Abstract
A unified approach for the construction of the potent marine antitumor agents (+)-tedanolide (1) and (+)-13-deoxytedanolide (2) is described. Highlights of the synthetic strategy include the development of a versatile bifunctional dithiane-vinyl iodide linchpin, the unorthodox use of the Evans–Tishchenko reaction, and a late-stage high-risk stereocontrolled introduction of the C(18,19) epoxide to achieve a total synthesis of (+)-13-deoxytedanolide (2).
Driven by cytotoxicity and in vivo tumor inhibition studies (1), Schmitz and coworkers (2) in 1984 reported the structure of the architecturally novel macrolide, (+)-tedanolide (1, Scheme 1), isolated from the abundant Caribbean sponge, Tedania ignis (fire sponge). Seven years later, the Fusetani group (3) disclosed the closely related congener, 13-deoxytedanolide (2), from the Japanese sponge, Mycale adhaerens. In vitro studies revealed that (+)-tedanolide (1) possesses an ED50 of 0.25 ng/ml and 1.6 pg/ml in KB and PS cells, respectively, and causes cell accumulation in the S phase of the cell cycle at concentrations as low as 0.01 μg/ml (2). Similarly, (+)-13-deoxytedanolide (2) expresses an IC50 of 94 pg/ml against P388 murine leukemia cells (3). Of considerable importance, 1 increases the lifespan of mice implanted with lymphocytic leukemia by 23% at 1.5 μg/kg of body weight (4), and 2 slows the growth rate of P388 tumors with a T/C value of 189% at a dose of 0.125 mg/kg [where T is the median time in days for the tumors of the treated group to reach 750 or 1,000 mg and C is the median time in days for the tumors of the control group to reach 750 or 1,000 mg (3)]. No information, however, on the biochemical mechanism of action of the tedanolides has been reported.
Scheme 1.
Intrigued with the structural complexity of the tedanolides, in conjunction with the significant biological profiles and the lack of a ready source for further biomedical studies, we initiated a synthetic program in the mid 1990s. Other groups have also been attracted by the synthetic challenge of the tedanolides (5–16).
Materials and Methods
General. Except as otherwise indicated, all reactions were carried out under an argon atmosphere in flame- or oven-dried glassware, all reagents were purchased from Aldrich, Acros, and Strem Chemicals (Newburyport, MA), and solvents were freshly distilled.
Chemical Synthesis. Details for the synthesis of (+)-13-deoxytedanolide and intermediates, as well as all spectroscopic/analytical data, are provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Synthetic Strategy
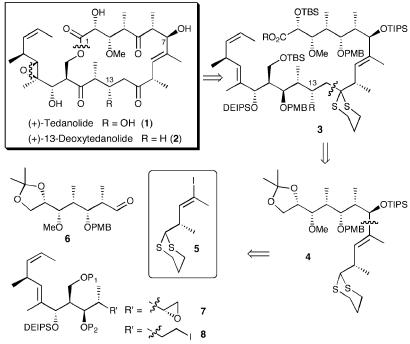
At the outset, we envisioned use of a bifunctional dithiane linchpin (cf. 5, Scheme 1), a tactic exploited to great advantage in our syntheses of the rapamycins (17–19). For the tedanolides, this scenario called for the construction of a common advanced C(1–11) fragment (4) containing the C(8–9) trisubstituted olefin. Reaction of 5 with common aldehyde 6 followed sequentially by dithiane union either with epoxide 7 or primary iodide 8, macrocyclization, and a late-stage high-risk introduction of the C(18,19) epoxide would, after functional group adjustments, lead, respectively, to (+)-tedanolide (1) and (+)-13-deoxytedanolide (2).
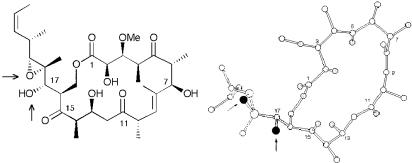
The clear danger (i.e., high risk) of this strategy was the proposed late-stage epoxidation event requiring both chemo- and stereoselectivity at the C(18,19) trisubstituted olefin. The alternative would be to carry forward what was thought to be a highly unstable trisubstituted epoxide through many synthetic operations. The electron-rich nature of the C(18,19) trisubstituted olefin, in conjunction with the free C(17) hydroxyl, suggested that the epoxide could be installed chemoselectively. Less certain was the stereochemical outcome. Here we envisioned taking advantage of the directing ability of the C(17) hydroxyl (20, 21). Support for this proposition derived from the crystal structure of (+)-tedanolide (1), wherein the C(17) hydroxyl is oriented appropriately to direct epoxidation (Fig. 1). Although we were aware that the solid-state conformation of tedanolide may not necessarily reflect the solution structure, we reasoned that the crystal structure implied a greater potential for successful epoxidation. An additional strategic element called for carrying the C(5) and C(15) ketones as hydroxyls to prevent epimerization at the α centers; judicious use of protecting groups would of course prove paramount to the success of this synthetic venture. Finally, the feasibility of generating the seco acid functionality in the presence of the dithiane moiety was of some concern (vide infra).
Fig. 1.
X-ray structure of tedanolide depicting the orientation of the C(17) hydroxyl.
Construction of the Northern Hemisphere of the Tedanolides
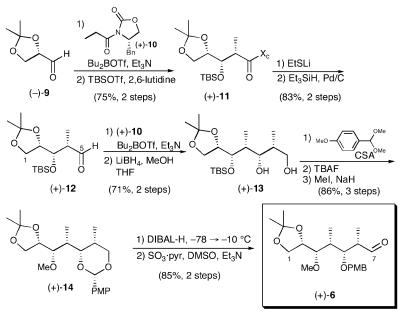
Analysis of aldehyde 6 revealed that the C(3–6) polypropionate motif was ideally suited for construction via successive Evans aldol condensations (22). We thus began the synthesis with (S)-(-)-glyceraldehyde acetonide (-)-9, available via a modification of the Hubschwerlen protocol (Scheme 2) (23, 24). Highlights of the sequence include reduction of amide (+)-11 to the requisite aldehyde without epimerization, exploiting the two-step Fukuyama thioester formation/palladium-catalyzed reduction protocol (25), and regioselective opening of the p-methoxybenzylidene acetal with diisobutylaluminum hydride (DIBAL-H) (26). The overall sequence required 11 steps and proceeded in 32% yield. The stereochemistry of (+)-13 was confirmed by selective acylation of the primary hydroxyl, removal of the TBS protecting group and acetonide formation. Analysis (13C NMR) a là Rychnovsky and Evans confirmed the depicted stereochemistry (27–29).
Scheme 2.
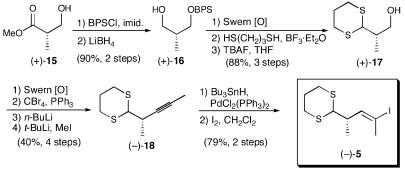
With the northern hemisphere aldehyde in hand, we turned to the construction of the bifunctional linchpin dithiane 5 (Scheme 3), beginning with commercially available (S)-3-hydroxy-2-methylpropionate [Roche's ester (+)-15]. This sequence proved equally straightforward, producing (-)-5 in 26% yield for the 11 steps. Of interest here was the Corey–Fuchs homologation (30) and the Guibé regioselective hydrostannylation (31). The resultant mixture of vinyl stannanes (6:1) was converted to a mixture of iodides readily separable by column chromatography. The vinylstannane was originally investigated as a linchpin; however, problems in the lithiation event made the vinyl iodide strategy much more attractive (24).
Scheme 3.
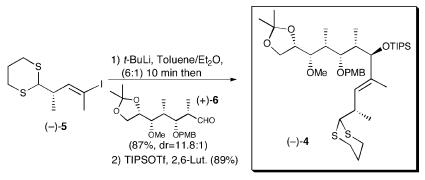
Elaboration of the bifunctional linchpin began with metal–halogen exchange (t-BuLi), followed by addition to aldehyde (+)-6, employing tetrahydrofuran (THF) as solvent (Scheme 4). A mixture of diastereomeric alcohols (58%) resulted. As expected, the desired β-epimer predominated, albeit with only a 2.7:1 advantage. Oxidative removal of the p-methoxybenzyl (PMB) group of the adduct and acetonide formation permitted determination of the stereochemistry via Rychnovsky–Evans acetonide analysis (27–29). All attempts to convert the undesired α-epimer to the β-epimer (Mitsunobu inversion, oxidation–reduction) proved unsuccessful. Aware that solvent can play a significant role in the stereoselectivity of such reactions (32), we explored alternative solvent systems. The best results were obtained with toluene:ether (6:1, vol/vol), which furnished the desired β-epimer both with high selectivity (11.8:1) and in excellent yield (87%). Silylation with triisopropylsilyl triflate (TIPSOTf) permitted facile chromatographic separation of the diastereomers. The linear synthetic sequence for (-)-4 required 13 steps and proceeded in 23% overall yield from glyceraldehyde acetonide (-)-9 (e.g., 89% average yield per step) (33).
Scheme 4.
Construction of the Tedanolide Southern Hemispheres
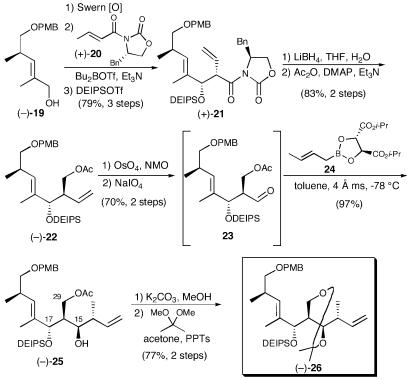
With (-)-4 in hand, we envisioned the requisite coupling partners, epoxide (7) and iodide (8), respectively, for (+)-tedanolide (1) and (+)-13-deoxytedanolide (2) to arise from a common, late-stage olefin such as (-)-26 (Scheme 5). The synthesis began with known alcohol (-)-19 (10). Swern oxidation, followed by an Evans aldol employing N-acylated oxazolidinone (+)-20 (34, 35), provided the aldol adduct as a single stereoisomer. Protection as the diethylisopropylsilyl (DEIPS) ether using freshly prepared DEIPSOTf (36) led to (+)-21 (95% yield). Reductive removal of the auxiliary and acylation (Ac2O, DMAP, and Et3N) of the resulting hydroxyl then furnished (-)-22. Although this transformation proved problematic because of competitive reduction of the oxazolidinone ring, the opened auxiliary byproduct could be readily recycled upon treatment with carbonyldiimidazole (CDI) and NaH in excellent yield (91%). Thus, the material could be recycled to afford the desired alcohol in 86% yield after one recycle. Dihydroxylation and diol cleavage provided aldehyde 23 (70% yield, two steps), which in turn was subjected to Brown crotylboration (37, 38). Forcing conditions, however, were required to hydrolyze the intermediate boronate. The Roush crotylboration alternative, a protocol known to proceed via the more labile tartrate-based boronic esters (39), furnished a single isomer in excellent yield; importantly, the boronic ester was readily hydrolyzed (1 M NaOH wash). Desilylation of (-)-25 and acetonide formation permitted confirmation of the stereochemistry via Rychnovsky–Evans acetonide analysis (27–29).
Scheme 5.
At this stage, careful analysis of the protecting group strategy for the common olefin (-)-26 appeared prudent. Indeed, the protecting group choice for the C(15), C(17), and C(29) hydroxyls proved to be one of the most critical aspects of the strategy for the C(12–23) fragments. After significant experimentation and analysis (40) we decided to retain the C(17) hydroxyl as the DEIPS ether. In addition, we envisioned installation of the C(21,22) Z-olefin later in the synthesis, while the C(15) and C(29) hydroxyls would be protected as a cyclic acetal. In turn, the cyclic acetal would be removed just before macrolactonization, to reveal the primary and secondary hydroxyls, which we anticipated could be readily differentiated during the macrolactonization.
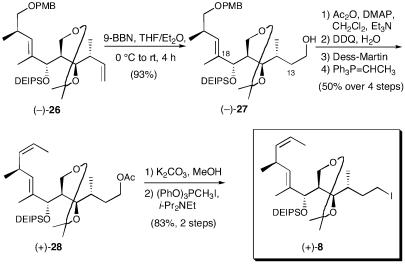
To this end, acetate (-)-25 was saponified and the acetonide was installed [Dess–Martin periodinane, acetone, and pyridinium p-toluenesulfonate (PPTs)] to furnish (-)-26, the common precursor for both southern hemisphere fragments (Scheme 5). We were now at the point of divergence in the synthetic strategy. The first step en route to (+)-13-deoxytedanolide (2) would entail chemoselective hydroboration. Several conditions were explored (Scheme 6) before we discovered that treatment with 9-borabicyclo[3.3.1]nonane (BBN) (41) in Et2O/THF (0°C to room temperature, 4 h), followed by oxidative work-up, would deliver the requisite primary alcohol as the sole product in excellent yield (93%).
Scheme 6.
Acylation of alcohol (-)-27, followed by oxidative removal of the PMB group and Dess–Martin oxidation (42) led to the corresponding aldehyde, which was immediately subjected to Wittig olefination to furnish Z-olefin (+)-28. Pleasingly, none of the E-isomer could be detected. Removal of the acetate in (+)-28 and conversion to iodide (+)-8 by using methyltriphenoxyphosphonium iodide (43) completed the coupling partner for (+)-13-deoxytedanolide (2). The overall yield of (+)-8 was 6.7%, requiring 22 steps from Roche's ester (+)-15, with an average yield per step of 88%.
With the coupling partner for 13-deoxytedanolide in hand, we next completed the construction of the related epoxide (7, Scheme 1) for the proposed (+)-tedanolide (1) synthesis. We continue here with our account of the (+)-13-deoxytedanolide (2) total synthesis.
Union of the Northern and Southern Hemispheres of (+)-13-Deoxytedanolide
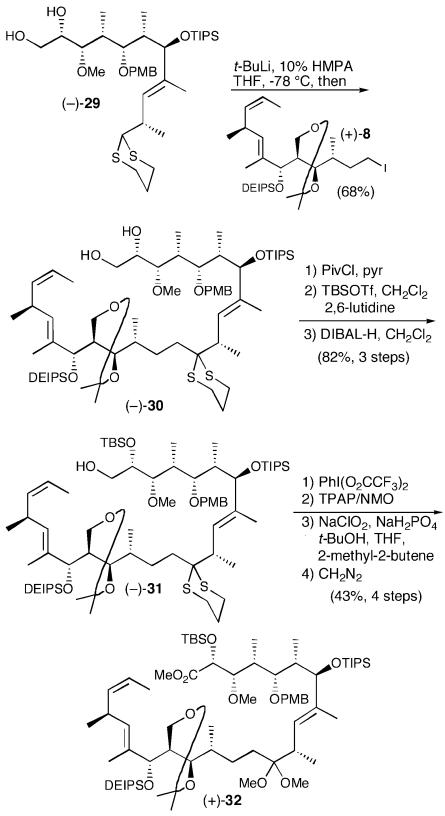
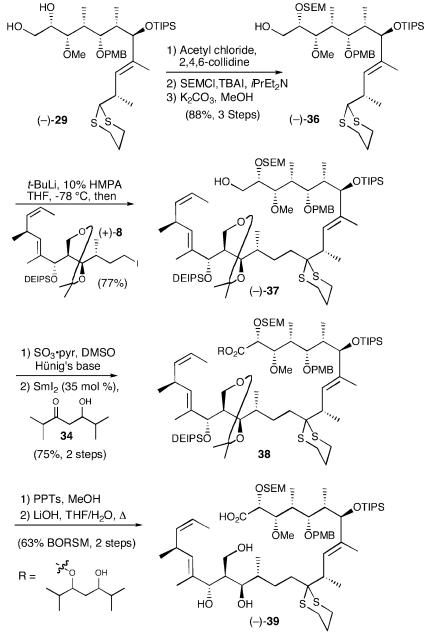
Because of our extensive experience with dithiane-based strategies, we were well aware that both the lithiation and reaction of complex dithianes can sometimes be challenging (44). Indeed, although we could document the formation of the dithiane anion derived from (-)-4 (Scheme 4) by deuterium incorporation, efficient coupling could only be accomplished with the simple model, benzyl (S)-(+)-glycidyl ether (33). Undaunted, we reexamined our synthetic strategy. We reasoned that the C(1,2) acetonide moiety in (-)-4 would not be workable as the endgame evolved (vide infra). We therefore explored a series of alternatively protected dithianes, as well as a range of solvent systems. After significant experimentation (40), we discovered that the tri-anion (!) of diol (-)-29 [t-BuLi, 10% hexamethylphosphoramide (HMPA)/THF] proved to be the optimal coupling partner, furnishing (-)-30, comprising the complete carbon skeleton of (+)-13-deoxytedanolide (2) in 68% yield (Scheme 7). The successful lithiation conditions comprised a slight modification of those introduced by Williams (45).
Scheme 7.
The seco-acid required for macrocyclization now seemed close at hand. All that would be required was differentiation of the C(1) and C(2) hydroxyls, oxidation of the C(1) hydroxyl to the carboxylic acid, and removal of the C(15,29) acetonide. To this end, selective formation of the primary pivaloate, followed by silylation (TBSOTf) and reductive removal of the pivaloyl group provided the desired alcohol (-)-31 in high yield (82%, three steps). Unfortunately, oxidation of the primary hydroxyl to the requisite carboxylic acid proved difficult in the extreme because of the oxidative lability of the dithiane moiety (vide infra).
The obvious solution appeared to be removal of the dithiane before oxidation of the C(1) hydroxyl. With this goal in mind, treatment of (-)-31 in MeOH with PhI(O2CCF3)2, Stork's hypervalent iodide protocol (46), converted the dithiane to the corresponding dimethyl ketal (93%). Ley oxidation (47), followed by Pinnick oxidation (48, 49) and immediate treatment with diazomethane, furnished the desired methyl ester (+)-32 in modest overall yield (46%, three steps).
Access to the seco-ester next required removal of the C(15,29) acetonide. Earlier studies indicated that acid-catalyzed hydrolysis would result in concomitant loss of the DEIPS protecting group. We anticipated that the resulting triol, however, would be synthetically useful, because all three hydroxyls would reside in significantly different steric environments. Unfortunately, attempts to access the triol via removal of the C(15,29) acetonide and the DEIPS group proved unsuccessful. Only complex mixtures of unproductive ketalization products at C(11) were obtained. These results, taken together with our inability to effect oxidation of the C(1) hydroxyl in the presence of the dithiane moiety, indicated that a new endgame scenario would be required.
Clearly, there existed a need for a reliable method to oxidize aldehydes to the corresponding carboxylic acid or derivative thereof in the presence of electron-rich elements such as sulfur. Standard oxidants such as Dess–Martin periodinane (42), tetrapropylammonium peruthenate/4-methylmorpholine N-oxide (TPAP/NMO) (47), pyridinium chlorochromate (PCC) (50), and 2,2,6,6-tetramethyl-1-piperidinyloxy/bleach (51) are simply not viable (40). On the other hand, the oxidation of primary alcohols to aldehydes in the presence of a dithiane is straightforward when using DMSO-based oxidations (52). These methods, however, are not usually capable of further oxidation.
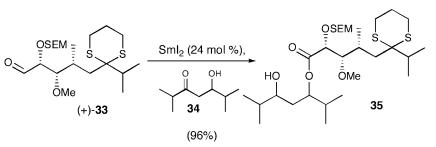
Having exhausted the standard arsenal of oxidants (53) by using model system (+)-33 (Scheme 8), we turned to an unorthodox implementation of the Evans–Tishchenko reaction (54, 55). This transformation, most often viewed as the reduction of a β-hydroxy ketone to the corresponding trans-1,3-diol system, involves intramolecular hydride transfer to generate the β-hydroxyl carrying a sacrificial acyl group. Why not sacrifice the trans-1,3-diol? Indeed the reaction upon development proved highly reliable, not only in the presence of a dithiane moiety, but also with other highly electron-rich heteroatoms (56). During this exercise, we also found the presence of the 2-(trimethylsilyl)ethoxymethyl (SEM) protecting group to be advantageous in the subsequent saponification of ester 35; the use of a TBS protecting group led to desilylation during saponification.
Scheme 8.
The lessons learned from the model studies quickly led to a new dithiane coupling partner, SEM ether (-)-36, as outlined in Scheme 9. Construction of the carbon skeleton of (+)-13-deoxytedanolide was then achieved by generation of the dianion of (-)-36 with 2.05 eq of t-BuLi, followed by addition of 1.1 eq of iodide (+)-8; the coupling proved quite efficient, with a yield of 77%. With (-)-37 in hand, we turned to the critical oxidation event. As now expected, Parikh–Doering oxidation (57) of (-)-37 provided the corresponding aldehyde, which upon treatment with 35 mol % SmI2 (58, 59) in the presence of β-hydroxy ketone 34 (54) afforded a diastereomeric mixture of esters (38), effectively achieving oxidation at C(1); the yield for the two steps was again good (75%). Removal of the acetonide, also as expected, led to concomitant loss of the DEIPS group. Attempts to access the macrocycle directly from the triol via transesterification employing KHMDS, LHMDS, or distannoxanes were unsuccessful (60). Saponification of the resultant triol-ester furnished the seco-acid in an overall yield of 63% for the two steps, based on recovered starting material (BORSM). The delicate nature of this intermediate was demonstrated by the requirement to carry the transformation to only partial conversion followed by recyclization.
Scheme 9.
Macrolactonization and Final Elaboration to (+)-13-Deoxytedanolide
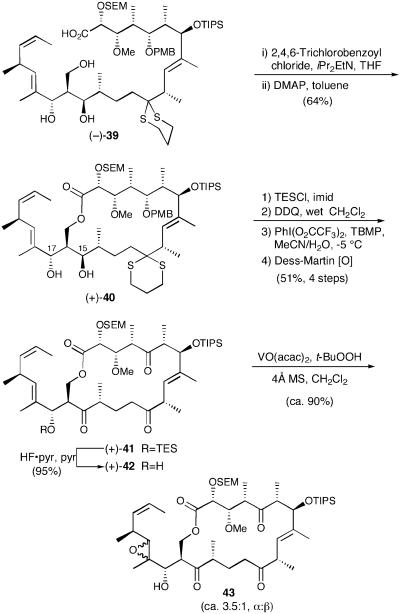
Pleasingly, the critical macrocyclization employing the Yonemitsu (61) modification of the Yamaguchi protocol (62) led to (+)-40 in 64% yield (Scheme 10). Confirmation that the macrocyclization event occurred at the C(29) hydroxyl was deduced via a series of careful 1H, 13C, 13C distortionless enhancement by polarization transfer, and heteronuclear multiple quantum correlation experiments. Completion of the synthesis of (+)-13-deoxytedanolide (2) would now require differentiation of the C(15) and C(17) hydroxyls. We reasoned that the C(17) allylic hydroxyl, required for the late-stage directed epoxidation of the C(18,19) olefin, was less sterically encumbered than the C(15) hydroxyl and therefore could be selectively protected. In the event, silylation (TESCl) furnished the desired silylated allylic alcohol as the sole product in excellent yield. Analysis (1H NMR) showed a significant change in olefinic resonances, thus providing evidence indicating that the C(17) allylic hydroxyl was indeed silylated preferentially. Oxidative removal of the PMB group, followed by removal of the dithiane with PhI(O2CCF3)2, again employing the Stork method (46), furnished an equilibrium mixture of the corresponding ketone and the hemiketal, involving the C(15) hydroxyl; treatment with Dess–Martin periodinane then led to triketone (+)-41. The overall yield for the four steps was 51%.
Scheme 10.
With the macrocycle intact and fully elaborated, installation of the C(18,19) trisubstituted epoxide was the next order of business. From the outset, we envisioned that the requisite chemo- and stereoselectivity could be achieved by a hydroxyl-directed epoxidation (20, 21). Toward this end, removal of the TES group in (+)-41 with HF·pyridine/pyridine furnished the requisite allylic alcohol in excellent yield. Unfortunately, epoxidation employing VO(acac)2 with t-BuOOH as the oxidant led to a diastereomeric mixture (≈3.5:1) of C(18,19) epoxides (43), favoring the undesired α-isomer. Assignment of the stereochemistry of the two epoxides was accomplished by comparing the 1H NMR spectrum of 43 to the spectrum of natural 13-deoxytedanolide. The yield, however, was excellent (>90%).
Although disappointed with the initial stereochemical outcome of the epoxidation, the availability of epoxides 43 proved quite valuable in our efforts to devise a viable endgame. Indeed, we quickly learned that removal of the SEM and TIPS ethers would not be straightforward. Acid-catalyzed conditions led rapidly to multiple undesired products. Analysis by 1H NMR implicated epoxide opening and subsequent cyclization with the C(21,22) olefin as one of the contributing factors. Fluoride anion-mediated processes were also not encouraging. Even the use of the relatively mild conditions of tetrabutylammonium fluoride (TBAF) in THF at 0°C led to almost instantaneous decomposition. Of some note, however, was the use of TBAF in wet N,N′-dimethylpropylene urea (DMPU), which led to selective removal of the TIPS ether before decomposition.
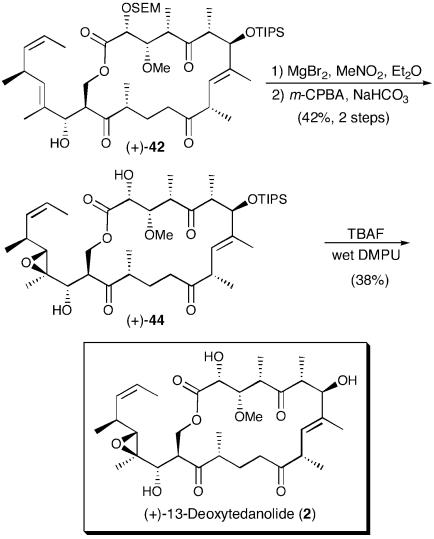
We therefore investigated removal of the SEM group before epoxidation (Scheme 11). The desired diol was cleanly obtained in high yield (88%), employing the conditions of Hoffmann and Vakalopoulos (63) (MgBr2, MeNO2/Et2O), known to remove selectively the SEM group in the presence of silyl ethers. Subsequent treatment with VO(acac)2 and t-BuOOH afforded a mixture of diastereomeric epoxides again favoring the undesired epoxide (2.5:1, α:β).
Scheme 11.
At this point, we examined alternative epoxidation protocols. The obvious choice was m-chloroperoxybenzoic acid (m-CPBA). At the time we were also aware of the work by Loh and coworkers (14), wherein they reported that the m-CPBA epoxidation of an advanced C(18,19) olefin in their synthetic approach to the tedanolides provided a single isomer in 54% yield. In our case, the standard conditions of m-CPBA and NaHCO3 furnished the desired β-epoxide (+)-44 as the only observable monoepoxide. The modest yield (48%) was presumably due to the formation of by-products attributed to overoxidation (Scheme 11). That the m-CPBA epoxidation gave the desired β-epoxide can be understood by analysis of the x-ray crystal structure of (+)-tedanolide (Fig. 1), noting that the dihedral angle between the C(17) hydroxyl and the C(18,19) C–C bond is nearly 120°, the optimal dihedral angle for m-CPBA oxidations (21). On the other hand, VO(acac)2 epoxidations require a dihedral angle of 40° (21), which would require the C(17) hydroxyl–C(18,19) C–C bond conformation to be significantly different from that observed in the x-ray structure. Such a conformation would induce significant A1,2 strain in a structure that positions the C(17) hydroxyl appropriately to direct the epoxidation to the desired face.
With epoxide (+)-44 in hand, all that remained to complete the synthesis of (+)-13-deoxytedanolide (2) was removal of the C(7) TIPS ether. Drawing from what we learned while investigating the deprotection of 43, treatment of (+)-44 with TBAF in wet DMPU at 0°C followed by warming to ambient temperature furnished synthetic (+)-13-deoxytedanolide in 38% yield (64) identical in all respects (e.g., 500 MHz 1H NMR, 125 MHz 13C NMR, high-resolution mass spectra, optical rotation, λmax, TLC in three different solvent systems) to a sample of the natural material. We attribute the modest yield in the final deprotection to the inherent instability of the natural product.
Summary
Total synthesis of the architecturally complex marine natural product (+)-13-deoxytedanolide (2) has been achieved via a highly convergent strategy. Highlights of the synthesis include the efficient use of a bifunctional dithiane-vinyl iodide linchpin to construct the carbon skeleton of the tedanolides, the unorthodox use of the Evans–Tishchenko reaction to generate the seco-ester, and a late-stage high-risk stereocontrolled introduction of the C(18,19) epoxide. The synthesis of (+)-2 was achieved with a longest linear sequence of 36 steps beginning with Roche's ester (+)-15. Full experimental details are provided in Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor Marisa Kozlowski (University of Pennsylvania) for suggesting the Evans–Tishchenko reaction, Dr. Robert Udal for optimizing the union of (-)-5 and (+)-6, and Professor Nobuhiro Fusetani (University of Tokyo) for supplying an authentic sample of (+)-2. This work was supported by National Institutes of Health (Institute of General Medical Sciences) Grant GM-29028.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DEIPS, diethylisopropylsilyl; PMB, p-methoxybenzyl; SEM, 2-(trimethylsilyl)-ethoxymethyl; THF, tetrahydrofuran; TIPS, triisopropylsilyl.
References
- 1.Schmitz, F. J., Vanderah, D. J., Hollenbeak, K. H., Enwall, C. E. L., Gopichand, Y., SenGupta, P. K., Hossain, M. B. & van der Helm, D. (1983) J. Org. Chem. 48, 3941-3945. [Google Scholar]
- 2.Schmitz, F. J., Gunasekera, S. P., Yalamanchili, G., Hossain, M. B. & van der Helm, D. (1984) J. Am. Chem. Soc. 106, 7251-7252. [Google Scholar]
- 3.Fusetani, N., Sugawara, T., Matsunaga, S. & Hirota, H. (1991) J. Org. Chem. 56, 4971-4974. [Google Scholar]
- 4.Schmitz, F. J., Gunasekera, S. P., Hossain, M. B., van der Helm, D. & Yalamanchili, G. (1988) U.S. Patent Application 87-7347.
- 5.Yonemitsu, O. (1994) J. Synth. Org. Chem. Jpn. 52, 946-955. [Google Scholar]
- 6.Matsui, K., Zheng, B.-Z., Kusaka, S.-I., Kuroda, M., Yoshimoto, K., Yamada, H. & Yonemitsu, O. (2001) Eur. J. Org. Chem. 3615-3624.
- 7.Liu, J.-F., Abiko, A., Pei, Z., Buske, D. C. & Masamune, S. (1998) Tetrahedron Lett. 39, 1873-1876. [Google Scholar]
- 8.Jung, M. E., Karama, U. & Marquez, R. (1999) J. Org. Chem. 64, 663-665. [Google Scholar]
- 9.Jung, M. E. & Lee, C. P. (2001) Org. Lett. 3, 333-336. [DOI] [PubMed] [Google Scholar]
- 10.Taylor, R. E., Ciavarri, J. P. & Hearn, B. R. (1998) Tetrahedron Lett. 39, 9361-9364. [Google Scholar]
- 11.Taylor, R. E., Hearn, B. R. & Ciavarri, J. P. (2002) Org. Lett. 4, 2953-2956. [DOI] [PubMed] [Google Scholar]
- 12.Roush, W. R. & Lane, G. C. (1999) Org. Lett. 1, 95-98. [DOI] [PubMed] [Google Scholar]
- 13.Roush, W. R. & Newcom, J. S. (2002) Org. Lett. 4, 4739-4742. [DOI] [PubMed] [Google Scholar]
- 14.Loh, T.-P. & Feng, L.-C. (2001) Tetrahedron Lett. 42, 3223-3226. [Google Scholar]
- 15.Loh, T.-P. & Feng, L.-C. (2001) Tetrahedron Lett. 42, 6001-6005. [Google Scholar]
- 16.Kalesse, M. & Hassfeld, J. (2002) Synlett, 2007-2010.
- 17.Smith, A. B., III, Condon, S. M., McCauley, J. A., Leazer, J. L., Jr., Leahy, J. W. & Maleczka, R. J., Jr. (1997) J. Am. Chem. Soc. 119, 947-962. [Google Scholar]
- 18.Smith, A. B., III, Condon, S. M., McCauley, J. A., Leazer, J. L., Jr., Leahy, J. W. & Maleczka, R. J., Jr. (1997) J. Am. Chem. Soc. 119, 962-973. [Google Scholar]
- 19.Smith, A. B., III, Condon, S. M. & McCauley, J. A. (1998) Acc. Chem. Res. 31, 35-46. [Google Scholar]
- 20.Hoveyda, A. H., Evans, D. A. & Fu, G. C. (1993) Chem. Rev. 93, 1307-1370. [Google Scholar]
- 21.Adam, W. & Wirth, T. (1999) Acc. Chem. Res. 32, 703-710. [Google Scholar]
- 22.Evans, D. A., Bartroli, J. & Shih, T. L. (1981) J. Am. Chem. Soc. 103, 2127-2129. [Google Scholar]
- 23.Hubschwerlen, C., Specklin, J.-L. & Higelin, J. (1995) Org. Synth. 72, 1-5. [Google Scholar]
- 24.Barbosa, S. A. L. (2000) Ph.D. dissertation (Univ. of Pennsylvania, Philadelphia).
- 25.Fukuyama, T., Lin, S.-C. & Li, L. (1990) J. Am. Chem. Soc. 112, 7050-7051. [Google Scholar]
- 26.Takano, S., Akiyama, M., Sato, S. & Ogasawara, K. (1983) Chem. Lett., 1593-1596.
- 27.Rychnovsky, S. D. & Skalitzky, D. J. (1990) Tetrahedron Lett. 31, 945-948. [Google Scholar]
- 28.Rychnovsky, S. D., Rogers, B. & Yang, G. (1993) J. Org. Chem. 58, 3511-3515. [Google Scholar]
- 29.Evans, D. A., Rieger, D. L. & Gage, J. R. (1990) Tetrahedron Lett. 31, 7099-7100. [Google Scholar]
- 30.Corey, E. J. & Fuchs, P. L. (1972) Tetrahedron Lett. 3769-3772.
- 31.Zhang, H. X., Guibé, F. & Balavoine, G. J. (1990) J. Org. Chem. 55, 1857-1867. [Google Scholar]
- 32.Mengel, A. & Reiser, O. (1999) Chem. Rev. 99, 1191-1224. [DOI] [PubMed] [Google Scholar]
- 33.Smith, A. B., III, & Lodise, S. A. (1999) Org. Lett. 1, 1249-1252. [DOI] [PubMed] [Google Scholar]
- 34.Evans, D. A., Sjorgren, E. B., Bartroli, J. & Dow, R. L. (1986) Tetrahedron Lett. 27, 4957-4960. [Google Scholar]
- 35.Evans, D. A., Chapman, K. T. & Bisaha, J. (1984) J. Am. Chem. Soc. 106, 4261-4263. [Google Scholar]
- 36.Toshima, K., Tatsuta, K. & Kinoshita, M. (1988) Bull. Chem. Soc. Jpn. 61, 2369-2381. [Google Scholar]
- 37.Brown, H. C. & Bhat, K. S. (1986) J. Am. Chem. Soc. 108, 5919-5923. [DOI] [PubMed] [Google Scholar]
- 38.Brown, H. C. & Bhat, K. S. (1986) J. Am. Chem. Soc. 108, 293-294. [DOI] [PubMed] [Google Scholar]
- 39.Roush, W. R., Palkowitz, A. D. & Ando, K. (1990) J. Am. Chem. Soc. 112, 6348-6359. [Google Scholar]
- 40.Adams, C. M. (2003) Ph.D. thesis (Univ. of Pennsylvania, Philadelphia).
- 41.Brown, H. C., Knights, E. F. & Scouten, C. G. (1974) J. Am. Chem. Soc. 96, 7765-7770. [Google Scholar]
- 42.Dess, D. B. & Martin, J. C. (1983) J. Org. Chem. 48, 4155-4156. [Google Scholar]
- 43.Verheyden, J. P. H. & Moffatt, J. G. (1970) J. Org. Chem. 35, 2319-2326. [DOI] [PubMed] [Google Scholar]
- 44.Smith, A. B., III, & Adams, C. M. (2003) Acc. Chem. Res., in press.
- 45.Williams, D. R. & Sit, S.-Y. (1984) J. Am. Chem. Soc. 106, 2949-2954. [Google Scholar]
- 46.Stork, G. & Zhao, K. A. (1989) Tetrahedron Lett. 30, 287-290. [Google Scholar]
- 47.Ley, S. V., Norman, J., Griffith, W. P. & Marsden, S. P. (1994) Synthesis, 639-666.
- 48.Kraus, G. A. & Taschner, M. J. (1980) J. Org. Chem. 45, 1175-1176. [Google Scholar]
- 49.Bal, B. S., Childers, W. E., Jr., & Pinnick, H. W. (1981) Tetrahedron 37, 2091-2096. [Google Scholar]
- 50.Corey, E. J. & Suggs, J. W. (1975) Tetrahedron Lett. 16, 2647-2650. [Google Scholar]
- 51.Anelli, P. L., Biffi, C., Montanari, F. & Quici, S. (1987) J. Org. Chem. 52, 2559-2562. [Google Scholar]
- 52.Mancuso, A. J. & Swern, D. (1981) Synthesis, 165-196.
- 53.Trost, B. M. & Fleming, I., eds. (1991) Comprehensive Organic Synthesis (Pergamon, Oxford), Vol. 7.
- 54.Evans, D. A. & Hoveyda, A. H. (1990) J. Am. Chem. Soc. 112, 6447-6449. [Google Scholar]
- 55.Törmakangas, O. P. & Koskinen, A. M. P. (2001) Rec. Res. Dev. Org. Chem. 5, 225-255. [Google Scholar]
- 56.Smith, A. B., III, Lee, D. J., Adams, C. M. & Kozlowski, M. C. (2002) Org. Lett. 4, 4539-4541. [DOI] [PubMed] [Google Scholar]
- 57.Parikh, J. R. & von Doering, E. W. (1967) J. Am. Chem. Soc. 89, 5505-5507. [Google Scholar]
- 58.Namy, J. L., Souppe, J., Collin, J. & Kagan, H. B. (1984) J. Org. Chem. 49, 2045-2049. [Google Scholar]
- 59.Molander, G. A. & Harris, C. R. (1996) Chem. Rev. 96, 307-338. [DOI] [PubMed] [Google Scholar]
- 60.Otera, J. (1993) Chem. Rev. 93, 1449-1470. [Google Scholar]
- 61.Matsushima, T., Nakajima, N., Zheng, B. & Yonemitsu, O. (2000) Chem. Pharm. Bull. 48, 855-860. [DOI] [PubMed] [Google Scholar]
- 62.Inanaga, J., Hirata, K., Saeki, H., Katsuki, T. & Yamaguchi, M. (1979) Bull. Chem. Soc. Jpn. 52, 1989-1993. [Google Scholar]
- 63.Vakalopoulos, A. & Hoffmann, H. M. R. (2000) Org. Lett. 2, 1447-1450. [DOI] [PubMed] [Google Scholar]
- 64.Smith, A. B., III, Adams, C. M., Barbosa, S. A. L. & Degnan, A. P. (2003) J. Am. Chem. Soc. 125, 350-351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.