen/Diels–Alder cascade preceded the A-ring Claisen rearrangement. Although we were unable to isolate the putative intermediate 25, in which only the Claisen/Diels–Alder cascade has occurred, we have obtained spectroscopic evidence suggesting its short-lived existence. Our results are highlighted in Scheme 3. Gradual heating of 17b produced a new doublet at 7.31 ppm (J = 6.7 Hz) that is indicative of the C8 vinylic proton of the 4-oxatricyclo[4.3.1.03,7]dec-8-en-2-one scaffold and specifically, of intermediate 25b. This signal appeared only transiently and gradually disappeared with concomitant appearance of a new doublet at 7.34 ppm (J = 7.0 Hz), which corresponds to the C8 vinylic proton of 1-O-acetylforbesione (1b). Other, less conspicuous, regions of the 1H NMR show the same trend and lead to the same conclusion: the C-ring Claisen/Diels–Alder rearrangement occurs first and is followed by the A-ring Claisen rearrangement, in this case, giving 1-O-acetylforbesione (1b). The spectroscopic data of this study are included in supporting information.
Selectivity of the C-Ring Claisen/Diels–Alder Rearrangement. The surprising results obtained during the cyclization of acyclic precursors 17a, 17b, and 17c (see Scheme 1) triggered several questions regarding the selectivity of the Claisen/Diels–Alder rearrangement. Notably, why do adducts 17a and 17b form, upon heating, exclusively the forbesione caged motif (regular caged scaffold), whereas methyl ether 17c produces both the regular caged scaffold and the isomeric the neo caged motif? Why does derivatization of the remote C1 hydroxyl group affect the selectivity of this cascade? Also why does the initial ortho Claisen rearrangement take place exclusively across the C5–C6 bond?
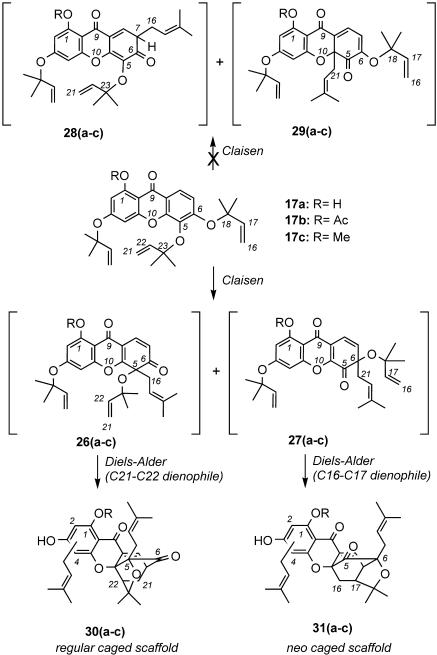
In principle, both the C5 and C6 allyl ethers can undergo the initial [3,3]-sigmatropic rearrangement to produce a set of 4 possible Claisen intermediates, represented by structures 26–29 (Scheme 4). The observed selectivity can be rationalized by the electronic effects of both the C9 carbonyl group and the xanthone oxygen (O10). Being para to the C6 allyloxy unit, the electronically deficient C9 carbonyl carbon can polarize the O–C18 bond and facilitate its rupture, leading to intermediate 26 (Scheme 4). This argument implies the development of partial charges during the transition state of the Claisen rearrangement. Such a polar transition state has been supported by both computational studies (27, 28) and kinetic isotope effects, showing that the O–Cα bond is more broken (50–60%) than the C–Cγ bond is formed (10–20%) (29). Polar solvents offset the development of such charges (30, 31), which are further stabilized by the presence of the geminal dimethyl unit at the C18 carbon center.
Scheme 4.
Potential Claisen rearrangement intermediates.
The effect of the C9 carbonyl group during the formation of the caged motif becomes evident when it is absent. For example, in related studies directed toward the synthesis of lateriflorone, the Nicolaou group reported a complete loss of site selectivity during the key Claisen/Diels–Alder rearrangement of a substrate that did not have the C9 carbonyl group (32), whereas a similar substrate containing the C9 carbonyl functionality underwent a site-selective rearrangement (33).
Equally important to the influence of the C9 carbonyl group on the Claisen/Diels–Alder's selectivity is the stability conferred to the C6 carbonyl group of intermediate 26 by the xanthone oxygen (O10). Stabilization of the C5 carbonyl functionality of intermediate 27 cannot be achieved in the same way. In a similar manner, stabilization of the C9 carbonyl group by the O10 xanthone oxygen may explain why we do not observe formation of Claisen adducts 28 and 29. This stabilization by resonance is possible in intermediates 26 and 27 but is interrupted in structures 28 and 29. This argument could explain why the C-ring Claisen rearrangement occurs exclusively across the C5–C6 bond (Scheme 4) (34). Similar observations have been reported during a recent application of the aromatic Claisen rearrangement (35).
A combination of the above effects can explain the origins of the site selectivity of the Claisen/Diels–Alder rearrangement. In the case of 17a (R = H) the C9 carbonyl group is bound to the C1 OH by a hydrogen bond, which increases its electronic deficiency. This leads to a high selectivity of the Claisen rearrangement producing intermediate 26a exclusively and, after Diels–Alder reaction with the pendant C21–C22 dienophile, affords only adduct 30a, and thus, only the regular caged scaffold. The trisubstituted alkene cannot participate as a dienophile during the Diels–Alder reaction because of its decreased reactivity. Similar results are obtained when 17b (R = Ac) is used as the acyclic xanthone precursor, suggesting that the inherent reactivity of the C9 carbonyl center suffices for the site selectivity of the Claisen/Diels–Alder rearrangement. However, a partial loss of the site selectivity is observed with the 1-O-methylated xanthone 17c (R = Me). This result can be explained if we consider that in 17c the presence of the C1 methyl ether attenuates the withdrawing effect of the C9 carbonyl group. This effect reduces the preference for cleavage of the O–C18 bond and allows a competing rearrangement using the C5 allyloxy ether to take place. In the case of 17c, competition of these processes produces a mixture of Claisen adducts 26c and 27c and thereby a mixture of final products 30c (regular caged scaffold) and 31c (neo caged scaffold) in an approximate ratio of 2:1 (Scheme 4). Similar results were also reported by Nicolaou and Li (16) during their synthesis of 1-O-methylforbesione, although the role of the O1 substitution was not identified.
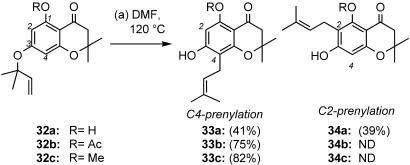
Selectivity of the A-Ring Claisen Rearrangement. Knowing the timing of the Claisen/Diels–Alder/Claisen cascade allowed us to focus on the site selectivity of the A-ring Claisen rearrangement. Because the NMR experiments suggest that the 4-oxatricyclo[4.3.1.03,7]dec-8-en-2-one system formed before the A-ring Claisen rearrangement, we decided to evaluate this reaction in a simpler system. Compounds 32a–c, having a single aromatic residue, were selected for this study. Experimental procedures and spectroscopic data for these compounds are included in supporting information.
Heating the model systems 32a–c at 125°C in DMF yielded results similar to those obtained during our synthesis of forbesione (Scheme 1). Using the underivatized 32a, we obtained a 1/1 mixture of C2 and C4 prenylated products (33a and 34a) (Scheme 5). When either the 1-O-acetyl or the 1-O-methyl model system was heated we saw exclusive formation of respective C4-prenylated products 33b and 33c in 75% and 82% yield. These results validate our use of these A-ring models to probe the site selectivity of the A-ring Claisen in our natural product systems and also support the conclusion of our high-temperature NMR studies.
Scheme 5.
Reagents and conditions: reaction a, DMF, 125°C, 1 h, 41% yield of 33a, 39% yield of 34a; 75% yield of 33b; 82% yield of 33c. ND, not detected.
The above results may be explained on the basis of increased steric hindrance by the R group during C2 prenylation. In the case of 32a the C1 phenolic hydrogen is bound to the adjacent carbonyl group and does not hinder the [3,3]-sigmatropic rearrangement at the C2 carbon. This, in turn, leads to a mixture of C2 and C4 prenylation in an equal ratio. However, in the case of 32b (R = Ac) and 32c (R = Me) the R group is forced to reside away from the carbonyl oxygen and hinders any approach at the C2 carbon center. In these cases the Claisen rearrangement proceeds exclusively at the more accessible C4 carbon center.
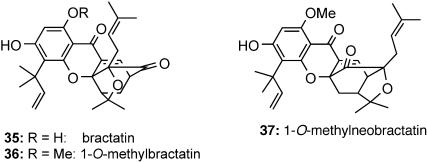
Potential Implications of the Claisen/Diels–Alder/Claisen Rearrangement for the Biosynthesis of Garcinia Natural Products. Our findings on the tandem Claisen/Diels–Alder/Claisen rearrangement in allyloxy xanthones could provide useful insights regarding the biosynthesis of all known caged Garcinia natural products. A literature survey indicates that all these naturally occurring compounds share a common caged structure exemplified by the simplest among them, forbesione (1a). The only exception to this trend is provided by the structure of 1-O-methylneobractatin (37), which contains the alternative, neo scaffold (3) (Fig. 4). This compound was isolated from extracts of the dried and powdered leaves of Garcinia bracteata as a minor constituent along with 1-O-methylbractatin (36) and bractatin (35) (3). The presence of the seemingly innocuous 1-O-methyl group may explain the concomitant biosynthesis of both 37 and 36. Guided by our results, we propose that, in the methylbractatin series, the 1-O-methyl group was incorporated by nature before the tandem Claisen/Diels–Alder/Claisen rearrangement.
Fig. 4.
Survey of natural products from Garcinia bracteata.
Conclusion
In conclusion, we have planned and put into action a unified strategy for the synthesis of caged Garcinia natural products, namely forbesione (1a), desoxygaudichaudione A (4), desoxymorellin (5), and gambogin (10). In addition to the total syntheses presented herein, the timing of the Claisen/Diels–Alder/Claisen cascade has been investigated by high-temperature 1H NMR and found to lead with the C-ring Claisen/Diels–Alder rearrangement and follow with the A-ring Claisen reaction. The site selectivity of both the A- and C-ring Claisen rearrangements have been rationalized, the former with models that mirror the reactivity of the natural product precursors to forbesione (1a), isoforbesione (23a), 1-O-acetylforbesione (1b), and 1-O-methylforbesione (1c).
It is apparent that every functionality present in the structure of xanthone precursor plays a crucial role in the biosynthesis of these natural products. The C9 carbonyl group, the O10 xanthone oxygen, and the C18 dimethylallyl substituent are strategically placed to facilitate the construction of the regular caged motif, by stabilizing the polar transition state of the C-ring Claisen rearrangement. Moreover, remote electronic effects influence the site selectivity of this rearrangement and may operate during the biosynthesis of the caged motif. In addition, the Claisen rearrangement at the A ring is controlled by steric effects that facilitate the C4 prenylation and pave the way for subsequent substitutions. The biological potential of the caged motif of these natural products still needs to be systematically evaluated. Our observations provide a testament to the unique opportunities offered by organic synthesis to decode information encrypted in the structures of natural products.
Supplementary Material
Acknowledgments
Financial support from the National Institutes of Health (Grant CA086079) is gratefully acknowledged. We also thank the San Diego Chapter of the Achievement Rewards for College Scientists (ARCS) Foundation for their support through graduate student fellowships to E.J.T.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: DMF, dimethylformamide.
References
- 1.Ollis, W. D., Redman, B. T., Sutherland, I. O. & Jewers, K. (1969) J. Chem. Soc. Chem. Commun., 879-880.
- 2.Kumar, P. & Baslas, R. K. (1980) Herba Hung. 19, 81-91. [Google Scholar]
- 3.Thoison, O., Fahy, J., Dumontet, V., Chiaroni, A., Riche, C., Tri, M. V. & Sevenet, T. (2000) J. Nat. Prod. 63, 441-446. [DOI] [PubMed] [Google Scholar]
- 4.Gruenwald, J., Brendler, T. & Jaenicke, C., eds. (2000) PDR for Herbal Medicines (Medical Economics, Montvale, NJ), 2nd Ed., pp. 325-326.
- 5.Kartha, G., Ramachandran, G. N., Bhat, H. B., Nair, P. M., Raghavan, V. K. V. & Venkataraman, K. (1963) Tetrahedron Lett. 4, 459-462. [Google Scholar]
- 6.Leong, Y.-W., Harrison, L. J., Bennett, G. J. & Tan, H. T.-W. (1996) J. Chem. Res. Synop., 392-393.
- 7.Venkataraman, K. (1974) Proc. Indian Natl. Sci. Acad. Part A Phys. Sci. 39, 365-381. [Google Scholar]
- 8.Karanjgaonkar, C. G., Nair, P. M. & Venkataraman, K. (1966) Tetrahedron Lett. 7, 687-691. [Google Scholar]
- 9.Ollis, W. D., Ramsay, M. V. J., Sutherland, I. O. & Mongkolsuk, S. (1965) Tetrahedron 21, 1453-1470. [Google Scholar]
- 10.Asano, J., Chiba, K., Tada, M. & Yoshii, T. (1996) Phytochemistry 41, 815-820. [DOI] [PubMed] [Google Scholar]
- 11.Cao, S.-G., Wu, X.-H., Sim, K.-Y., Tan, B. K. H., Pereira, J. T., Wong, W. H., Hew, N. F. & Goh, S. H. (1998) Tetrahedron Lett. 39, 3353-3356. [Google Scholar]
- 12.Ahmad, S. A., Rigby, W. & Taylor, R. B. (1966) J. Chem. Soc. C, 772-779.
- 13.Zhang, H.-Z., Kasibhatla, S., Wang, Y., Herich, J., Guastella, J., Tseng, B., Drewe, J. & Cai, S.-X. (2004) Bioorg. Med. Chem. 12, 309-317. [DOI] [PubMed] [Google Scholar]
- 14.Wu, X., Cao, S., Goh, S., Hsu, A. & Tan, B. K. H. (2002) Planta Med. 68, 198-203. [DOI] [PubMed] [Google Scholar]
- 15.Quillinan, A. J. & Scheinmann, F. (1971) J. Chem. Soc. Chem. Commun., 966-967.
- 16.Nicolaou, K. C. & Li, J. (2001) Angew. Chem. Int. Ed. Engl. 40, 4264-4268. [DOI] [PubMed] [Google Scholar]
- 17.Tisdale, E. J., Chowdhury, C., Vong, B. G., Li, H. & Theodorakis, E. A. (2002) Org. Lett. 4, 909-912. [DOI] [PubMed] [Google Scholar]
- 18.Tisdale, E. J., Slobodov, I. & Theodorakis E. A. (2003) Org. Biomol. Chem. 1, 4418-4422. [DOI] [PubMed] [Google Scholar]
- 19.Ishiguro, K., Nagareya, N., Suitani, A. & Fukumoto, H. (1997) Phytochemistry 44, 1065-1066. [Google Scholar]
- 20.Chung, M.-I., Weng, J.-R., Lai, M.-H., Yen, M.-H. & Lin, C.-N. (1999) J. Nat. Prod. 62, 1033-1035. [DOI] [PubMed] [Google Scholar]
- 21.Burling, E. D., Jefferson, A. & Scheinmann, F. (1965) Tetrahedron 21, 2653-2669. [Google Scholar]
- 22.Pettus, T. R. R. & Hoarau, C. (2003) Synlett, 127-137. [DOI] [PMC free article] [PubMed]
- 23.Perrin, R., Muyard, F., Bevalot, F., Tillequin, F. & Vaquette, J. (2000) J. Nat. Prod. 63, 245-247. [DOI] [PubMed] [Google Scholar]
- 24.Hlubucek, J., Ritchie, E. & Taylor, W. C. (1971) Aust. J. Chem. 24, 2355-2363. [Google Scholar]
- 25.Tisdale, E. J., Kochman, D. A. & Theodorakis, E. A. (2003) Tetrahedron Lett. 44, 3281-3284. [Google Scholar]
- 26.Tisdale, E. J., Vong, B. G., Li, H., Kim, S. H., Chowdhury, C. & Theodorakis, E. A. (2003) Tetrahedron 59, 6873-6887. [Google Scholar]
- 27.Coates, R. M., Rogers, B. D., Hobbs, S. J., Peck, D. R. & Curran, D. P. (1987) J. Am. Chem. Soc. 109, 1160-1170. [Google Scholar]
- 28.Gajewski, J. J., Jurayi, J., Kimbrough, D. R., Gande, M. E., Ganem, B. & Carpenter, B. K. (1987) J. Am. Chem. Soc. 109, 1170-1186. [Google Scholar]
- 29.Kupczyk-Subotkowska, L., Saunders, W. H., Jr., & Shine, H. J. (1988) J. Am. Chem. Soc. 110, 7153-7159. [Google Scholar]
- 30.Gajewski, J. J. (1997) Acc. Chem. Res. 30, 219-225. [Google Scholar]
- 31.Wipf, P. & Rodriguez, S. (2002) Adv. Synth. Catal. 344, 434-440. [Google Scholar]
- 32.Nicolaou, K. C., Sasmal, P. K., Xu, H., Namoto, K. & Ritzen, A. (2003) Angew. Chem. Int. Ed. Engl. 42, 4225-4229. [DOI] [PubMed] [Google Scholar]
- 33.Tisdale, E. J., Li, H., Vong, B. G., Kim, S. H. & Theodorakis, E. A. (2003) Org. Lett. 5, 1491-1494. [DOI] [PubMed] [Google Scholar]
- 34.Murray, R. D. H., Sutcliffe, M. & Hasegawa, M. (1975) Tetrahedron 31, 2966-2972. [Google Scholar]
- 35.Pettus, T. R., Inoue, M., Chen, X.-T. & Danishefsky, S. J. (2000) J. Am. Chem. Soc. 122, 6160-6168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.