Abstract
Background:
Central nervous system (CNS) infections present a grave health care challenge due to high morbidity and mortality. Clinical findings and conventional laboratory assessments are not sufficiently distinct for specific etiologic diagnosis. Identification of pathogens is a key to appropriate therapy.
Aim:
In this retrospective observational study, we evaluated the efficacy and clinical utility of syndrome evaluation system (SES) for diagnosing clinically suspected CNS infections.
Materials and Methods:
This retrospective analysis included inpatients in our tertiary level neurointensive care unit (NICU) and ward from February 2010 to December 2013. Cerebrospinal fluid (CSF) samples of 70 patients, clinically suspected of having CNS infections, were subjected to routine laboratory tests, culture, imaging, and SES. We analyzed the efficacy of SES in the diagnosis of CNS infections and its utility in therapeutic decision-making.
Results:
SES had a clinical sensitivity of 57.4% and clinical specificity of 95.6%. Streptococcus pneumoniae and Pseudomonas aeruginosa were the top two bacterial pathogens, whereas Herpes simplex virus (HSV) was the most common viral pathogen. Polymicrobial infections were detected in 32.14% of SES-positive cases. SES elicited a change in the management in 30% of the patients from initial empiric therapy. At discharge, 51 patients recovered fully while 11 patients had partial recovery. Three-month follow-up showed only six patients to have neurological deficits.
Conclusion:
In a tertiary care center, etiological microbial diagnosis is central to appropriate therapy and outcomes. Sensitive and accurate multiplex molecular diagnostics play a critical role in not only identifying the causative pathogen but also in helping clinicians to institute appropriate therapy, reduce overuse of antimicrobials, and ensure superior clinical outcomes.
Keywords: Acute encephalitic syndrome (AES), meningitis, meningoencephalitis, molecular diagnostics, multiplex polymerase chain reaction, syndrome evaluation system (SES)
Introduction
Infections of the central nervous system (CNS) such as meningitis, encephalitis, and meningoencephalitis pose a serious health care challenge due to high morbidity and mortality. The mortality associated with bacterial meningitis is approximately 6–14% worldwide and 16–32% in India and other developing countries, and of those patients who recover, approximately 11–19% have permanent sequelae, imposing heavy societal and economic burdens. Worldwide, the three major meningeal pathogens, Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae account for approximately 75–80% of the cases of meningitis but the proportion varies among geographies.[1,2,3,4,5,6,7] Aseptic meningitis in India is caused by a variety of viruses and Mycobacterium tuberculosis. Distinguishing the aseptic and bacterial forms of the disease is extremely critical for therapy and outcomes. In most cases, the clinical findings are not sufficiently distinct to allow a specific etiologic diagnosis. Sporadic and epidemic viral encephalitis clinically encountered in India are caused by Herpes simplex virus (HSV), Varicella zoster virus (VZV), Japanese encephalitis virus (JEV), dengue, measles, and mumps.[8] Accurate etiological diagnosis is the key to institute specific as well as supportive therapeutic measures and prevent disastrous sequelae.[9,10] A major challenge in patients with encephalitis is to distinguish between infectious encephalitis and postinfectious or postimmunization encephalitis, encephalomyelitis, or encephalopathy.
The routine laboratory practices of cerebrospinal fluid (CSF) cytology, CSF culture, Gram staining, India ink staining, latex agglutination tests, etc., have limited use in a tertiary care center such as ours where a majority of the patients come with prior antibiotic therapy. Molecular methods for the diagnosis of CNS infections are now well-established in the diagnosis of HSV and enteroviruses.[11,12,13,14] Multiplex polymerase chain reaction (PCR) has been successfully used to diagnose viral CNS infections[15,16,17,18,19,20,21,22,23,24,25] and bacterial meningitis.[25,26]
In this retrospective observational study, we evaluated the efficacy and clinical impact of a commercially available multiplex molecular (PCR-based) diagnostic system––the syndrome evaluation system (SES) developed by XCyton Diagnostics Pvt. Ltd., Bengaluru, Karnataka, India for diagnosing (ruling in or ruling out) clinically suspected CNS infections in our tertiary care hospital. SES is a multiplex PCR system that can identify 14 bacteria, 3 fungi, 17 viruses, and 1 parasite distributed across separate clinically relevant panels such as bacterial meningitis, meningoencephalitis, and acute encephalitic syndrome (AES) in a single test from a single sample, with a processing time of 7 h. In addition to the detection rates, we studied the clinical correlation of SES results with our laboratory findings, clinical suspicion, and patient outcomes. To the best of our knowledge, this is the first and most comprehensive study using multiplex PCR-based diagnosis, which can simultaneously detect bacteria, virus, fungi, and parasite on CNS infections in India.
Materials and Methods
Setting
This study was conducted in our tertiary level neurointensive care unit (NICU) and ward from February 18, 2010 to December 28, 2013.
Out of the 70 patients, 67 were from the state of Tamil Nadu while there was 1 patient each from Kerala, West Bengal, and Tripura. At the time of conducting SES test, we routinely made clear cut demarcation for ruling in or ruling out a suspected CNS infection. In case of ruling out, the clinical suspicion was of a noninfectious etiology. The clinical suspicion was based on a) clinical presentation, signs, and symptoms, b) routine biochemical tests, and c) clinical history of patients, whereas in case of ruling in, the clinical suspicion was that of infectious etiology, again based on the same parameters.
Inclusion and exclusion criteria
All consecutive patients who were clinically warranted and thus were prescribed the SES test during the abovementioned period were included in the study. Patients who were not prescribed SES test or who could not afford SES test during this period were excluded from this retrospective analysis.
Routine diagnostics
The freshly tapped CSF samples of 70 patients were subjected to routine biochemistry, cell counts, staining, and culture in our laboratory. All necessary aseptic precautions were taken to collect 1–2 mL of CSF at the same time in EDTA vacutainer, and analyzed by SES at XCyton Diagnostics, Bengaluru, Karnataka, India. Diagnostic imaging such as magnetic resonance imaging (MRI) and computed tomography (CT) of the brain were performed as deemed clinically appropriate for deserving patients. The standard therapy was provided to all patients based on diagnostic information from our laboratory and XCyton Diagnostics.
Syndrome Evaluation System procedure
The SES assay procedure as described by XCyton Diagnostics is given in details below.
Primer and probe design
Primers and probes used in SES were designed using full length genome sequences or complete coding sequence obtained from GenBank of National Center for Biotechnology Information (NCBI).
Syndrome Evaluation System assay
Nucleic acid extraction
Nucleic acid was extracted from 0.2 mL of EDTA CSF sample using commercial columns (Qiagen, USA) as per the procedure specified in the instruction manual provided by the manufacturer.
Complementary DNA preparation
Complementary DNA (cDNA) was prepared using a cDNA Archive kit (ABI, USA) using a multiplexed pathogen specific primers.
Nucleic acid amplification
Nucleic acid amplification was standardized in a 50-μl volume containing 4 mM magnesium chloride, 0.2 mM deoxynucleoside triphosphates, 50–300 nM concentration of each primer set (biotin labeled), and 1U of Taq polymerase (ABI, USA). The initial denaturation step was carried out at 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 45 s, annealing at 60°C for 45 s and extending at 72°C for 45 s in a thermal cycler (Bio-Rad, UK).
Hybridization
The signature gene sequences chosen as probes for each of the pathogens were commercially synthesized. 20 μM of probes for each of the pathogens was transferred on to a predetermined position on the SES platform according to the templates. The SES platform comprised a plastic frame mounted on a charged membrane on to which probes were arrayed. For each gene amplified, a single probe was used for hybridization. In order to monitor the amplification and the subsequent hybridization, ß-globin was used as an internal control.
The amplified products were denatured at 95°C for 10 min. They were then incubated at 50°C for 30 min on the SES platform in the hybridization buffer. Unbound amplicons were removed by washing the device thrice with a preheated wash buffer. Following the washes, conjugate (streptavidin peroxidase, Thermo Fisher) diluted in 1% BSA in PBS, along with 0.05% tween 20 was added and incubated for 15 min at room temperature. The SES platform was washed thrice with the conjugate buffer at room temperature. Subsequently, freshly prepared substrate (0.5 mg/mL of diaminobenzidine with 0.03% of H2O2) was added and incubated for 10 min at room temperature. SES platforms were then washed with water and the signal observed with the naked eye under adequate illumination. A semi-quantitative scale was developed in order to minimize interreader variability in the interpretation of results. Only signals with intensity 1 and above were classified as positive.
Controls
XCyton Diagnostics uses three different controls, negative, positive, and method controls for each batch of sample. In addition, internal control is used to monitor amplification in each sample. These controls ensure the following: (i) no amplicon contamination, (ii) no contaminant has been introduced during the assay, (iii) all the reagents have worked according to the standard process, and (iv) all the reagents have performed to the satisfaction in each sample tested.
SES validation was done on proficiency panels of Quality Control for Molecular Diagnostics (QCMD), UK.[27]
Statistical analysis
Descriptive statistics was used to analyze qualitative and discrete variables. The data are represented in terms of percentage and central tendency.
The institutional review board (IRB) and institutional ethics committee (IEC) approvals that were necessary were taken from KG Hospital before conducting the study.
Results
Seventy patients (53% males, 47% females; mean age: 35.6 years) clinically suspected of CNS infections between February 2010 and December 2013 were part of this study. The major clinical presentations at the time of admission were fever, headache, neck stiffness, vomiting, and seizures. While 40 patients had neck stiffness, 23 out of 70 patients had all three presentations of neck stiffness, headache, and fever. Thirty-one patients had at least one episode of seizure. Among them, three patients had headache, fever, neck stiffness and vomiting, along with seizure. Seven and eight patients had at least three and two of the top five presentations, along with seizure, respectively. Figure 1 shows the distribution of clinical presentations.
Figure 1.
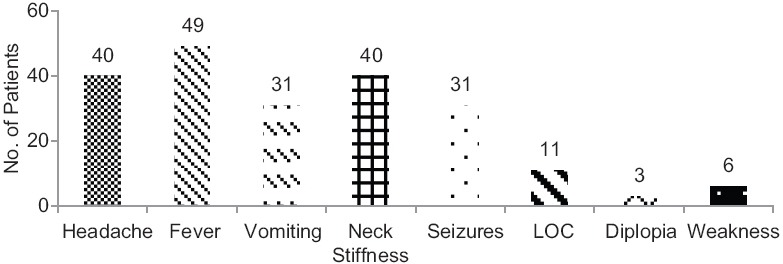
Distribution of clinical presentation at the time of admission
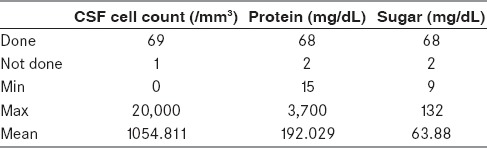
CSF cytology was performed at our laboratory. Table 1 provides the details.
Table 1.
Routine CSF cytology
Routine laboratory diagnostic tests such as CSF culture, acid-fast bacillus (AFB), and Gram staining were performed for all patients (except for one patient with bilateral basifrontal and left anterior temporal cortical encephalomalacia and on the ventricular shunt where CSF culture was not performed). However, diagnostic information for the causative agent could not be ascertained by these tests. Except for three patients where Gram staining was positive for nonspecific pus cells, other diagnostic tests were negative. However, SES was positive in 28 out of 70 cases (40%). Figure 2 provides the details.
Figure 2.
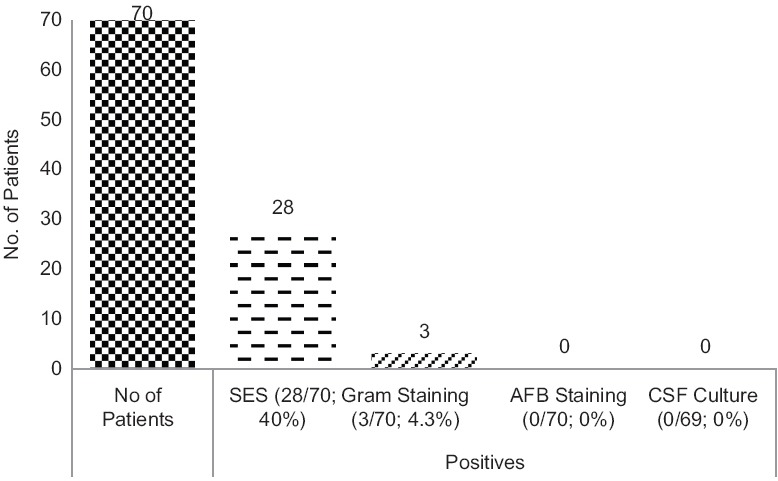
Positivity of different diagnostic tests
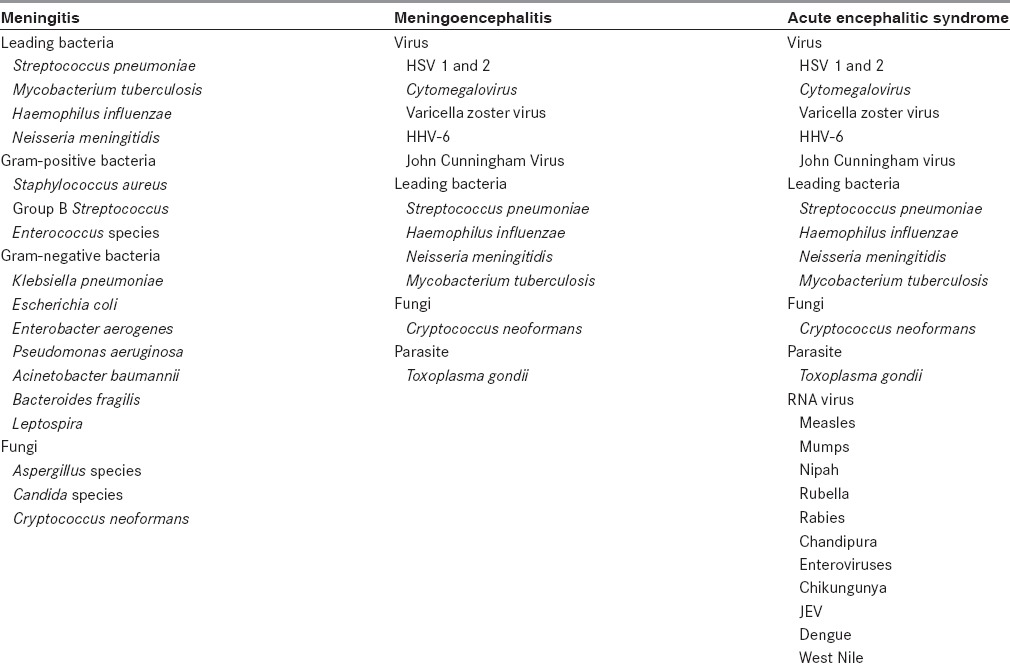
Out of 23 samples sent for ruling out an infection, 22 were negative in SES. This gives a 95.6% clinical correlation with the ruling out of cases. One was actually found to have Acinetobacter baumannii. This patient had neck stiffness and fever for 10 days. He was treated with intravenous (IV) ceftriaxone and recovered fully. However, out of 47 cases where SES test was done to rule in an infection, 27 were positive. This means that in cases of strong clinical suspicion of an infection, SES demonstrated 57.4% clinical sensitivity, which is the percentage of SES-positive cases where we clinically suspected an infectious etiology. Similarly, in cases where we wanted to confirm our clinical suspicion of a noninfectious etiology, SES results were 95.6% specific, as mentioned above. Figure 3 provides the details.
Figure 3.
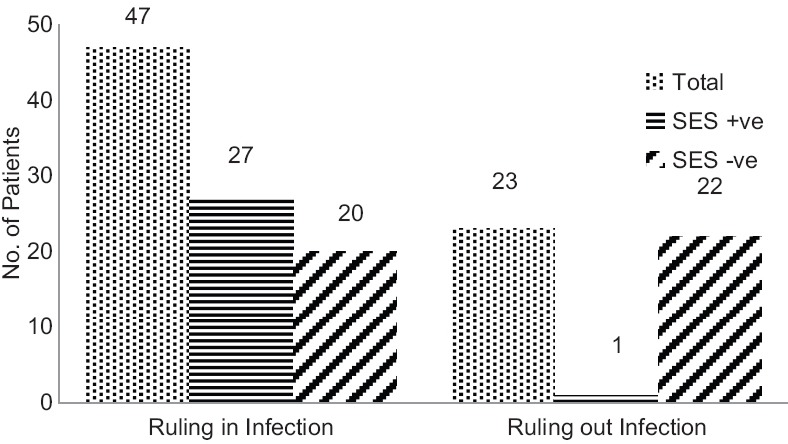
Concordance of SES results with ruling or ruling out infection
In this study, we used three panels of SES for the purpose of microbial diagnosis. The selection of panels was based on our clinical suspicion and to rule in or rule out a suspected CNS infection. The composition of each panel is provided in Table 2.
Table 2.
Composition of SES panels
Panelwise detection rates of SES, based on the decision to conduct the test to rule in or rule out, are provided in Table 3.
Table 3.
Panelwise detection rates of SES
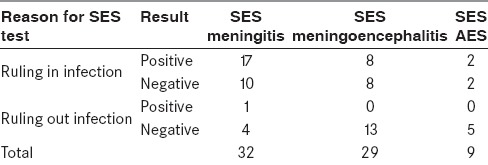
The rank order of organisms detected by SES is provided in Figure 4.
Figure 4.
Rank order of organisms detected by SES
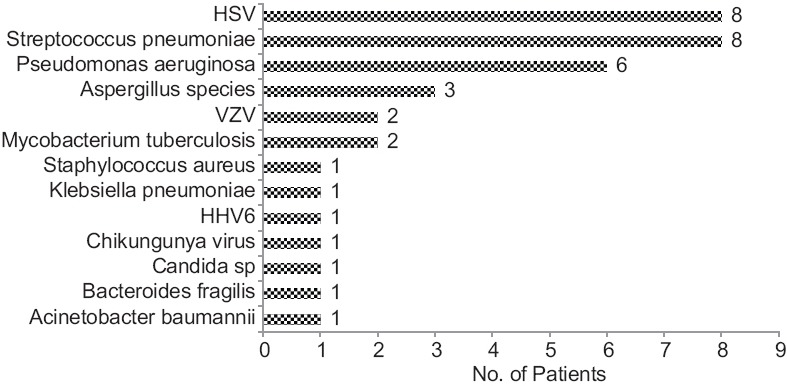
Streptococcus pneumoniae and Pseudomonas aeruginosa were found to be the most common bacterial pathogens, whereas HSV remained the most common virus responsible for CNS infections. Interestingly, we detected three cases of Aspergillus and one case of Candida. In nine cases, we detected polymicrobial infections (32.14% of SES-positives). Details of these polymicrobial infections are illustrated in Figure 5.
Figure 5.
Polymicrobial infection detected by SES
In terms of clinical correlation, a majority of SES results seemed to be in accordance with existing knowledge of CNS infections although there are cases where new light has been thrown, as is expected from a new technology. In eight HSV-positive cases (either HSV alone or HSV as a part of polymicrobial infection), the average CSF cell count was 160 cells. However, there are two cases of HSV-positives with no cells found in CSF cytology. Five patients presented with at least one episode of seizure, whereas one patient had neck stiffness. Five patients had fever, among whom two also had vomiting. Out of the eight HSV cases, four patients had neurological deficits. No deaths were reported in these eight patients. Two HSV-positive patients were discharged against medical advice, one among them a mixed infection of both HSV and VZV. The patient had post Varicella Guillain-Barre Syndrome.
In the four cases where we detected fungus (three cases of Aspergillus and one case of Candida, either alone or as a part of polymicrobial infection), the average CSF cell count was 296 cells. The patient in whom Candida and Pseudomonas aeruginosa were detected did not survive.
In 17 cases where at least one bacterium was detected, either alone or as a part of polymicrobial infection, the average CSF cell count was 3,228 cells. Interestingly, in one case, Acinetobacter baumannii was detected in spite of no cells being found in CSF cytology. This is the same case that we have already described wherein SES was positive for the “ruling out” category. The patient improved after treatment with IV ceftriaxone.
SES detected Mycobacterium tuberculosis in two cases. In the first case, CSF cytology showed 83 cells, CT of the abdomen showed mild ascites with high attenuation of mesentery, and MRI was suggestive of inflammatory granuloma (tuberculoma). The second case had 200 cells while MRI showed the presence of tubercles in brain parenchyma. Both were negative on AFB staining.
There were 40 patients who presented with neck stiffness as at least one of the symptoms. Twenty-three among them also had both fever and headache, along with neck stiffness. In these 40 patients, there were eight cases where CSF cytology did not show any cell. One among them turned out to be Acinetobacter baumannii, as described above. For the remaining 32 cases where cells were detected in CSF cytology, the average CSF cell count was 2,152. Among these 32 cases, 15 were found to be positive on SES, with an average CSF cell count of 3,573, whereas 17 were negative on SES with an average cell count of 899. Interestingly, in 2 of these 17 cases, there were 2 patients with cell counts of 6,250 and 4,733, respectively, where infectious etiology could not be ascertained. The patient with 6,250 cells in CSF had osteomyelitis in the left temporal bone and had a temporal abscess. The MRI of the patient with 4,733 cells in CSF was normal and the patient fully recovered. Barring these 2 cases, the average CSF cell count in the remaining 15 SES-negative patients was 286.6. Among the 15 cases where CSF cytology has shown the presence of cells and SES was positive, at least one bacterial pathogen, either alone or as a part of polymicrobial infection, was detected in 11 cases, with an average cell count of 4,832. In the remaining four cases, either a virus or Mycobacterium tuberculosis was detected with an average cell count of 114.
In 21 patients, who accounted for 30% of the cases, SES results elicited a change in the management from initial empiric therapy. Importantly, 19 out these 21 cases were deescalations. These deescalations included stopping of acyclovir in six patients. Five of these six patients fully recovered while one patient recovered partially. He had Streptococcus pneumoniae meningitis. Ceftriaxone was stopped in 10 cases (both acyclovir and ceftriaxone and vancomycin and ceftriaxone were stopped in one case each) and vancomycin was stopped in four cases, with all of them except one patient having full recovery.
Overall, at the time of discharge 51 out of 70 patients recovered completely from the episode for which they were admitted, 11 patients partially recovered from the episode, 3 of them died while the other 5 patients were discharged against medical advice. The details of SES detected microbial etiology and corresponding cell cytology; the change in treatment and outcome has been described in Table 4. After 3 months, follow-up could be done for 56 patients; three among 51 fully recovered patients and three among 11 partially recovered patients were lost to follow-up. Out of these 56 patients, 6 showed signs of neurological deficit; 1 among 51 fully recovered patients and 5 among 11 partially recovered patients. Out of these six patients who had neurological deficits, four had seizure as one of the presentations and all four were HSV-positives. One patient with neurological deficit had Streptococcus pneumoniae while the other patient was negative on SES. However, this patient had a communicating hydrocephalus with syndrome of inappropriate antidiuretic hormone secretion, suggestive of tubercular meningitis with sequelae. A subanalysis of patient outcomes with SES results has been illustrated in [Figure 6].
Table 4.
SES results and their corresponding cell cytology, change in treatment, and outcome
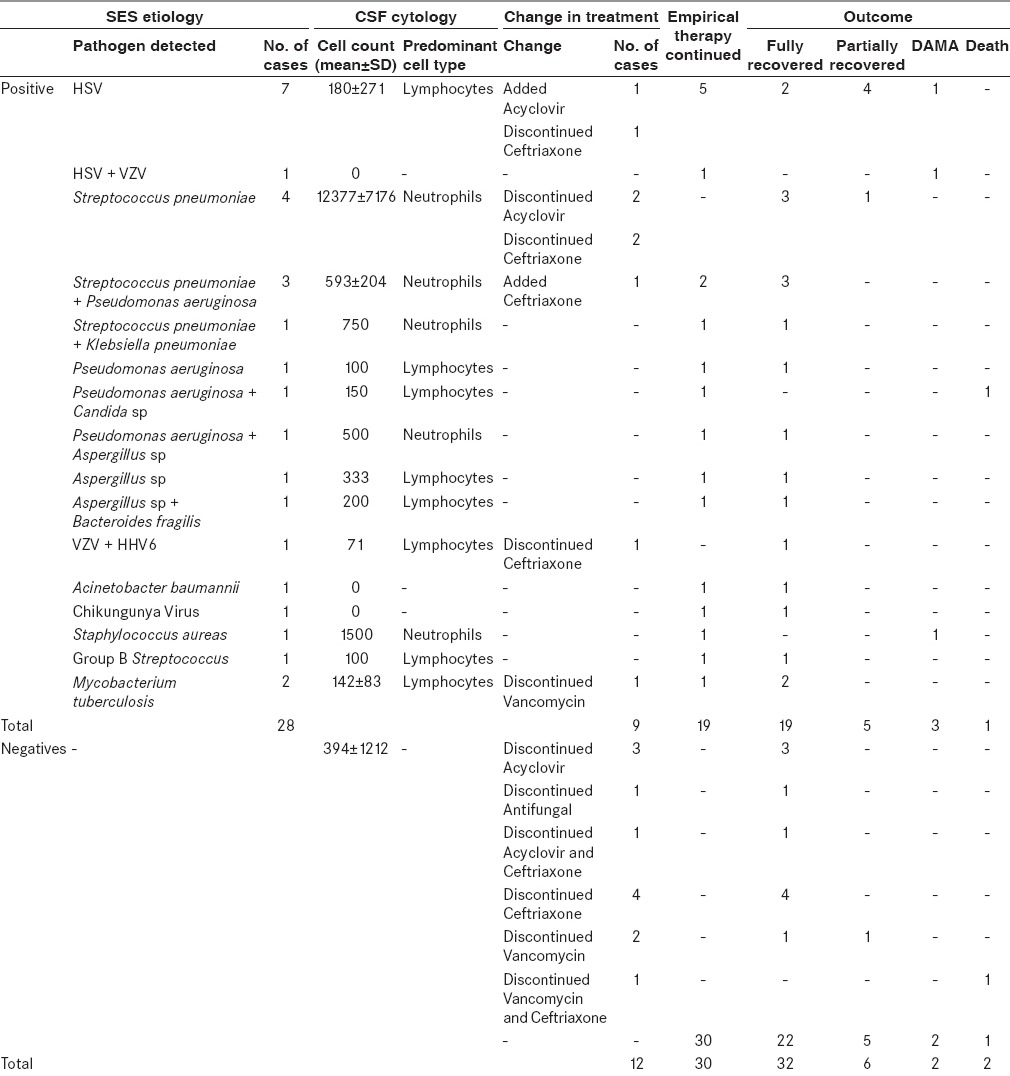
Figure 6.
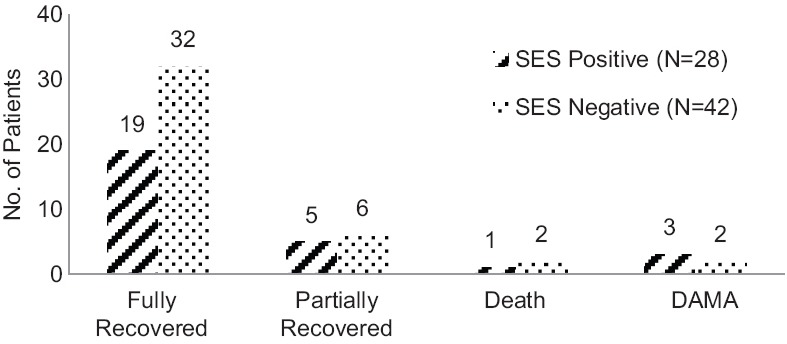
SES results and patient outcomes
While the case where SES was positive for Candida and Pseudomonas aeruginosa and the patient expired has been mentioned above, the other two deaths in this study were in the SES-negative category. One among them was diagnosed with Creutzfeldt–Jakob disease (CJD) while the other patient was diagnosed with autoimmune encephalitis.
Discussion
In a tertiary care center such as ours, classical textbook scenarios of clinical presentations in CNS infections are a rarity. This is primarily because of prior empirical antibiotic therapy. At the time of admission, the presentations are often atypical, thereby adding urgency as a dimension to the diagnostic dilemma. Hence, there is a need for rapid, sensitive, and specific microbial diagnostics which, coupled with cytological and clinical parameters, can be relied upon in order to take therapeutic decisions.
PCR-based diagnosis of viral etiology in CNS infections has long been accepted as the gold standard. In this study, we have used SES, a multiplex molecular diagnostic platform, to diagnose microbial etiology in suspected CNS infections such as meningitis, meningoencephalitis, and acute encephalitis. In this study, SES test was performed for two diagnostic objectives, either to confirm an infection or to rule out an infection. The fact that only one of the 23 cases wherein SES was performed to rule out an infection was positive points to the specificity of the test. This helps us to explore further for noninfectious etiologies causing the same CNS manifestations. In fact, two out of three deaths in this study occurred in this category, both due to noninfectious CNS diseases such as CJD and autoimmune encephalitis. In the category of confirming/ruling in an infection, SES had a clinical sensitivity of 57.4%. This detection rate is far higher than conventional techniques available, including CSF culture, Gram staining, and latex agglutination tests, considering that all our patients have been administered antibiotics before being referred to us.[28] This helped us shift to a targeted therapy from the initial empirical therapy within 24 h, leading to deescalations in 19 cases and escalation in 2 cases. Deescalation is of particular importance in a tertiary care hospital such as ours where unnecessary antimicrobials in the absence of microbial diagnosis lead to increased morbidity, hospital stay, cost, and antibiotic resistance. Among these 21 cases where SES results induced a change in the therapy, 16 patients recovered fully. Four patients had a partial recovery while one patient died. In these four patients with partial recovery, antibiotics were stopped in two patients positive for HSV; acyclovir was stopped for a patient positive for Streptococcus pneumoniae while acyclovir was started for a patient positive for HSV. Overall, SES had a very profound impact on patient management.
In 42.6% of the cases where SES was negative in the category of ruling in an infection, no death was reported. Only one case had a neurological deficit at 3 months’ review. In other words, SES did not miss any clinically significant infection.
In terms of the most common organisms detected by SES, Streptococcus pneumoniae (28.5%) and Pseudomonas aeruginosa (21.4%) were the top two bacterial species identified among the positive cases. Previous studies conducted in India have shown both these organisms in CNS infections. HSV (32.14%) is the most common virus detected by SES. The rank order of organisms detected by SES is in concordance with previously reported organisms in India.[8,29,30,31,32,33,34,35] Although multiplex PCR-based diagnostics have been used in CNS infections before, they are mostly confined to the detection of single or very limited families of viruses.[18,36] SES, unlike other multiplex systems available, detects a variety of organisms such as viruses, bacteria, and fungi in a single assay. To the best of our knowledge, ours is the first study in India to use a validated multiplex diagnostic platform.
Polymicrobial infections have been reported in CNS, either in the form of coinfection of viruses or multiple bacteria.[37,38,39,40,41,42,43] In our study, we had nine cases (32.14%) of polymicrobial infections, which is slightly higher than the previous studies. This is understandable as SES, unlike conventional techniques used in previous studies, is a molecular diagnostic platform with higher sensitivity. The detection of polymicrobials helped in instituting appropriate therapy as in two cases, both Gram-negative and Gram-positive organisms were found while in three cases, a bacterium and a fungus was detected.
Currently, in a clinical setting where the epidemiology of a syndrome such as AES or meningitis is known, multiplex PCR is faster and more cost effective than newer developments such as next generation sequencing (NGS). However, NGS is a great tool to identify newer and emerging pathogens, which are not described in a clinical setting yet. It is also a great tool for academic projects researching into rare outbreaks of unknown infectious etiology.
Although sensitive tests such as PCR may pose a challenge in terms of specificity, we have been routinely using this technology for the last few years and found SES to be in correlation with clinical suspicion. SES technology is being routinely used in other hospitals in India and has been shown to increase the diagnostic yield as well as reduce the time to antibiotic therapy modification including deescalation.[44] The technology has also been used to study the etiology of community-acquired pneumonia among children in India.[45]
Conclusion
In conclusion, in a tertiary care center such as ours, etiological microbial diagnosis is indispensable for appropriate therapy and desirable outcomes. Sensitive and accurate multiplex molecular diagnostics play a critical role in not only identifying the causative pathogen but also in helping clinicians to institute appropriate therapy, reduce overuse of antimicrobials, and ensure superior clinical outcomes.
The limitations of our study include the lack of randomization or control since ours is a retrospective analysis. Limited sample size and patients lost to discharge against medical advice as well as to follow-up are the other drawbacks of this study. However, a larger, prospective, multicenter, controlled study would pave the way for better understanding of the clinical utility of SES in the management of CNS infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
I thank Mr. Bharath N from XCyton Diagnostics Pvt. Ltd for his help in database management and analytics.
References
- 1.Mani R, Pradhan S, Nagarathna S, Wasiulla R, Chandramuki A. Bacteriological profile of community acquired acute bacterial meningitis: A ten-year retrospective study in a tertiary neurocare centre in South India. Indian J Med Microbiol. 2007;25:108–14. doi: 10.4103/0255-0857.32715. [DOI] [PubMed] [Google Scholar]
- 2.Erleena Nur H, Jamaiah I, Rohela M, Nissapatorn V. Bacterial meningitis: A five year (2001-2005) retrospective study at university Malaya medical center (UMMC), Kuala Lumpur, Malaysia. Southeast Asian J Trop Med Public Health. 2008;39:73–7. [Google Scholar]
- 3.van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Meningitis. Clinical information for healthcare professionals. [Last accessed on 2015 Aug 20]. Available from: http://www.cdc.gov/meningitis/index.html .
- 5.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467–92. doi: 10.1128/CMR.00070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirsch EA, Barton P, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: A review and recent experience. Pediatr Infect Dis J. 1996;15:967–79. doi: 10.1097/00006454-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Edwards MS, Baker CJ. Complications and sequelae of meningococcal infections in children. J Pediatr. 1981;99:540–5. doi: 10.1016/s0022-3476(81)80250-8. [DOI] [PubMed] [Google Scholar]
- 8.Jain P, Jain A, Kumar A, Prakash S, Khan DN, Singh KP, et al. Epidemiology and etiology of acute encephalitis syndrome in North India. Jpn J Infect Dis. 2014;67:197–203. doi: 10.7883/yoken.67.197. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy PG. Viral encephalitis: Causes, differential diagnosis, and management. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 1):i10–5. doi: 10.1136/jnnp.2003.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Shukla D, Kumar R, Idris MZ, Misra UK, Dhole TN. An epidemic of encephalitis associated with human enterovirus B in Uttar Pradesh, India, 2008. J Clin Virol. 2011;51:142–5. doi: 10.1016/j.jcv.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Read SJ, Jeffery KJ, Bangham CR. Aseptic meningitis and encephalitis: The role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691–6. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang YW, Mitchell PS, Espy MJ, Smith TF, Persing DH. Molecular diagnosis of herpes simplex virus infections in the central nervous system. J Clin Microbiol. 1999;37:2127–36. doi: 10.1128/jcm.37.7.2127-2136.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yerly S, Gervaix A, Simonet V, Catflisch M, Perrin L, Wunderli W. Rapid and sensitive detection of enteroviruses in specimens from patients with aseptic meningitis. J Clin Microbiol. 1996;34:199–201. doi: 10.1128/jcm.34.1.199-201.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev. 2004;17:903–25. doi: 10.1128/CMR.17.4.903-925.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read SJ, Mitchell JL, Fink CG. LightCycler multiplex PCR for the laboratory diagnosis of common viral infections of the central nervous system. J Clin Microbiol. 2001;39:3056–9. doi: 10.1128/JCM.39.9.3056-3059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read SJ, Kurtz JB. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J Clin Microbiol. 1999;37:1352–5. doi: 10.1128/jcm.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markoulatos P, Georgopoulou A, Siafakas N, Plakokefalos E, Tzanakaki G, Kourea-Kremastinou J. Laboratory diagnosis of common herpesvirus infections of the central nervous system by a multiplex PCR assay. J Clin Microbiol. 2001;39:4426–32. doi: 10.1128/JCM.39.12.4426-4432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leveque N, Van Haecke A, Renois F, Boutolleau D, Talmud D, Andreoletti L. Rapid virological diagnosis of central nervous system infections by use of a multiplex reverse transcription-PCR DNA microarray. J Clin Microbiol. 2011;49:3874–9. doi: 10.1128/JCM.01214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankuntaw N, Sukprasert S, Engchanil C, Kaewkes W, Chantratita W, Pairoj V, et al. Single tube multiplex real-time PCR for the rapid detection of herpesvirus infections of the central nervous system. Mol Cell Probes. 2011;25:114–20. doi: 10.1016/j.mcp.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Mannonen L, Vainionpää R, Kauppinen J, Lienhard R, Tritten ML, Cannon G, et al. Evaluation of multiplex polymerase chain reaction and microarray-based assay for rapid herpesvirus diagnostics. Diagn Microbiol Infect Dis. 2012;73:74–9. doi: 10.1016/j.diagmicrobio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Del Prete R, Di Taranto AM, Lipsi MR, Natalicchio MI, Antonetti R, Miragliotta G. Simultaneous detection of viruses and Toxoplasma gondii in cerebrospinal fluid specimens by multiplex polymerase chain reaction-based reverse hybridization assay. New Microbiol. 2009;32:143–6. [PubMed] [Google Scholar]
- 22.Bergallo M, Costa C, Margio S, Sidoti F, Terlizzi ME, Cavallo R. Development of a multiplex polymerase chain reaction for detection and typing of major human herpesviruses in cerebrospinal fluid. Can J Microbiol. 2007;53:1117–22. doi: 10.1139/w07-074. [DOI] [PubMed] [Google Scholar]
- 23.Tafreshi NK, Sadeghizadeh M, Amini-Bavil-Olyaee S, Ahadi AM, Jahanzad I, Roostaee MH. Development of a multiplex nested consensus PCR for detection and identification of major human herpesviruses in CNS infections. J Clin Virol. 2005;32:318–24. doi: 10.1016/j.jcv.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Ramamurthy M, Alexander M, Aaron S, Kannangai R, Ravi V, Sridharan G, et al. Comparison of a conventional polymerase chain reaction with real-time polymerase chain reaction for the detection of neurotropic viruses in cerebrospinal fluid samples. Indian J Med Microbiol. 2011;29:102–9. doi: 10.4103/0255-0857.81777. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti P, Das BK, Kapil A. Application of 16S rDNA based seminested PCR for diagnosis of acute bacterial meningitis. Indian J Med Res. 2009;129:182–8. [PubMed] [Google Scholar]
- 26.Balganesh M, Lalitha MK, Nathaniel R. Rapid diagnosis of acute pyogenic meningitis by a combined PCR dot-blot assay. Mol Cell Probes. 2000;14:61–9. doi: 10.1006/mcpr.2000.0287. [DOI] [PubMed] [Google Scholar]
- 27.External Quality Assessment Program from Quality Control for Molecular Diagnostics. [Last accessed on 2015 Aug 20]. Available from: http://www.qnostics.com/molecular-controls/
- 28.Shameem S, Vinod Kumar CS, Neelagund YF. Bacterial meningitis: Rapid diagnosis and microbial profile: A multicentered study. J Commun Dis. 2008;40:111–20. [PubMed] [Google Scholar]
- 29.Debnath DJ, Wanjpe A, Kakrani V, Singru S. Epidemiological study of acute bacterial meningitis in admitted children below twelve years of age in a tertiary care teaching hospital in Pune, India. Med J DY Patil Univ. 2012;5:28–30. [Google Scholar]
- 30.Chinchankar N, Mane M, Bhave S, Bapat S, Bavdekar A, Pandit A, et al. Diagnosis and outcome of acute bacterial meningitis in early childhood. Indian Pediatr. 2002;39:914–21. [PubMed] [Google Scholar]
- 31.Satishchandra P, Nandini M, Shankar SK, Vasudevarao T, Ravi V, Shenoy PK, et al. Herpes simplex encephalitis: A diagnostic and therapeutic reapprisal. J Assoc Physicians India. 1993;41:277–8. [PubMed] [Google Scholar]
- 32.Gambhir IS, Singh NN, Singh DS, Gulati AK. Herpes simplex virus-1 encephalitis in eastern Uttar Pradesh. J Assoc Physicians India. 1999;47:1149–51. [PubMed] [Google Scholar]
- 33.Nagaveni S, Rajeshwari H, Oli AK, Patil SA, Chandrakanth RK. Widespread emergence of multidrug resistant pseudomonas aeruginosa isolated from CSF samples. Indian J Microbiol. 2011;51:2–7. doi: 10.1007/s12088-011-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juhi T, Bibhabati M, Archana T, Poonam L, Vinita D. Pseudomonas aeruginosa meningitis in post neurosurgical patients. Neurology Asia. 2009;14:95–100. [Google Scholar]
- 35.Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65:257–67. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Correia C, Costa I, Oleastro M, Simoes MJ. Viral infections of the central nervous system-use of a multiplex pcr microarray for its diagnosis. Madrid, Espanha: 15th Annual Meeting of the European Society for Clinical Virology (ESCV) and Joint Meeting with the European Society for Veterinary Virology (ESVV) 2012:141. [Google Scholar]
- 37.Sperber AD, Alkan M, Stemmer S, Avnon L, Schlaeffer F. Polymicrobial central nervous system infection in the acquired immunodeficiency syndrome. J Neurol Neurosurg Psychiatry. 1988;51:998–9. doi: 10.1136/jnnp.51.7.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche M, Humphreys H, Smyth E, Phillips J, Cunney R, McNamara E, et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003;9:803–9. doi: 10.1046/j.1469-0691.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 39.Prasad RS, Khuraijam GS. An unusual case of mixed bacterial meningitis in an immunocompetent adult. J Infect. 1999;39:98. doi: 10.1016/s0163-4453(99)90112-3. [DOI] [PubMed] [Google Scholar]
- 40.Herweg JC, Middelkamp JN, Hartmann AF. Simultaneous mixed bacterial meningitis in children. J Pediatr. 1963;63:76–83. doi: 10.1016/s0022-3476(63)80305-4. [DOI] [PubMed] [Google Scholar]
- 41.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, Kaczmarski EB. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–8. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chokephaibulkit K, Leo YS, Wittek AE. Mixed bacterial meningitis in a 4-year-old girl. West J Med. 1995;162:59–60. [PMC free article] [PubMed] [Google Scholar]
- 43.Marchandin H, Ventura V, Alonso JM, Van de Perre P. Mixed bacterial meningitis due to Streptococcus pneumoniae and Neisseria meningitidis in an 18-month-old child. J Clin Microbiol. 2005;43:1477–9. doi: 10.1128/JCM.43.3.1477-1479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sircar M, Ranjan P, Gupta R, Jha OK, Gupta A, Kaur R, et al. Impact of bronchoalveolar lavage multiplex polymerase chain reaction on microbiological yield and therapeutic decisions in severe pneumonia in intensive care unit. J Crit Care. 2016;31:227–32. doi: 10.1016/j.jcrc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Mathew JL, Singhi S, Ray P, Hagel E, Saghafian-Hedengren S, Bansal A, et al. Etiology of community acquired pneumonia among children in India: Prospective, cohort study. J Glob Health. 2015;5:050418. doi: 10.7189/jogh.05.020418. [DOI] [PMC free article] [PubMed] [Google Scholar]


