Abstract
Background:
We aimed to compare the efficacy of fixed doses of bupropion and ropinirole and iron alone for the treatment of restless legs syndrome (RLS) and to look for the tolerability of these medications.
Materials and Methods:
Patients diagnosed with RLS were randomly divided into three groups with thirty patients in each group (Group A: Bupropion [300 mg/day], Group B: Ropinirole [0.25–0.5 mg/day], and Group C: Oral iron [150 mg elemental iron] along with folic acid [500 μg]). Each participant was then assessed for severity of RLS, as well as RLS-related quality at the baseline, and thereafter, every 14th day till 6 weeks based on the International Restless Legs Scale (IRLS) severity rating scale and Restless Legs Syndrome Quality of Life (RLSQoL) Questionnaire, respectively.
Results:
IRLS scores differed significantly from baseline visit to last (F = 4.85; P = 0.01). The interaction between the time x treatment group was significant (F = 10.37; P < 0.001) showing an improvement with the therapy in all the groups. Pair-wise comparison depicted that ropinirole group differed from other two groups in IRLS score (F = 7.06; P = 0.001), which were comparable to each other. Regarding quality of life of these cases, within each group scores differed among all the four visits (F = 5.12; P = 0.002). Unlike IRLS, there was no significant difference among the RLSQOL scores between groups at any point of time (F = 1.2; P = 0.28).
Conclusion:
RLS severity decreased across time in all three groups; however, the ropinirole treatment was better than the bupropion and iron-folate therapy. Moreover, RLS-related quality of life although improved among all groups, it was comparable among three groups.
Keywords: Bupropion, efficacy, restless legs syndrome, ropinirole, tolerability, Willis-Ekbom disease
Introduction
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is characterized by an irresistible urge to move the legs, which may begin or worsen during periods of rest or inactivity and often adversely affects sleep.[1,2] Alteration in the dopamine and/or iron metabolism in brain have been suggested as the underlying mechanism of this disease.[2] Disruption of central and subcortical dopaminergic pathway in RLS has been supported by clinical observations which showed alleviation of symptom with dopaminergic drugs and precipitation or exacerbation of RLS by dopamine antagonist.[3] The most frequently used drugs in RLS are dopamine agonists with the highest level of evidence.[4] The nonergot derived dopamine agonists (NEDAs) are the mainstay of the treatment of RLS.[4] They are highly effective in relieving the symptoms of RLS, but many patients experience some residual symptoms, as well as side effects. Few of them discontinue the treatment due to augmentation of RLS and appearance of impulse control disorders.[4,5] Among dopamine agonists, NEDAs such as ropinirole, pramipexole, and rotigotine have been approved by the US Food and Drug Administration (FDA).[4] Recently, gabapentin enacarbil has also been approved by FDA for use in RLS.[4,6] In addition, treatment with iron (oral as well as IV) has also been reported to improve or resolve symptoms of RLS but lacks evidence.[4]
It has been observed in previous studies that RLS is commonly associated with depression though most of the commonly prescribed antidepressant drugs exacerbate the symptoms of RLS.[7,8] However, there is mounting evidence that bupropion, a selective noradrenergic-dopaminergic reuptake inhibitor, does not exacerbate the symptoms of RLS.[8] In some studies, bupropion has been found to relieve symptoms of RLS with an added advantage of fewer side effects, tolerable adverse drug reactions (ADR) ADRs, and reasonable cost compared with dopamine agonists.[8,9]
Considering these facts, it would be worthwhile to compare the efficacy of fixed-dose schedules of bupropion and ropinirole and iron alone for the treatment of RLS. To best of our knowledge, no such study is available so far. Thus, the present study was planned to compare the efficacy by assessing the improvement in severity of RLS over 6 weeks of therapy in patients receiving bupropion or ropinirole or oral iron therapy alone. Another aim of the study was to look for the tolerability of these medications among three groups.
Materials and Methods
Participants of either sex aging 18 years or more who attended the sleep clinic were screened for the presence of RLS according to the international RLS study group criteria.[10] Patients with the diagnosis of RLS were explained the rationale of the study and were requested to participate. The study was approved by the Institutional Ethics Committee and a written informed consent was taken from each of the participants.
However, cases with comorbid conditions such as moderate to severe depression, sciatica, osteoarthritis of knee, rheumatoid arthritis, myelopathies and neuropathies, those using opioids/opiates or experiencing its withdrawal, diagnosed of alcohol dependence, those using neuroleptics, antidepressants or antiparkinsonian drugs for at least 1 week, and cases with uremia or end-stage renal disease were excluded from the study. Similarly, cases where history was suggestive of obstructive sleep apnea, parasomnia, hypersomnia, and neurocognitive disorders were also excluded from the study. In addition, participants with either restrictive or obstructive pulmonary diseases or pregnant women were also not included in this study. All these conditions were diagnosed based on history, clinical examination, and laboratory investigations, wherever required.
Therapy
Cases who consented to participate in the study were randomly divided into three groups till we had thirty patients in each group who have completed 6 weeks of trial. After randomization, medicines were given in a double-blinded manner. Cases fell in one of the following groups: Group A: Bupropion (150 mg/day once daily for first 5 days followed by 300 mg/day in two divided doses), Group B: Ropinirole (0.25 mg before bedtime during first 2 weeks that was increased to 0.5 mg/day before bedtime afterwards), and lastly, Group C: Oral iron (150 mg elemental iron) along with folic acid (500 μg) once a day before bedtime. These doses remained fixed till the end of the study. The night-time doses in all groups were given 2 h before the bedtime.
Schedule of visits
Each case was assessed at the baseline, and thereafter, every 14th day with the margin of 1 day till 6 weeks. Thus, all the cases had four visits. Biochemical parameters were examined at the baseline and on the last visit. The severity of RLS and RLS-related quality of life (see below) were assessed on each visit. Adverse effects of the medications were recorded on second, third, and fourth visits.
Severity of restless legs syndrome
Severity of RLS was assessed using Hindi version of the international RLS severity rating scale.[11,12] The severity symptoms were assessed at the time of randomization, as well as at the time of follow-up once every 2-week for 6 weeks. It is a validated scale containing ten questions, each scoring from 0 to 4 (0 = absence of a problem, 4 = very severe problem). The lower the score means, the less-severe is the disease.[11] Based on the score, cases may be categorized into mild (1–10), moderate (11–20), severe (21–30), and very severe (31–40).
Restless legs syndrome-related quality of life
The patients were also assessed for their quality of life using Restless Legs Syndrome Quality of Life (RLSQoL) questionnaire[13,14] on every visit. It is a validated tool consisting of 18 questions based on how RLS affects the quality of life and measures three basic parameters that may affect the overall quality of life score, work statuses, and sexual interest. Higher scores of RLSQoL score indicate a better quality of life.[13,14]
Laboratory investigations
Complete hemogram was ordered at the baseline and at the end of the study. In addition, serum ferritin and serum total iron binding capacity (TIBC) levels were also assessed at the baseline and at the end of the study.
Monitoring of adverse effects and augmentation of restless legs syndrome
On each follow-up visit, patients were also monitored for the possible adverse effects using the WHO-Central Drugs Standard Control Organization (CDSCO) form. It is a suspected Adverse Drug Reaction (ADR) Reporting Form for voluntary reporting of suspected ADRs by health care professionals. This is under Pharmacovigilance Programme of India National Coordinating Centre, Indian Pharmacopoeia Commission Ministry of Health and Family Welfare, Government of India.[15] Augmentation was assessed by a sleep physician.
Statistical analysis
Statistical analysis was done with the help of SPSS version 22.0 (IBM Corporation Released 2012, IBM SPSS Statistics for Windows, Version 22.0, Armonk, NY: IBM Corporation, USA). Descriptive statistics was calculated. Categorical variable was presented as proportions. For continuous variables, skewing was calculated. Chi-square test was used to compare proportions between groups. Continuous variables across three groups were compared using one-way ANOVA. The response to therapy at various points was measured using mixed ANOVA with covariates. Baseline hemoglobin, serum ferritin, and serum folate were considered as covariates as they have been described in association with RLS. While comparing the change in RLS severity and RLS-related quality of life across four points of measurements, test of sphericity could not be met in this sample; hence, the Greenhouse-Geisser corrected degrees of freedom were used during interpretation of results. Leven's test depicted that variance was homogeneous.
Results
This study included a total of 103 patients, out of which thirty cases were assigned to each group. Thirteen patients were lost to follow-up during the course of the study.
Females (68.8%) outnumbered males (31.1%) in this study; however, they were equally distributed in each group (χ2 = 0.415; P = 0.81). The average age of the cases in this study was 40.3 ± 12.8 years (range: 18–75 years), and the average duration of illness was 4.1 ± 4.7 years. All the groups were comparable with regards to age and duration of illness (F = 1.698; P = 0.189 and F = 3.099; P = 0.050, respectively). All the three groups were also comparable with regards to prevalence of family history of RLS (χ2 = 4.229; P = 0.1206).
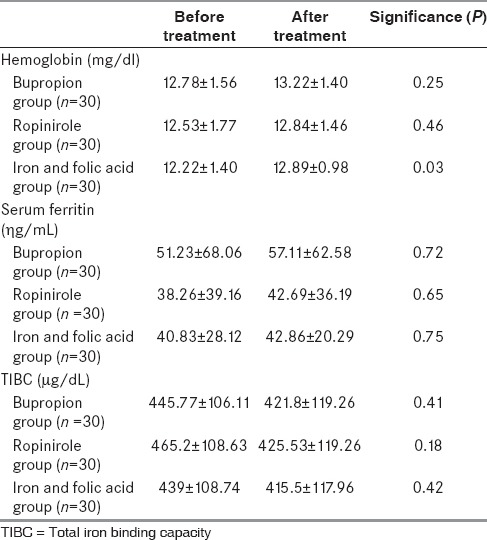
Baseline characteristics of the cases included in all three groups are depicted in Table 1. All the three groups were comparable with regards to most of the parameters except the fact that bupropion group had a shorter duration of symptoms, and serum folate level was highest in the iron-folate group. Table 2 depicts the concentration of hemoglobin, serum ferritin, and TIBC at the baseline and at the end of 6 weeks. There was no clinically significant change in hemoglobin or serum ferritin or TIBC in any of the groups from baseline to the end of the study.
Table 1.
Baseline characteristics of the study groups
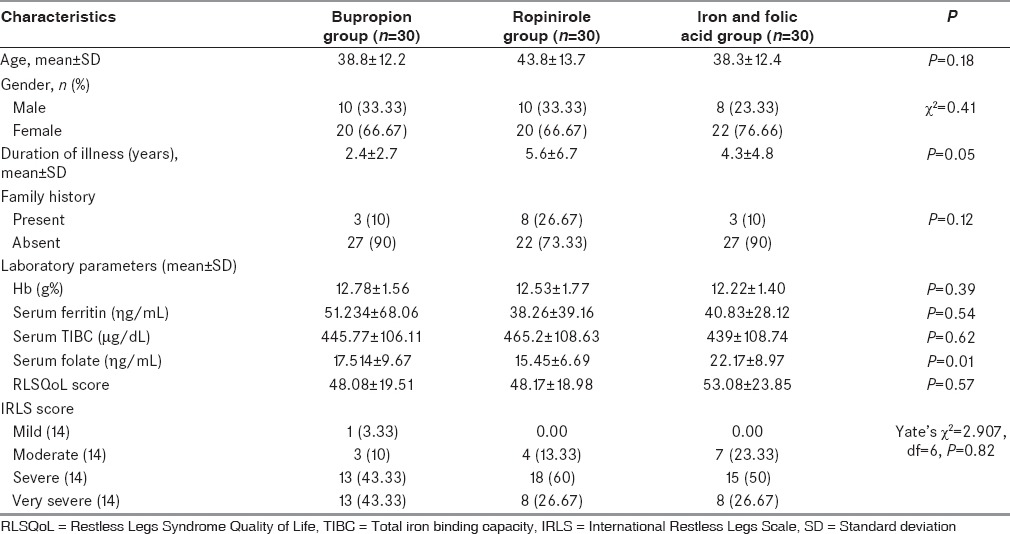
Table 2.
Change in biochemical parameters over the study period
Change in severity of restless legs syndrome over time
The results showed that case's scores differed significantly at the four points of measurement, i.e., from baseline visit to last (F = 4.85; P = 0.01). The interaction between the time x treatment group was significant (F = 10.37; P < 0.001) suggesting that all the groups had an improvement with the therapy with the observed power of 0.99. However, we could not find any interaction between time x hemoglobin, time x ferritin, and time x folate measures. All the three groups differed significantly from each other (F = 7.06; P = 0.001) with the observed power of 0.92 with regards to the International Restless Legs Scale (IRLS) score. Figure 1 shows the scores of all the groups on each visit. It was also found that ropinirole group had the lowest score on the IRLS on the final visit. Pair-wise comparison depicted that this group differed from other two groups which were comparable to each other.
Figure 1.
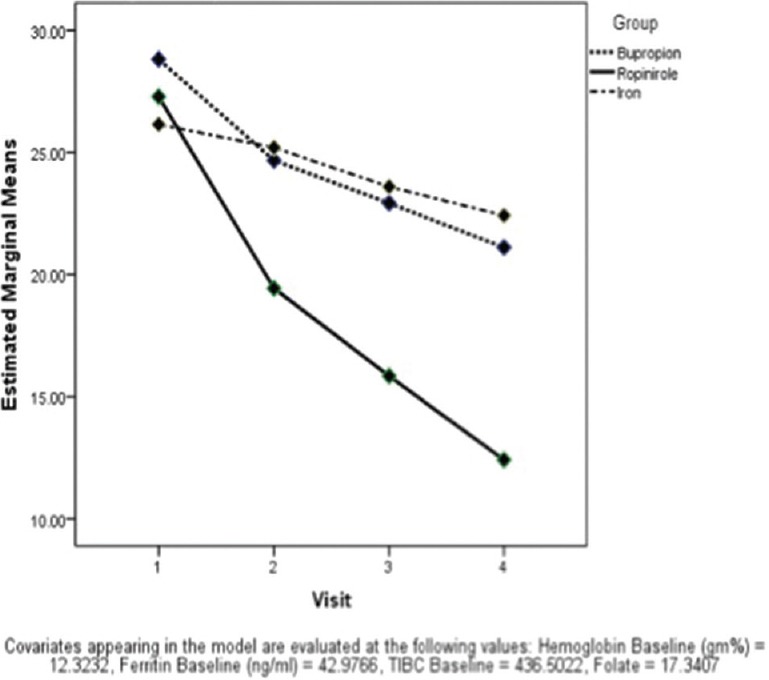
Comparison of International Restless Legs Scale score over time among groups
Change in restless legs syndrome-related quality of life
Results showed that cases in each group had a difference in the scores on all of their visits (F = 5.12; P = 0.002). However, the interaction of the time with hemoglobin, folate, and ferritin was not significant in any of the treatment arms. After controlling the covariates, pair-wise comparison was made which showed that RLSQoL score of baseline visit were significantly different from remaining three visits (P < 0.001); scores of the second visit were different from that of subsequent visits (P = 0.004 and P < 0.001, respectively), and that of the third visit were also different from the final visit (P < 0.001). Unlike IRLS, we could not find a significant difference among the RLSQoL scores between groups at any point of time (F = 1.2; P = 0.28) as depicted in Figure 2. Thus, though the RLSQoL scores differed across time in each group, they were not different between groups.
Figure 2.
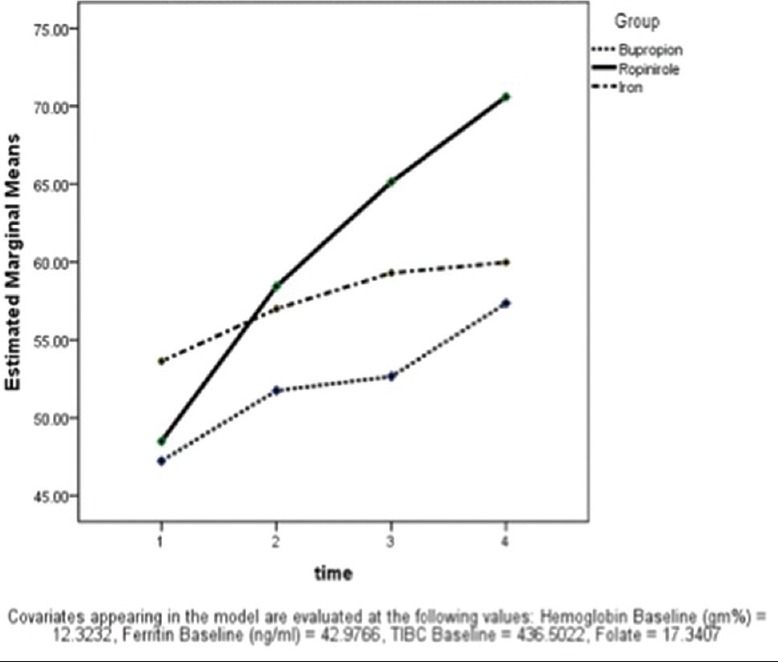
Changes in restless legs syndrome-related quality of life among groups
Adverse drug reactions
ADRs were recorded for 6 weeks of the study period in all the patients using the WHO-CDSCO form. A total of 13 ADRs were observed in nine patients out of ninety patients. In the ropinirole group, around 16.67% of patients (five out of thirty patients) presented with ADRs. These were nausea, dizziness, tremor, tingling sensation in palm and sole, gastritis, constipation, and weight gain. In the bupropion group, only gastritis was reported in a single patient. In the iron and folic acid group, 10% of the patients (three out of thirty) presented with ADRs such as constipation, gastritis, and headache. The side effects in all the groups were mild, transient, and did not necessitate stoppage of the treatment. None of the patients opted out of the study because of the adverse drug events.
Patients who did not complete the study
Thirteen cases did not complete the study. Six patients from the iron therapy, four from bupropion, and three from ropinirole. Reasons for drop out were due to no improvement in the initial week of the study.
Discussion
This study showed that in the middle-aged cases with RLS, 6 weeks treatment with ropinirole, bupropion, and oral iron therapy reduce the severity of RLS without any major adverse effect. Moreover, the maximum improvement was noticed in the ropinirole group as compared to the bupropion and oral iron therapy. On the other hand, RLSQOL score improved in all three groups across time, however, no difference among the groups was observed.
Multiple studies have shown that ropinirole is effective for the management of RLS both in the short term and also in the long term using the IRLS score as the measurement of severity.[16,17,18,19,20,21,22,23,24,25,26] Some of these studies have included cases which were older in age as compared to the cases included in the present study.[20,25,26] The methodology of the studies conducted in past some of them was open label,[23] some randomized,[17,19,20,21,25,26] and few were single blind,[22] while others were double blinded.[19,20,21,25,26] Some had placebo control,[17,18,19,20,21,25,26] while others were cross-over trials.[18] Having trials with diverse methodologies make the comparison difficult. As far as the dose of ropinirole is concerned, most of them had prescribed ropinirole in the dose range of 0.25–4 mg/day[17,19,21,25,26] except one where doses as high as 6 mg/day were prescribed.[18] All the studies have found that ropinirole was well tolerated with minimal and mild adverse effects[17,18,19,20,21,22,23,26] and in one study treatment, discontinuation was seen in only 9% due to adverse effects.[23] These studies have reported that adverse effects were dose related, and hence the lower incidence of adverse effects in the present study could be related to low dosage.
Bupropion has also been shown to improve RLS although the evidence is mostly anecdotal saving one randomized, placebo-controlled trial that measured improvement using IRLS.[9,27,28] However, in this trial, bupropion was prescribed in low doses (150 mg/day). Only available randomized, placebo-controlled trial by Bayard et al.[9] included cases who were older than the cases in the present study. Moreover, this study had shown that bupropion was effective in reducing the RLS severity by the 3rd week, which was then maintained at the end of 6th week. This occurred even when the score on depression rating scale did not show a clinically meaningful change across the study.[9] However, we found that bupropion group showed a sustained reduction in the IRLS score over 6 weeks.
Oral iron therapy has been shown to be effective in the management of RLS among children, as well as adults, as compared to the placebo in some studies, but contradictory data are also available.[29,30,31,32,33,34] One study compared the oral iron therapy with pramipexole and reported equal improvement in both groups.[32] Improvement in the RLS with iron therapy has been found to correlate with the improvement in serum ferritin.[29,30] Although we did not find a change in serum ferritin or TIBC in any of the groups over the study period, yet we have found a significant improvement in RLS severity over the study period.
However, we could not find any study that has head-to-head compared three modalities that have been found effective in the management of RLS across various studies. A sizable number of RLS patients show placebo response.[16] We have found that ropinirole group had a greater reduction of IRLS score as compared to other two groups. Considering these two facts, can we consider that bupropion and iron therapy merely acted as a placebo? It appears unlikely if we take into account the studies that have found that iron therapy and bupropion had shown greater improvement as compared to the placebo in the past.[9,29,30] Moreover, both bupropion and iron are at least theoretically involved in the pathophysiology of RLS by reducing the reuptake of dopamine and improving the dopamine synthesis, respectively.[2,35,36] Still, in the absence of the placebo arm, this notion cannot be completely ruled out.
Like earlier reports, the present study has also found improvement in the RLS-related quality of life in all three groups across time; however, in contradiction to previous studies, we did not find any difference between groups on this measure.[17,18,21,22,23,29] It could be related to the fact that earlier studies have compared the ropinirole or oral iron with placebo. Ropinirole is a known dopamine agonist, and thus it may reduce the burden of symptoms and thereby improves the quality of life. Other two molecules that we studied were also pathophysiologically related to the RLS and thus, they could not be considered as placebo.[2,35,36] This could be one reason why we found improvement in RLS-related quality of life among all groups. The absence of difference among groups could be related to the fact that IRLS and RLSQoL address separate issues. Although Allen et al.[37] have reported a correlation between these two scales, Vishwakarma et al.[13] did not find the same in the Indian population.
One of the major implications of this study is the use of bupropion in patients with RLS who also have comorbid depression.[9] However, it must be noted that RLS may clinically mimic depression or may be found comorbid with depression or as an adverse effect of antidepressant therapy.[38] As another implication, we also reinforce the conclusion that bupropion should be tested further as an agent to manage RLS in larger randomized, double-blind, controlled trials, especially those patients who are not able to tolerate dopamine agonists or alpha-2-delta ligand agonists.[9]
However, like any other study, this study also had some methodological limitations. First, the sample size was small. Second, the study period was short. Third, doses of the ropinirole were small as compared to the doses mentioned in literature, leaving a possibility that symptoms could be better managed with higher doses. Fourth, we did not control the groups for the presence of depressive symptoms. Fifth, disease duration was short. Sixth, cases included in this study were younger as compared to many of the earlier studies. Lastly, we did not have the placebo arm.
Despite, all these limitations, this is the first study to compare the efficacy of ropinirole with bupropion and oral iron therapy in a randomized, double-blind manner.
Conclusion
This study showed that ropinirole improves the symptoms of RLS, yet bupropion and oral iron therapy were also effective. These treatment modalities also improve the RLS-related quality of life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are thankful to MAPI research trust for allowing us to use Hindi version of IRLS and RLSQoL.
References
- 1.Babu RH, Kaza R, Nagarju R. Restless leg syndrome. JITPS. 2010;1:1–8. [Google Scholar]
- 2.Earley CJ, Connor J, Garcia-Borreguero D, Jenner P, Winkelman J, Zee PC, et al. Altered brain iron homeostasis and dopaminergic function in Restless legs syndrome (Willis-Ekbom disease) Sleep Med. 2014;15:1288–301. doi: 10.1016/j.sleep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Chahine LM, Chemali ZN. Restless legs syndrome: A review. CNS Spectr. 2006;11:511–20. doi: 10.1017/s1092852900013547. [DOI] [PubMed] [Google Scholar]
- 4.Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults – an update for 2012: Practice parameters with an evidence-based systematic review and meta-analyses: An American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35:1039–62. doi: 10.5665/sleep.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker WL, White CM, Coleman CI. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Ann Fam Med. 2008;6:253–62. doi: 10.1370/afm.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilt TJ, MacDonald R, Ouellette J, Khawaja IS, Rutks I, Butler M, et al. Pharmacologic therapy for primary restless legs syndrome: A systematic review and meta-analysis. JAMA Intern Med. 2013;173:496–505. doi: 10.1001/jamainternmed.2013.3733. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Lahan V, Goel D. A study examining depression in Restless legs syndrome. Asian J Psychiatr. 2013;6:308–12. doi: 10.1016/j.ajp.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Durmaz O, Ateþ MA. Bupropion XL use in comorbidity of depression and restless leg syndrome: A case report. Klinik Psikofarmakol Bulteni. 2014;24:84–8. [Google Scholar]
- 9.Bayard M, Bailey B, Acharya D, Ambreen F, Duggal S, Kaur T, et al. Bupropion and restless legs syndrome: A randomized controlled trial. J Am Board Fam Med. 2011;24:422–8. doi: 10.3122/jabfm.2011.04.100173. [DOI] [PubMed] [Google Scholar]
- 10. [Last cited on 2015 Nov 05]. Available from: http://www.irlssg.org/diagnostic-criteria/
- 11.Gupta R, Lahan V, Goel D. Translation and validation of International Restless leg syndrome study group rating scale in Hindi language. Ann Indian Acad Neurol. 2011;14:257–61. doi: 10.4103/0972-2327.91939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 13.Vishwakarma K, Lahan V, Gupta R, Goel D, Dhasmana DC, Sharma T, et al. Translation and validation of restless leg syndrome quality of life questionnaire in Hindi language. Neurol India. 2012;60:476–80. doi: 10.4103/0028-3886.103188. [DOI] [PubMed] [Google Scholar]
- 14.Abetz L, Arbuckle R, Allen RP, Mavraki E, Kirsch J. The reliability, validity and responsiveness of the restless legs syndrome quality of life questionnaire (RLSQoL) in a trial population. Health Qual Life Outcomes. 2005;3:79. doi: 10.1186/1477-7525-3-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [Last cited on 2015 Nov 15]. Available from: http://www.cdsco.nic.in/writereaddata/ADR%20form%20PvPI.pdf .
- 16.Estivill E, de la Fuente V. The efficacy of ropinirole in the treatment of chronic insomnia secondary to restless legs syndrome: Polysomnography data. Rev Neurol. 1999;29:805–7. [PubMed] [Google Scholar]
- 17.Trenkwalder C, Garcia-Borreguero D, Montagna P, Lainey E, de Weerd AW, Tidswell P, et al. Ropinirole in the treatment of restless legs syndrome: Results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Adler CH, Hauser RA, Sethi K, Caviness JN, Marlor L, Anderson WM, et al. Ropinirole for restless legs syndrome: A placebo-controlled crossover trial. Neurology. 2004;62:1405–7. doi: 10.1212/01.wnl.0000120672.94060.f1. [DOI] [PubMed] [Google Scholar]
- 19.Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K TREAT RLS (Therapy with Ropinirole: Efficacy and Tolerability in RLS) Study Group. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: A 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23. doi: 10.1002/mds.20257. [DOI] [PubMed] [Google Scholar]
- 20.Bliwise DL, Freeman A, Ingram CD, Rye DB, Chakravorty S, Watts RL. Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med. 2005;6:141–7. doi: 10.1016/j.sleep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Bogan RK, Fry JM, Schmidt MH, Carson SW, Ritchie SY TREAT RLS US Study Group. Ropinirole in the treatment of patients with restless legs syndrome: A US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81:17–27. doi: 10.4065/81.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Montplaisir J, Karrasch J, Haan J, Volc D. Ropinirole is effective in the long-term management of restless legs syndrome: A randomized controlled trial. Mov Disord. 2006;21:1627–35. doi: 10.1002/mds.21050. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Borreguero D, Grunstein R, Sridhar G, Dreykluft T, Montagna P, Dom R, et al. A 52-week open-label study of the long-term safety of ropinirole in patients with restless legs syndrome. Sleep Med. 2007;8:742–52. doi: 10.1016/j.sleep.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Allen RP, Ritchie SY. Clinical efficacy of ropinirole for restless legs syndrome is not affected by age at symptom onset. Sleep Med. 2008;9:899–902. doi: 10.1016/j.sleep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Kushida CA, Geyer J, Tolson JM, Asgharian A. Patient- and physician-rated measures demonstrate the effectiveness of ropinirole in the treatment of restless legs syndrome. Clin Neuropharmacol. 2008;31:281–6. doi: 10.1097/WNF.0b013e31815a3eec. [DOI] [PubMed] [Google Scholar]
- 26.Giorgi L, Asgharian A, Hunter B. Ropinirole in patients with restless legs syndrome and baseline IRLS total scores=24: Efficacy and tolerability in a 26-week, double-blind, parallel-group, placebo-controlled study followed by a 40-week open-label extension. Clin Ther. 2013;35:1321–36. doi: 10.1016/j.clinthera.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Erdos J, Wilkosz MF, LaPlante R, Wagoner B. Bupropion as a possible treatment option for restless legs syndrome. Ann Pharmacother. 2009;43:370–4. doi: 10.1345/aph.1L035. [DOI] [PubMed] [Google Scholar]
- 28.Kim SW, Shin IS, Kim JM, Yang SJ, Shin HY, Yoon JS. Bupropion may improve restless legs syndrome: A report of three cases. Clin Neuropharmacol. 2005;28:298–301. doi: 10.1097/01.wnf.0000194706.61224.29. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, O’Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–5. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Mohri I, Kato-Nishimura K, Kagitani-Shimono K, Kimura-Ohba S, Ozono K, Tachibana N, et al. Evaluation of oral iron treatment in pediatric restless legs syndrome (RLS) Sleep Med. 2012;13:429–32. doi: 10.1016/j.sleep.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Tilma J, Tilma K, Norregaard O, Ostergaard JR. Early childhood-onset restless legs syndrome: Symptoms and effect of oral iron treatment. Acta Paediatr. 2013;102:e221–6. doi: 10.1111/apa.12173. [DOI] [PubMed] [Google Scholar]
- 32.Lee CS, Lee SD, Kang SH, Park HY, Yoon IY. Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol. 2014;21:260–6. doi: 10.1111/ene.12286. [DOI] [PubMed] [Google Scholar]
- 33.Davis BJ, Rajput A, Rajput ML, Aul EA, Eichhorn GR. A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol. 2000;43:70–5. doi: 10.1159/000008138. [DOI] [PubMed] [Google Scholar]
- 34.Trotti LM, Bhadriraju S, Becker LA. Iron for restless legs syndrome. Cochrane Database Syst Rev. 2012;5:CD007834. doi: 10.1002/14651858.CD007834.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino H, Obata H, Nakajima K, Mieda R, Saito S. The antihyperalgesic effects of intrathecal bupropion, a dopamine and noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg. 2015;120:460–6. doi: 10.1213/ANE.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 36.Egerton A, Shotbolt JP, Stokes PR, Hirani E, Ahmad R, Lappin JM, et al. Acute effect of the anti-addiction drug bupropion on extracellular dopamine concentrations in the human striatum: An [11C] raclopride PET study. Neuroimage. 2010;50:260–6. doi: 10.1016/j.neuroimage.2009.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen R, Oertel W, Walters A, Benes H, Schollmayer E, Grieger F, et al. Relation of the International Restless Legs Syndrome Study Group rating scale with the Clinical Global Impression severity scale, the restless legs syndrome 6-item questionnaire, and the restless legs syndrome-quality of life questionnaire. Sleep Med. 2013;14:1375–80. doi: 10.1016/j.sleep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Hornyak M, Benes H, Eisensehr I, Haan J, Kassubek J, Peglau I, et al. Depression in restless legs syndrome. Pathogenesis, assessment, and implications for treatment. Nervenarzt. 2009;80:1160. doi: 10.1007/s00115-009-2710-8. [DOI] [PubMed] [Google Scholar]


