Abstract
The thapsigargins are a family of complex guaianolides with potent and selective Ca2+-modulating properties. This article documents the evolution of a synthetic route through several iterations to a final practical and scaleable synthetic route capable of generating both unnatural and natural products based around the guaianolide skeleton.
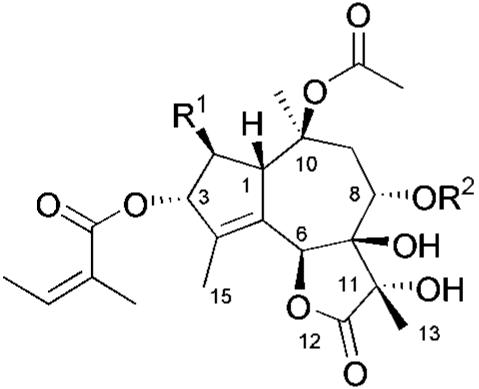
Over the centuries, preparations from the root of the Mediterranean plant Thapsia garganica L. have been used to treat conditions ranging from rheumatic pains to pulmonary disorders. Although knowledge of the efficacy of this plant in the ancient botanical pharmacopoeia was widespread, it was not until recently that the biologically active components of Thapsia were elucidated and determined to be thapsigargin 1, and 15 closely related guaianolides, collectively termed “thapsigargins” (Table 1 and refs. 1 and 2). An extensive review of these sesquiterpenoids encompassing the taxonomy, chemistry, isolation, and structural determination of this interesting class of natural products has been published (3). In this review we reexamine these structures as targets for total synthesis and analog generation in view of their potent biological activity (4).
Table 1. The known range of thapsigargins found in Thapsia.
Ang, angeloyl; Sen, senecioyl; iVal, iso-valeroyl.
These 16 natural compounds (1–16) fall into two series of molecules differentiated by the presence of an oxygen substituent at the C-2 position (Table 1). Compounds 1–16 can be broadly categorized as the thapsigargins, containing an oxygen substituent at C-2 or the trilobolide series in which this substituent is absent.
The therapeutic effects of the thapsigargins are well described in that they are recognized as potent histamine liberators (5) and selective and irreversible inhibitors of sarcoendoplasmic reticulum Ca2+ ATPase (SERCA)-dependent pumps at subnanomolar concentrations (6, 7). Thapsigargin itself penetrates intact cells and binds the SERCA into a conformation that has poor affinity for both Ca2+ and ATP (8). As such, they have become powerful tools in the study of Ca2+ signaling pathways (9). In recent times they have been shown to restore apoptotic function in cancer lines (ref. 10 and references therein) and consequently have been developed as potential treatments for prostate cancer (11). This approach utilizes a prodrug conjugate that is cleaved and activated by prostate-specific antigen (PSA) (12). This antigen is an enzyme, the normal function of which causes the breakdown of seminal gel proteins; however, increased levels of PSA are used as a specific diagnostic for prostate cancer (13). There is also evidence that PSA plays an important part in tumor cell growth and their later metastasis, and consequently they have become targets for potential antitumor drugs (14).
As targets for a total synthesis program, these materials present a significant challenge. Thapsigargin itself possesses a polyoxygenated 5-7-5 tricyclic core functionalized with four different ester groups and eight stereogenic centers. Through a series of elegant degradation studies (15), information regarding the relative reactivities of the oxygen substituents on the ring system has been elucidated. In designing a potential route, we were guided by the requirement for a flexible and scaleable process that would enable access to all members of the thapsigargin series through a common strategy. Such a synthetic route should be adaptable to the preparation of analogs not available through degradation processes to enable exploration of detailed structure–activity relationships.
Disconnections
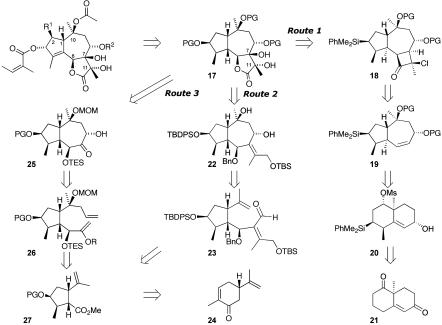
During our synthetic efforts on the thapsigargin family, three potential routes have been explored, all of which are outlined from a disconnection point of view in Scheme 1. In all three, we opted to leave out the C-2 oxygen as well as the internal five-ring double bond because we expected to be able to introduce these at a later point in the synthesis. In this way we hoped to gain access to relatively large quantities of the major fragment, which could serve as a divergent intermediate to either the trilobolide or the thapsigargin series. In route 1, we envisaged utilizing a silyl group as a masked C-3 hydroxyl and believed that we could introduce the dihydroxylactone moiety through a regio- and stereoselective Baeyer–Villiger oxidation of a cyclobutanone 18. This could be accessed through a facially selective cycloaddition process from the hydroazulene 19, which we hoped to prepare from the decalin derivative 20 through a Grob fragmentation, transannular cyclization. This could be generated from the readily available Weiland–Miescher ketone 21. Route 2 is an almost unrelated disconnection and relies on a series of oxidations to generate the lactone and a Prins cyclization to close the seven-membered ring in 22 from the aldehyde 23. This material could be accessed by a carbometallation-addition protocol on a derivative of the cyclopentane 27, available through a Favorskii rearrangement (16) from (S)-carvone 24. Our final and ultimately successful route is outlined in Route 3 and incorporates many of the lessons learned during route 2. It relies on a series of oxidations and an intramolecular Horner–Emmons reaction to control stereochemistry during introduction of the lactone functionality in 17. The seven-membered ring in 25 is accessed by a ring-closing metathesis of an enol-ether derivative 26 that can be generated from the same Favorskii-derived cyclopentane 27 by a series of organometallic additions.
Scheme 1.
Retrosynthetic plans. PG, protecting group; TBDPS, tert-butyldiphenylsilyl; TBS, tert-butyldimethylsilyl.
Route 1: Grob Fragmentation, Transannular Cyclization
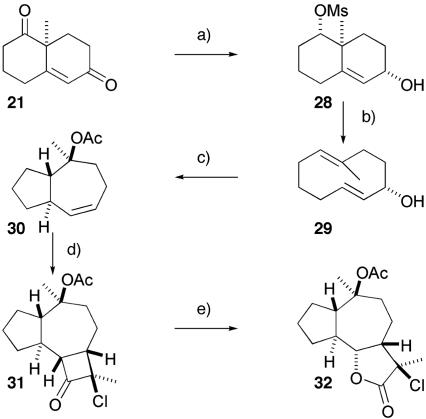
Our first attempts at the synthesis of the guaianolide ring system began within a model system aimed at generating the requisite 5-7-5 ring system. Starting from rac-ketone 21, treatment with sodium borohydride enabled reduction of the more reactive unconjugated ketone to give an alcohol that was activated as a mesylate (Scheme 2). Reduction with tri tert-butoxyaluminum hydride in tetrahydrofuran (THF) gave the equatorial alcohol 28 as a single diastereomer; other hydrides were less diastereoselective. Treatment of this material with borane·THF gave an intermediate alkyl borane that underwent a Grob-type fragmentation after treatment with sodium methoxide to give the cyclodecadiene 29. This hydroboration-fragmentation sequence was remarkably efficient, generating the desired ring-expanded compound 29 in 74% yield. The remaining alcohol in 29 was activated as a p-nitrobenzoyl ester in preparation for the solvolytic rearrangement to the hydroazulene ring system. Treatment of this material with aqueous bicarbonate at reflux initiated generation of an allylic cation that underwent 1,7-cyclization to give a tertiary carbocation that was trapped by water to afford the observed product 30 in 66% yield. The double bond in 30 reacted with the ketene derived from 2-propionyl chloride in the presence of triethylamine in a facially selective [2 + 2] cycloaddition process to afford the cyclobutanone 31 in 28% yield (plus 20% of the other regioisomer). Baeyer–Villiger oxidation of the cyclobutanone was accomplished with hydrogen peroxide in trifluoroethanol to give the requisite γ-butyrolactone 32 in 53% yield and complete the synthesis of the 5-7-5 ring system. However, although the cycloaddition process was facially selective, the cyclobutanone was formed exclusively on the wrong face to lead to the natural material. Extensive investigations of this reaction on more highly substituted azulene ring systems did not lead to the required stereochemistry at the lactone ring junction. This failure, coupled with difficulties in the efficient synthesis of more highly substituted decalin ring systems, led us to reevaluate this as a potential route to the thapsigargin family of natural products.
Scheme 2.
Grob fragmentation: transannular cyclization approach. a, 1: NaBH4, EtOH (91%); 2: MsCl, Py (91%); 3: Li(Ot-Bu)3AlH, THF, 0°C (61%). b, 1: BH3·THF; 2: NaOMe, MeOH, reflux (74%). c, 1: p-Nitrobenzoyl chloride, Et3N, 4-(N,N-dimethylamino)pyridine (DMAP), DCM (92%); 2: NaHCO3, H2O, reflux (66%); 3: Ac2O, TMSCl (72%). d, 2-Chloropropionoyl chloride, Et3N, room temperature (RT) (28%, +20% of other regioisomer). e, H2O2, CF3CH2OH (53%).
Route 2: Favorskii Rearrangement, Prins Cyclization Approach
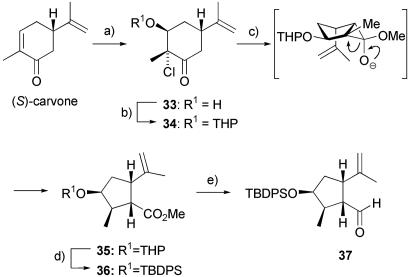
Our redesigned route was essentially linear in nature and relied extensively on substrate stereocontrol. In this approach we opted to form the five-membered ring first and then use it to induce the desired stereochemistry in the rest of the molecule. The synthesis began with the conversion of (S)-carvone to an intermediate epoxide (17) that was ring-opened by lithium chloride in the presence of trifluoroacetic acid (18). This gave the chlorohydrin 33 as the only product, which was protected as its tetrahydropyranyl ether 34; other protecting groups were less satisfactory in the subsequent rearrangement reaction. Treatment of 34 with sodium methoxide promoted a regio- and stereoselective Favorskii rearrangement to afford methyl ester 35 in 95% yield (Scheme 3).
Scheme 3.
Synthesis of left-hand fragment 37. a, 1: H2O2, NaOH, MeOH, 10°C, 88%; 2: LiCl, trifluoroacetic acid, RT, 95%. b, Dihydropyran, pyridinium p-toluenesulfonate catalytic (cat.), CH2Cl2, RT, 87%. c, NaOMe, MeOH, 0°C, 95%, d.r. > 95:5. d, 1: Pyridinium p-toluenesulfonate cat., MeOH, 40°C, 84%; 2: t-butyl diphenylsilyl chloride, imidazole, N,N-dimethylformamide (DMF), RT, 98%. e, 1: LiAlH4, THF, 0°C; 2: DMSO, oxalyl chloride, CH2Cl2, Et3N, 95%. THP, tetrahydropyranyl; TBDPS, t-butyl diphenylsilyl.
At this point, the tetrahydropyranyl protecting group in 35 was switched to the more robust t-butyldiphenylsilyl group to afford 36, and the methyl ester in 36 was reduced with lithium aluminum hydride and reoxidized to the aldehyde 37 under Swern conditions. As an important aside, in these opening five reactions, we were able to avoid chromatography of any intermediate compound, and 36 was simply obtained after a brief passage through a silica gel pad to remove salts and other minor impurities. These reactions were also readily performed on significant scales (i.e., up to 100 g), and consequently, large stocks of 36 could be accumulated.
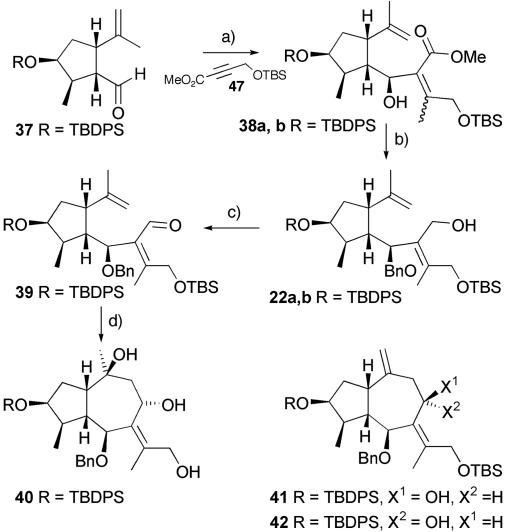
Felkin–Anh analysis of the aldehyde indicated that nucleophilic additions should occur from a single face to generate the correct C-6 stereochemistry. With this in mind, carbocupration of the alkyne 47 with the Gilman reagent and subsequent addition into the aldehyde 37 gave 38 in good yield but poor selectivity for the geometry of the double bond (5:4, Z/E) (Scheme 4). These materials were inseparable by conventional chromatography, but the alcohols 22a and 22b, generated after benzyl protection of the C-6 alcohol, and diisobutylaluminum hydride reduction of the esters 38a and 38b were easily separable. The alcohol with the correct geometry was oxidized with tetra-n-propyl ammonium perruthenate (TPAP) in the presence of N-methylmorpholine-N-oxide (NMO) to give an unstable aldehyde 39 that was used immediately after preparation. A range of Lewis acids were investigated to facilitate the Prins reaction, and extensive experimentation demonstrated that dimethylaluminum chloride was capable of generating the azulene skeleton, isolated as the triol 40 (which bears the correct stereochemistry for the natural product) and the alcohols 41 and 42. However, the yields for this process were modest at best and were significantly worse when the reaction was scaled up to even relatively small scales. Faced with the inability to generate the seven-ring system in an efficient fashion and the lack of stereoselectivity in the carbocupration reaction, we decided to take an alternative approach that would hinge around a different approach to generating the azulene ring system while also incorporating elements that had proven to be successful in this route.
Scheme 4.
Prins cyclization approach. a, 1: Me2CuLi·LiBr, Et2O, -78°C (81%, 5:4, Z/E); 2: BnBr, NaH, DMF (91%). b, Diisobutylaluminum hydride, CH2Cl2, -78°C (40% of 22a plus Z isomer 22b). c, TPAP, 4-Å sieve, NMO, CH2Cl2 (89%). d, Me2AlCl, CH2Cl2, 0°C (8% of 40, 27% of 41, and 18% of 42).
Route 3: Favorskii Rearrangement, Ring-Closing Metathesis Approach
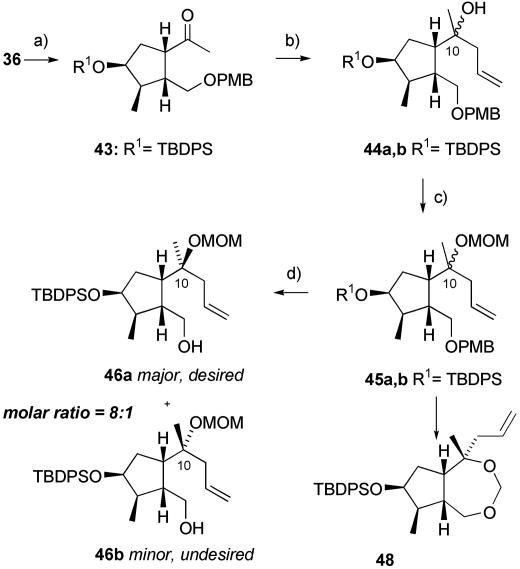
The ease and efficiency with which we were able to generate the cyclopentane portion of the molecule and its success in inducing the correct C-6 configuration motivated us to incorporate these elements into our modified route, which was built around a ring-closing metathesis approach to forming the seven-membered ring. With this in mind, the ester 36 was reduced with lithium aluminum hydride, protected with p-methoxy benzyl chloride, and the double bond was cleaved by osmylation followed by periodate treatment to give 43 (Scheme 5).
Scheme 5.
Synthesis of the C-10 epimeric alcohols 46a and 46b. a, 1: LiAlH4, THF, 0°C; 2: NaH, p-methoxybenzyl chloride, DMF, RT; 3: OsO4 cat., NMO, acetone, H2O, RT; 4: NaIO4, RT, 74% over four steps. b, AllylMgBr, dimethoxyethane, -78°C, 99%, d.r. = 8:1. c, MOMCl, N,N-diisopropylethylamine, DMAP cat., CH2Cl2, RT, 88%. d, 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, aqueous pH 7 phosphate buffer, CH2Cl2, RT, 93%.
However, stereoselective allylation of the ketone 43 proved difficult. We investigated a large number of allylating agents, temperatures, and solvents before finding satisfactory conditions. It was eventually discovered that allylmagnesium bromide in dimethoxyethane at -78°C gave an 8:1 ratio of the desired product 44a to the minor C-10 diastereoisomer 44b. These products could not be separated easily at this stage, but they could be converted by way of methoxymethyl ether (MOM) protection of the tertiary alcohol to compounds 45a and 45b. The p-methoxybenzyl protecting group could then be removed by the use of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in the presence of a pH 7 buffer; frustratingly, we discovered the hard way that application of these conditions on a large scale (45 g) without the buffer led to the cyclic acetal 48 (as an 8:1 mixture at the C-10 stereocenter), presumably by intramolecular trapping of a MOM-derived oxonium cation. The alcohols 46a and 46b are more easily separable, and this step required the first chromatographic purification in the synthesis thus far, but it was also a convenient point at which to store material due to the stability of these structures.
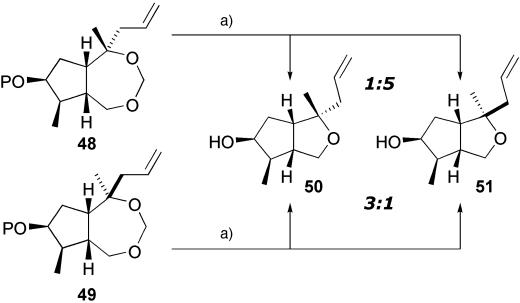
Our attempts to recover material from the inadvertent large-scale in situ acetal formation proved fruitless (Scheme 6).
Scheme 6.
Acetal recovery attempt. a, TsOH, MeOH, reflux.
Attempts to hydrolyze the formyl acetal in 48 demonstrated that relatively harsh conditions were required; refluxing methanolic tosic acid led to hydrolysis of the acetal, but only the cyclic ethers 50 and 51 in which the C-10 stereochemistry had been predominantly inverted (1:5, 50/51) was isolated. Interestingly, applying the same conditions to the C-10 epimer 49 also led to a mixture of the same products in which the stereocenter had been inverted (3:1, 50/51).
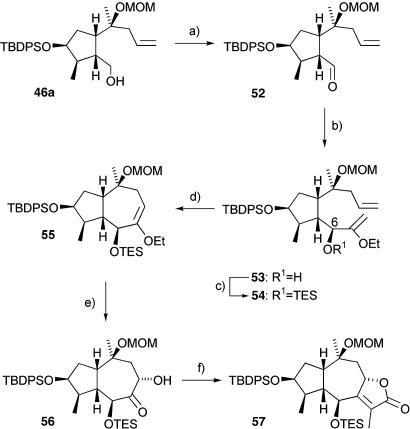
The synthesis continued with oxidation of the primary alcohol in 46a with catalytic TPAP in the presence of NMO to give the aldehyde 52 (Scheme 7). The lithium anion of ethyl vinyl ether was added to this to generate the alcohol 53 as a single diastereoisomer in >96% yield; the stereochemical outcome of this addition process is consistent with the Felkin–Anh model, as we observed in our previous route (Scheme 4).
Scheme 7.
Synthesis of the α,β-unsaturated lactone 53. a, TPAP cat., NMO, 4-Å MS, CH2Cl2, RT. b, CH2=CHOEt, t-BuLi, THF, -78°C, 96%, d.r. > 95:5. c, triethylsilyl chloride, imidazole, DMF, RT, 95%. d, 2.5 mol % Grubbs' dihydroimidazolidine Ru cat., CH2Cl2, reflux, 92%. e, K2OsO2(OH)4 cat., K3Fe(CN)6, NaHCO3, MeSO2NH2, K2CO3, t-BuOH, H2O, RT, 90%, d.r. = 16:1. f, 1: HO2CCH(Me)P(O)(OEt)2, triethylsilyl chloride, CH2Cl2, RT; 2: NaH, reflux, 79% over two steps.
The C-6 alcohol was protected by using triethylsilyl (TES) chloride to give 54, which underwent a ring-closing metathesis using 2.5 mol % of the Grubbs' dihydroimidazolidine ruthenium catalyst, to afford the required cyclic enol ether 55 in high yield (92%). The next crucial reaction involved the facially selective osmylation of 55 using catalytic potassium osmate to give the product in a diastereomeric ratio (d.r.) of 16:1 in favor of the desired isomer 56. The high facial selectivity in this reaction was expected from force-field calculations, which suggested that the triethylsilyloxy group blocks the convex face of the molecule, thereby forcing attack from the concave face. Even better results were obtained by using the Sharpless AD-mix-α conditions in a chirality-matched case that gave 56 as the exclusive product in 87% yield. By way of comparison, the mismatched AD-mix-β gave a poor yield of 56% and only a 1.3:1 ratio of products. The C-8 hydroxyl group of 56 was then esterified with 2-(diethylphosphoryl)propionic acid and subjected to an intramolecular Horner–Wadsworth–Emmons reaction to give the butenolide 57 (Scheme 7).
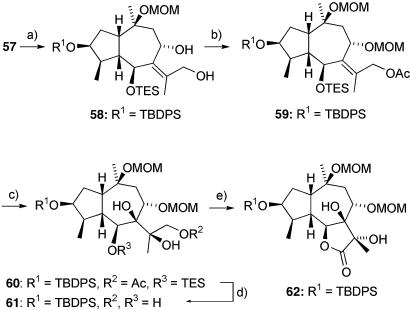
This material proved to be extremely resistant toward oxidation, presumably due to steric and electronic reasons, and consequently we were unable perform a cis-dihydroxylation under any conditions. However, after reduction with lithium borohydride to 58, followed by acylation of the primary alcohol and orthogonal MOM protection of the secondary hydroxyl group, we were able to obtain the alkene 59. This compound underwent clean (but rather slow) dihydroxylation to give the diol 60 as a single diastereoisomer (Scheme 8).
Scheme 8.
Completion of the tricyclic framework. a, LiBH4, THF, reflux, 92%. b, 1: Ac2O, DMAP, 2,6-lutidine, CH2Cl2, RT, 95%; 2: MOMCl, N,N-diisopropylethylamine, DMAP, CH2Cl2, RT, 92%. c, 20 mol % K2OsO2(OH)4, quinuclidine, K2CO3, MeSO2NH2, K3Fe(CN)6, t-BuOH, H2O, RT, d.r. > 95:5. d, K2CO3, MeOH, RT, 85% over two steps. e, 10 mol % TPAP, 40 eq of NMO, 4-Å MS, MeCN, RT, 73%.
Some migration of the acetate and TES groups occurred under the reaction conditions but was inconsequential as the next step involved hydrolysis with methanolic potassium carbonate to remove both the acetate and TES groups to give the tetraol 61. Completion of the required carbon framework was neatly accomplished by a selective TPAP oxidation that facilitated intermediate lactol formation followed by a second oxidation to the required lactone 62 in a single overall transformation (Scheme 8).
Although some 23 steps were required to assemble 62, the process was efficient (11% overall yield) and required only five chromatographic steps. In this way we were quickly able to generate quantities of 62 in excess of 30 g. This is clearly an important pivotal point in the synthesis, because material can be driven forward to the natural products or usefully diverted to analog programs.
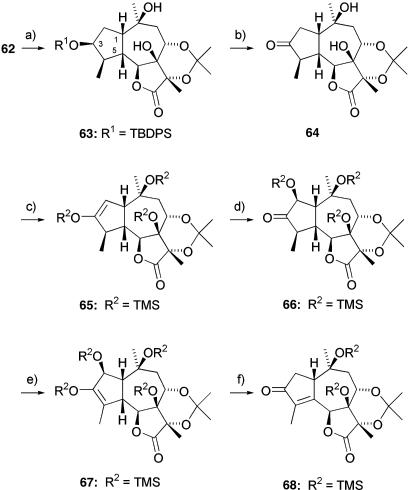
Compound 62 was treated with acidic amberlyst-15 resin in acetone, which effected both MOM hydrolysis and simultaneous acetonide formation to give 63; the acetonide imparts remarkable stability to this series of materials and renders them crystalline. This property greatly aids purification and allows full structural characterization through x-ray analysis. For example, after removal of the silyl protection in 63 and oxidation with TPAP, the ketone 64 was obtained as a white, highly crystalline material, the x-ray crystal analysis of which confirmed the configuration of all stereocenters.
To introduce the required C-4 to C-5 unsaturation present in thapsigargin, several direct methods were investigated without success. However, an alternative approach delineated in a series of model studies was also investigated. This approach required the conversion of the ketone 64 to the corresponding kinetic silyl enol ether 65, which in turn was oxidized with dimethyldioxirane to the α-silyloxy ketone 66. X-ray structural analysis confirmed the regio- and stereochemistry of this transformation. With an oxygen substituent in the C-2 position, 66 can be utilized further to form the trimethylsilylenol ether 67 in excellent yield (Scheme 9).
Scheme 9.
Synthesis of ketone 68. a, Amberlyst-15, acetone, RT, 85%. b, 1: Tetrabutylammonium fluoride, THF, RT, 98%; 2: TPAP cat., NMO, 4-Å MS, CH2Cl2, RT, 93%. c, TMSCl, Et3N, DMF, 120°C, 88%. d, Dimethyldioxirane, acetone, CH2Cl2, 0°C, 99%, d.r. > 95:5. e, TMSCl, Et3N, DMF, 150°C, 90%. f, 10 mol % PhSeBr, CH2Cl2, 0°C to RT, 94%.
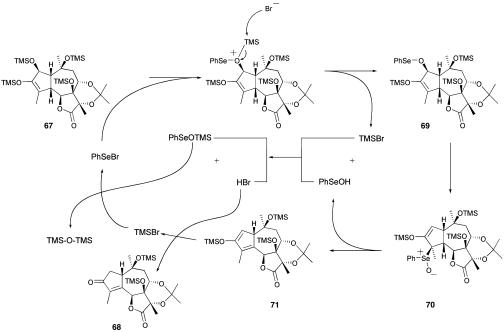
The oxidation pattern in 67 is now set up for conversion to the thapsigargin family of materials. However, we discovered an un-anticipated catalytic selenium reaction by using 67 as a precursor, which leads directly to 68 after treatment with 10 mol % of phenylselenyl bromide in dichloromethane (DCM) at 0°C. This is a useful reaction for the synthesis of the trilobolide series, generating the required unsaturation at C-4 to C-5 and simultaneously effecting deoxygenation at C-2. A tentative mechanistic proposal that accounts for the catalytic nature of this reaction is outlined in Scheme 10. The reaction starts with selenation of the trimethylsilyl (TMS)-protected secondary alcohol in 67; it can be expected that selenyl ether 69 would be formed, because this alcohol is the least hindered. This material could undergo a 2,3-sigmatropic rearrangement to generate the selenoxide 70 that should eliminate to give 71. The hydrolysis of this enol ether with in situ generated HBr would generate the observed product 68, and lead (through TMSBr) to regeneration of the phenylselenium bromide.
Scheme 10.
Mechanistic proposal for the synthesis of ketone 68.
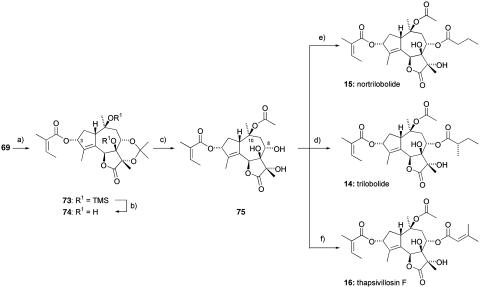
To complete the total synthesis of the trilobolide series of molecules, a stereoselective reduction of the carbonyl group in 68 with sodium borohydride was effected to give the required alcohol in >4:1 selectivity. Treatment with angelic acid in the presence of 2,4,6-trichlorobenzoyl chloride gave the ester 72. From this point, we were able to progress the synthesis with the minimum number of steps, taking advantage of the anticipated order of reactivity of the hydroxyl groups. Accordingly, the silyl groups in 72 were removed by using tetra-n-butylammonium fluoride to give 73. This material was treated with isopropyl acetate and polymer-supported tosic acid to selectively generate the C-10 monoacetate, and removal of the acetonide gave triol 74, which can now be transformed to three natural products: trilobolide 14, nortrilobolide 15, and thapsivillosin F 16 (Scheme 11).
Scheme 11.
Completion of the synthesis of trilobolide, nortrilobolide, and thapsivillosin F. a, 1: NaBH4, MeOH, O°C, 86%, d.r. = 4:1; 2: angelic acid, Et3N, 2,4,6-trichlorobenzoyl chloride, toluene, 80°C, 76%. b, Tetrabutylammonium fluoride, THF, RT, quantitative c, 1: Isopropenyl acetate, polymer-supported-TsOH, CH2Cl2, RT, 68%; 2: HCl (aqueous), MeOH, 40°C. d, (S)-2-Methylbutyric anhydride, DMAP, CH2Cl2, RT, 78% over two steps. e, Butyric anhydride, DMAP, CH2Cl2, RT, 72% over two steps. f, Senecioic anhydride, DMAP, CH2Cl2, RT, 73% over two steps.
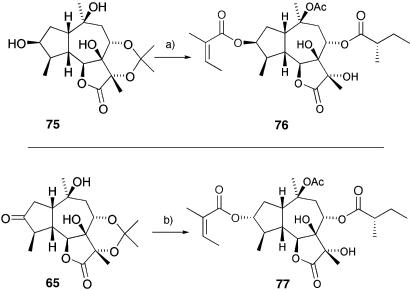
The divergent nature of this synthesis and the ease with which much of this chemistry can be scaled up alludes to the possibility that other members of the thapsigargins and their structural analogs will now be available for the exploration of structure–activity relationships. We initiated an analog program, and our first efforts are outlined in Scheme 12.
Scheme 12.
Analog synthesis. a, 1: 2,4,6-trichlorobenzoyl chloride, angelic acid, Et3N, toluene, 75°C (85%); 2: isoprenyl acetate, polymer-supported TsOH, 4-Å sieve, DCM, RT (95%); 3: MeOH, 3 M HCl; 4: (S)-2-methyl butyric anhydride, DCM, DMAP, RT (90%, two steps). b, 1: Ac2O, DMAP, DCM (99%); 2: NaBH4, MeOH, RT; 3: 2,4,6-trichlorobenzoyl chloride, angelic acid, Et3N, toluene, 75°C; 4: MeOH, 3 M HCl, 40°C; 5: (S)-2-methyl butyric anhydride, DCM, DMAP, RT (60% over four steps).
These materials omit the internal double bond in the five-membered ring and explore both C-3 configurations and, as a result, are significantly easier to synthesize. We anticipated (on the basis of modeling studies) that the analog 76 would be the more active one, because its overall conformation is more like that of thapsigargin than the epimer 77. To our surprise, preliminary biological evaluation in a rabbit skeletal muscle Ca2+ ATPase assay indicated that 77 is at least as active as thapsigargin, whereas 76 is some 170 times less potent. These preliminary results indicate that the goal of generating simplified analogs of the thapsigargin family is real and viable and that future structure–activity relationships should be elucidated by using total synthesis as a primary tool.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: THF, tetrahydrofuran; TPAP, tetra-n-propyl ammonium perruthenate; NMO, N-methylmorpholine-N-oxide; MOM, methoxymethyl ether; TES, triethylsilyl; d.r., diastereomeric ratio; TMS, trimethylsilyl; DMAP, 4-(N,N-dimethylamino)pyridine; DCM, dichloromethane; cat., catalytic; RT, room temperature; N,N-DMF, dimethylformamide; TBDPS, tert-butyldiphenylsilyl.
References
- 1.Rasmussen, U. S. B., Christensen, S. B. & Sandberg, F. (1978) Acta Pharm. Suec. 15, 133-140. [PubMed] [Google Scholar]
- 2.Christensen, S. B. & Rasmussen, U. (1980) Tetrahedron Lett. 21, 3829-3830. [Google Scholar]
- 3.Christensen, S. B., Andersen, A. & Smitt, U. W. (1997) Fortschr. Chem. Org. Naturst. 71, 129-167. [DOI] [PubMed] [Google Scholar]
- 4.Oliver, S. F., Högenauer, K., Simic, O., Antonello, A., Smith, M. D. & Ley, S. V. (2003) Angew. Chem. Int. Ed. Engl. 42, 5996-6000. [DOI] [PubMed] [Google Scholar]
- 5.Patkar, S. A., Rasmussen, U. & Diamant, B. (1979) Agents Actions 9, 53-57. [DOI] [PubMed] [Google Scholar]
- 6.Lytton, J., Westlin, M. & Hanley, M. (1991) J. Biol. Chem. 266, 17067-17071. [PubMed] [Google Scholar]
- 7.Caspersen, C. & Treiman, M. (1995) FEBS Lett. 377, 31-36. [DOI] [PubMed] [Google Scholar]
- 8.Sagara, Y., Wade, J. B. & Inesi, G. (1992) J. Biol. Chem. 267, 1286-1292. [PubMed] [Google Scholar]
- 9.Treiman, M., Caspersen, C. & Christensen, S. B. (1998) Trends Pharmacol. Sci. 19, 131-135. [DOI] [PubMed] [Google Scholar]
- 10.Furuya, Y., Lundmo, P., Short, A. D., Gill, J. D. L. & Isaacs, T. (1994) Cancer Res. 54, 6167-6175. [PubMed] [Google Scholar]
- 11.Christensen, S. B., Andersen, A., Kromann, H., Trieman, M., Tombal, B., Denmeade, S. & Isaacs, J. T. (1999) Bioorg. Med. Chem. Lett. 7, 1273-1280. [DOI] [PubMed] [Google Scholar]
- 12.Denmeade, S. R., Jakobsen, C. M., Janssen, S., Khan, S. R., Garrett, E. S., Lilja, H., Christensen, S. B. & Isaacs, J. T. (2003) J. Natl. Cancer Inst. 95, 990-1000. [DOI] [PubMed] [Google Scholar]
- 13.Denmeade, S. R. & Isaacs, J. T. (1996) in Advances in Pharmacology, eds. Thomas, J. T., Anders, M. W., Murad, F. & Coyles, J. T. (Academic, New York).
- 14.Jakobsen, C. M., Denmeade, S. R., Isaacs, J. T. Gady, A., Olsen, C. E. & Christensen, S. B. (2001) J. Med. Chem. 44, 4696-4703. [DOI] [PubMed] [Google Scholar]
- 15.Andersen, A., Cornett, C., Lauridsen, A., Olsen, C. E. & Christensen, S. B. (1994) Acta Chem. Scand. 48, 340-346. [Google Scholar]
- 16.Lee, E., Lim, J. W., Yoon, C. H., Sung, Y. & Kim, Y. K. (1997) J. Am. Chem. Soc. 119, 8391-8392. [Google Scholar]
- 17.Nicolaou, K. C., Xu, J. Y., Kim, S., Pfefferkorn, J., Ohshima, T., Vourloumis, D. & Hosokawa, S. (1998) J. Am. Chem. Soc. 120, 8661-8673. [DOI] [PubMed] [Google Scholar]
- 18.Bajwa, J. S. & Anderson, R. C. (1991) Tetrahedron Lett. 32, 3021-3024. [Google Scholar]