Abstract
Context:
Fatigue is one of the most frequent nonmotor manifestations in Parkinson's disease (PD), having a major effect on quality of life but is not reported in Indian patients.
Aims:
To evaluate the frequency of fatigue in a cohort of PD population and its correlation with disease.
Settings and Design:
Fatigue Severity Scale (FSS) was translated and validated in local vernacular language. All patients of PD visiting neurology outpatient department of a tertiary care hospital.
Subjects and Methods:
A total of 150 patients were screened, and 104 were included in this study. They were divided into – Group I with fatigue (score of >4 in each item) and Group II without fatigue.
Statistical Analysis:
Data were analyzed by SPSS software version 20.0. Spearman correlation was used to evaluate the convergent validity of the FSS-Ind score with PD-related variables. The principal components analysis was applied to detect the domain structure of the FSS.
Results:
Of the total 104 patients, 68 (65.3%) patients experienced fatigue. The duration of disease was significantly more (P = 0.021) in Group I (4.39 ± 3.8 years) than in the Group II (3.13 ± 1.6 years). The severity of disease also showed a positive correlation with fatigue with 50.9% patients in H and Y stage >3 experiencing fatigue. 69.1% patients of tremor phenotype experienced fatigue as compared to 32.3% of rigid phenotype. There was no relation of fatigue with age, gender, H and Y stage, levodopa equivalent dose and mean Unified PD Rating Scale motor III score.
Conclusions:
Translated version of the FSS, FSS-Ind has high internal consistency and validity which supports its application as an effective tool in detecting fatigue in patients with PD. Fatigue in PD was related to duration and phenotype of the disease.
Keywords: Fatigue, Fatigue Severity Scale, Indian, Parkinson's disease
Introduction
Nonmotor symptoms (NMS) in Parkinson's disease (PD) greatly affect the quality of life (QOL). Hence, in recent years, they have received significant attention and have become the focus of care by many physicians attending to PD patients. Fatigue is one of the most frequent nonmotor manifestations noted in PD, affecting 35–68% of the total PD population.[1,2] Patients do not spontaneously use the word “fatigue” to describe their symptom; however, it is defined as a feeling of lack of energy, exhaustion or overwhelming tiredness, distinct from normal tiredness. This “nondopaminergic” symptom has significant negative impact on QOL as it does not respond satisfactorily to dopaminergic replacement therapy.[3,4]
Fatigue is a subjective symptom and patient's self-reported questionnaires remain the mainstay of diagnosing fatigue and measuring its severity.[5] Fatigue Severity Scale (FSS) is a self-reported scale which assesses the physical aspects and impact of fatigue on patient's daily functioning. This scale is also recommended by the International Movement Disorder Society and has been validated in different languages.[6,7,8] However, there is no study of its use in any of the Indian languages. The aim of this study is to assess fatigue in PD patients using translated FSS (FSS-Ind) in their vernacular language (Hindi/Punjabi) and to assess the relationship of fatigue with various patient-related factors.
Subjects and Methods
Data collection
The study was carried out in Department of Neurology at Dayanand Medical College and Hospital, Ludhiana, Punjab, India from January 2014 to June 2015. The Local Ethical Committee approved the study protocol. A total of 150 patients of idiopathic PD were recruited from the outpatient department.
Two independent professionals translated the items and response categories of FSS individually into Hindi and Punjabi. Then a consolidated version of the scale was prepared. Another professional translated this questionnaire back into English to check for the discrepancies between Hindi/Punjabi version and the original questionnaire. After a careful review and a few changes, the provisional version of the questionnaire was finalized. In the next phase, this questionnaire was pilot-tested by administration to a sample of twenty patients of acute coronary syndrome who complained of fatigue and twenty healthy individuals as controls (the age-gender matched family members of patients). The FSS questionnaire in English was completed with the help of the doctor and the Hindi/Punjabi version by the patient himself/herself. The comparison of FSS scale in English and Hindi/Punjabi in this pilot group yielded similar results [Table 1]. After pilot testing, a few more changes were made and then a final version of the scale, (FSS-Ind) was prepared and used in the study.
Table 1.
Comparison of Fatigue Severity Scale in English and Hindi/Punjabi in 20 (controls) already known individuals with fatigue

Subjects
A total of 150 adult patients were included in the study after confirmation of diagnosis by neurologist using the United Kingdom Brain Bank criteria. Informed consent was obtained from all the patients who participated in the study. Patients with diseases such as multiple system atrophy, progressive supranuclear palsy, vascular PD, drug-induced PD and PD associated with dementia (Mini-Mental State Examination < 24) were excluded from the study. Patients who had depression and were rated >9 on the Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire (PRIME-MD PHQ)[9] a depression rating scale were also excluded from the study.
Methodology
Data were collected on a predesigned performa regarding basic demographic information including age, gender, baseline educational status, and comorbidities. PD-related characteristics including age of onset, disease duration (time elapsed after diagnosis), measures of disease severity such as Hoehn and Yahr stage (H and Y), the Unified PD Rating Scale (UPDRS) motor III score and levodopa cumulative daily dosage were recorded. PRIME-MD PHQ (depression rating scale) score was also recorded.
PD subjects were classified into phenotypes as akinetic/rigid predominant PD (AR) or tremor-predominant PD (T) using the modified ratio developed by Schiess et al., based on the UPDRS-III.[10]
Fatigue Severity Scale questionnaire
FSS is a nine-item scale for assessing physical aspects of fatigue and its impact on the QOL. It is used to evaluate the effect of fatigue on routine activities as exercise, physical functioning, performance of duties, responsibilities, and interference with routine family and social life.[11]
Each item which is in the form of a short statement is scored on a Likert scale from one to seven, (Grade-1 indicates completely disagrees, and Grade 7 indicates completely agrees). The total FSS score represents the mean score of nine items, with higher scores indicating severe fatigue.
Although FSS has been validated in different languages in PD population, there is no study of its use in Indian languages. The Hindi/Punjabi translated version of the FSS (FSS-Ind) was filled by the patient himself and based on the total FSS score the PD patients were categorized into two groups: Group I or who experienced fatigue (total FSS > 36 with a mean score >4 on each individual item) and Group II or who did not experience fatigue (total FSS < 36 with a mean score <4 on each individual item).[7,8]
Statistical analysis
Data were analyzed by SPSS software version 20.0 (Chicago, IL, USA). Mean (standard deviation) and frequency (percentage) were used to describe continuous and qualitative variables, respectively. The minimum and maximum values and coefficient of variation (CV) were also reported for each of the items in FSS-Ind questionnaire. Spearman correlation was used to evaluate the convergent validity of the total score of the FSS-Ind questionnaire in association with the baseline and PD-related variables. The principal components analysis (factor analysis) was applied to explore the best-fit factors with an Eigen value of >1 to detect the domain structure of the FSS.
Results
Baseline characteristics
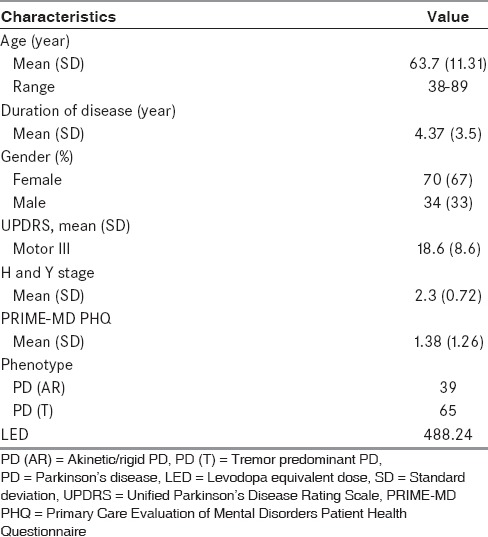
We evaluated 150 patients with a diagnosis of PD. Thirty-eight patients had severe depression (PRIME-MD PHQ > 9) and eight did not give consent to participate, hence, 46 patients were excluded from this study. The remaining cohort of 104 PD patients included 70 (67%) males and 34 (33%) females with mean age 63.7 ± 11.31 years (age range from 38 to 89 years) and mean H and Y stage of 2.3 ± 0.72. Mean duration of disease in our cohort was 4.37 ± 3.5 years. In this study, 68 patients (65.3%) experienced fatigue. The mean fatigue score in Group I was 46.07 ± 4.70 whereas in Group II was 24.83 ± 8.70. These characteristics are depicted in Table 2.
Table 2.
Baseline, clinical characteristics of Parkinson's disease patients (n=104)
Fatigue Severity Scale characteristics
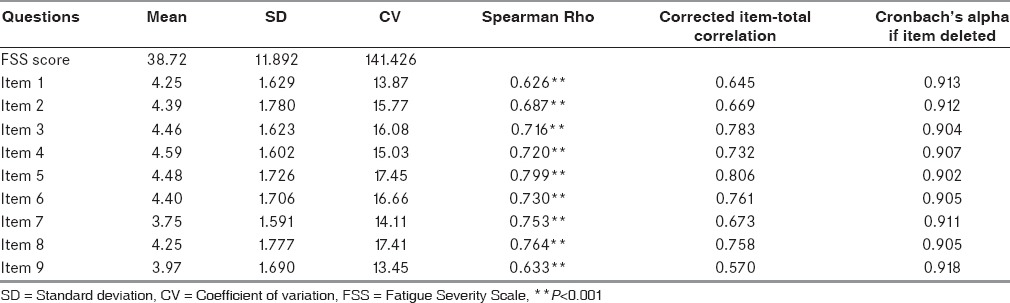
The highest score was seen for item 5 “fatigue causes frequent problems for me” and showed a value of 5.48 ± 1.60, and item 4 “interference of fatigue with physical functioning” showed the second highest score (5.32 ± 1.42) whereas lowest score was noted for item 9 on the scale “fatigue interferes with my work, social and family life” with value of 4.60 ± 1.59. The largest and smallest CV was observed in items 5 (17.45) and 9 (13.45), respectively. As shown in Table 3, the Spearman Rho was more than 0.9 for all items with all P < 0.001. The correlation coefficients were highest for items 9 (r = 0.918) and 1 (r = 0.913) respectively while item 5 (r = 0.902) and item 3 (r = 0.904) showed the lowest correlation coefficient. The whole FSS questionnaire had statistically significant reliability with the Cronbach's alpha coefficient of 0.91.
Table 3.
Descriptive characteristics and the Spearman correlation of each item of Fatigue Severity Scale in Parkinson's disease patients depicting the mean score, standard deviation, and coefficient of variation each of the nine items in Fatigue Severity Scale
Correlation of fatigue with clinical variables
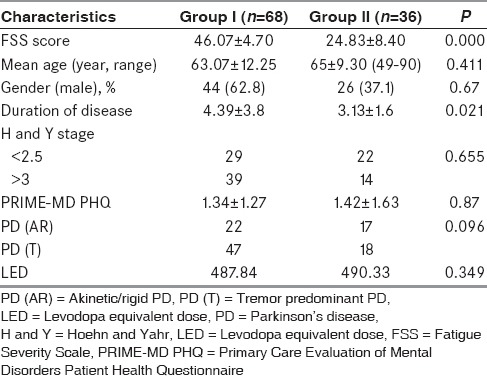
The mean age and age of onset of disease was comparable in both groups. The mean age and age of onset of disease in Group I and Group II was 63.07 ± 12.25 and 65 ± 9.30 years and 59.28 ± 12.8 years and 61.42 ± 9.27 years respectively. The duration of disease was more in Group I (4.39 ± 3.8 years) than in the Group II (3.13 ± 1.6 years). The difference was statistically significant (P = 0.021) suggesting that there is a linear relationship between the duration of disease and incidence of fatigue. The severity of disease as measured by H and Y stage also showed a trend of correlation with fatigue. There were 53 (50.9%) patients in H and Y stage >3 out of which 39 (73.5%) patients experienced fatigue as compared to 29 (56.8%) with H and Y stage <3. The PD patients were divided into two phenotypes (PD [AR] and PD [T] types) based on UPDRS score. Among the subtype of PD, 47 (69.1%) patients of tremor phenotype experienced fatigue as compared to 22 (32.3%) of AR phenotype. This data signifies that the frequency of fatigue was more in the tremor predominant phenotype (47 patients; 69.1%) as compared to rigid phenotype in our patient population though this was not statistically significant [Table 4]. This study could not reveal any relation of fatigue with respect to L-dopa equivalent dose and PRIME-MD PHQ score (depression score).
Table 4.
Comparison between Group I and Group II regarding clinical profile and disease characteristics
Discussion
Ours is the first study to use Indian translation of FSS questionnaire (Punjabi/Hindi) for evaluation of fatigue in 104 PD patients. Patients self-reported questionnaires remains the mainstay of measuring and diagnosing fatigue, as patients do not spontaneously use the word fatigue to describe these symptoms. For this purpose, we translated the FSS into their vernacular language (Hindi/Punjabi). The reliability analysis of this translated FSS-Ind showed an acceptable internal consistency and high intra-class correlation (Cronbach's alpha > 0.91).
The study population was grouped as Group I, who experience fatigue (FSS > 4 per item) and Group II who do not experience fatigue.[7,8] In this study, 68 (65%) patients experienced fatigue as shown in Figure 1 out of the total of 104 patients. The largest holistic study of NMS in 1072 PD patients, the PRIAMO study, reported a similar incidence of fatigue of about 58.1%.[12]
Figure 1.
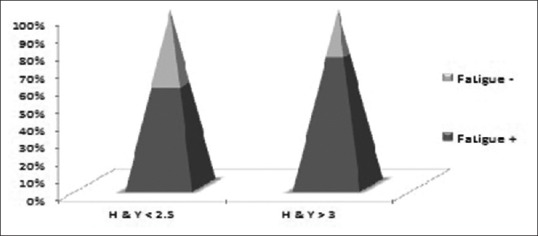
Bar diagram showing correlation of fatigue with disease severity (H and Y <2.5- mild disease; H and Y >3-moderate to severe disease)
The slightly higher incidence in our study may be due to the translation of the FSS questionnaire in the vernacular language, which may have inadvertently detected fatigue also. Small sample size and regional differences may be additional factors. Other studies in literature have suggested a prevalence rate from 33% to 58%. This variation can be contributed to heterogeneity in the methods of diagnosis, scales used and definition of “fatigue.”[13,14,15]
A significant clinical factor predicting the presence of fatigue in this study was the duration of PD, being 4 years (P = 0.021). This observation is in line with Japanese and Brazilian studies which also showed a positive correlation between the duration of disease and fatigue, suggesting fatigue to be intrinsic to the PD itself.[14,16,17] Thus, as the duration of disease increases, the nonmotor complications such as fatigue, a key NMS in PD patients also increases proportionately.
The other clinical parameter which also had a direct correlation with fatigue was the severity of disease. As the severity increases so does the incidence of fatigue, 56.8% in patients with mild disease to 73.5% in patients with moderate-severe disease (H and Y > 3) experienced fatigue. Our results echo with the PRIMO study where fatigue levels were reported to rise incrementally as H and Y stages increased, with 37.7% patients experiencing fatigue in their early stage and 81.6% having fatigue in moderate to severe stage of the disease.[12,18] Although it is difficult to establish which variable independently contributes to fatigue whether disease duration or severity, our results show that Indian population had more fatigue even in the early stage of disease.
In this study, males experienced more fatigue than females though the difference was statistically not significant and therefore needs to be further confirmed with larger samples. Although the exact reason for male preponderance of fatigue is not known it may be related to the agrarian culture and more physical and outdoor activities in Punjabi culture. This is in contrary to previous studies that found female gender experienced more fatigue. This observation of sex differences is unlikely to simply be a function of reporting bias but may be a result of a complex spectrum of factors contributing to gender differences in responses, as hormone levels in cycling women have a substantial impact on the perception of sensations like pain.
The results of this study show that there are differences in the fatigue score between the subtypes of PD, though they may be due to small sample size and are statistically not significant. These results need further validation in larger samples. Some reports in literature show that tremor dominant types experience more fatigue as compared to akinesia type of PD, though Metta et al. in their study of 135 patients of PD found no difference in the incidence of fatigue between the subtypes of PD.[2] Differential involvement of striato- and cerebello-thalamocortical pathways in tremor and AR-predominant PD may be the reason for the variation in the presence of fatigue. Tremors are generally less responsive to dopaminergic treatment highlighting the widespread degenerative process involving the nondopaminergic pathways.[19] Differences in the physical mobility patterns between the two phenotypes of PD may also be another factor responsible for variation in the prevalence of fatigue.
Literature search has resulted in conflicting relation of UPDRS motor score III with fatigue[7,19] but this study did not find any relation of fatigue with UPDRS motor III, Levodopa equivalent dose, PRIME-MDQ depression scores. Isolating depression from fatigue in PD can be challenging, but PRIME-MD PHQ score (screening questionnaire) was comparable between the two groups in this study with no statistically significant difference. Hence, we conclude that occurrence of fatigue is independent of depression and levodopa dosage suggesting that it is an independent nondopaminergic symptom.
As we conducted a unicentric study, it has some limitations like referral bias. With patients of varying disease severity and duration, subgroup analysis with sufficient power of the study could not be performed.
Conclusions
Fatigue is a common (65%) yet poorly understood NMS which may be easily overlooked as PD is primarily a motor disease. Patient's self-reported questionnaire remains the mainstay of measuring and diagnosing fatigue. High internal consistency and validity support the application of FSS-Ind as an easily administered sensitive tool to evaluate fatigue in Indian PD patients. Understanding its role in lives of individuals with PD will have favorable impact on QOL, vis-à-vis facilitating more therapeutic interactions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Schrag A, Sauerbier A, Chaudhuri KR. New clinical trials for nonmotor manifestations of Parkinson's disease. Mov Disord. 2015;30:1490–504. doi: 10.1002/mds.26415. [DOI] [PubMed] [Google Scholar]
- 2.Metta V, Logishetty K, Martinez-Martin P, Gage HM, Schartau PE, Kaluarachchi TK, et al. The possible clinical predictors of fatigue in Parkinson's disease: A study of 135 patients as part of international nonmotor scale validation project. Parkinsons Dis 2011. 2011:125271. doi: 10.4061/2011/125271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havlikova E, Rosenberger J, Nagyova I, Middel B, Dubayova T, Gdovinova Z, et al. Impact of fatigue on quality of life in patients with Parkinson's disease. Eur J Neurol. 2008;15:475–80. doi: 10.1111/j.1468-1331.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JH, Friedman H. Fatigue in Parkinson's disease: A nine-year follow-up. Mov Disord. 2001;16:1120–2. doi: 10.1002/mds.1201. [DOI] [PubMed] [Google Scholar]
- 5.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 6.Schiehser DM, Ayers CR, Liu L, Lessig S, Song DS, Filoteo JV. Validation of the modified fatigue impact scale in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:335–8. doi: 10.1016/j.parkreldis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Valderramas S, Feres AC, Melo A. Reliability and validity study of a Brazilian-Portuguese version of the fatigue severity scale in Parkinson's disease patients. Arq Neuropsiquiatr. 2012;70:497–500. doi: 10.1590/s0004-282x2012000700005. [DOI] [PubMed] [Google Scholar]
- 8.Fereshtehnejad SM, Hadizadeh H, Farhadi F, Shahidi GA, Delbari A, Lökk J. Reliability and validity of the Persian version of the fatigue severity scale in idiopathic Parkinson's disease patients. Parkinsons Dis 2013. 2013:935429. doi: 10.1155/2013/935429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–15. [Google Scholar]
- 10.Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson's disease subtypes: Clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6:69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk JP, Havlikova E, Rosenberger J, Nagyova I, Skorvanek M, Gdovinova Z, et al. Influence of disease severity on fatigue in patients with Parkinson's disease is mainly mediated by symptoms of depression. Eur Neurol. 2013;70:201–9. doi: 10.1159/000351779. [DOI] [PubMed] [Google Scholar]
- 12.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009;24:1641–9. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 13.Schifitto G, Friedman JH, Oakes D, Shulman L, Comella CL, Marek K, et al. Fatigue in levodopa-naive subjects with Parkinson disease. Neurology. 2008;71:481–5. doi: 10.1212/01.wnl.0000324862.29733.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka K, Wada-Isoe K, Yamamoto M, Tagashira S, Tajiri Y, Nakashita S, et al. Clinical evaluation of fatigue in Japanese patients with Parkinson's disease. Brain Behav. 2014;4:643–9. doi: 10.1002/brb3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulart FO, Godke BA, Borges V, Azevedo-Silva SM, Mendes MF, Cendoroglo MS, et al. Fatigue in a cohort of geriatric patients with and without Parkinson's disease. Braz J Med Biol Res. 2009;42:771–5. doi: 10.1590/s0100-879x2009000800014. [DOI] [PubMed] [Google Scholar]
- 16.Kummer A, Scalzo P, Cardoso F, Teixeira AL. Evaluation of fatigue in Parkinson's disease using the Brazilian version of Parkinson's fatigue scale. Acta Neurol Scand. 2011;123:130–6. doi: 10.1111/j.1600-0404.2010.01364.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson's disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–9. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 18.Falup-Pecurariu C. Fatigue assessment of Parkinson's disease patient in clinic: Specific versus holistic. J Neural Transm (Vienna) 2013;120:577–81. doi: 10.1007/s00702-013-0969-1. [DOI] [PubMed] [Google Scholar]
- 19.Lewis MM, Du G, Sen S, Kawaguchi A, Truong Y, Lee S, et al. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–9. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]


