Abstract
Background:
The modified Atkins diet (MAD) has been used predominantly in older children, adolescents, and adults. There is a paucity of data on the use of the MAD in refractory epilepsy in young children.
Objectives:
This study was planned to evaluate the efficacy and tolerability of the MAD in refractory epilepsy in young children.
Methods:
This study recruited children aged 9 months to 3 years with refractory seizures. Children received MAD for 6-month with the on-going anticonvulsant medications being continued unchanged. Reduction in seizure frequency was the primary outcome measure. Adverse effects were also studied.
Results:
Thirty-one children with daily seizures were studied with a median age of 18-month (range 9–30 months). West syndrome was the most common epilepsy syndrome (26, 86.6%). Twenty-one children remained on diet at 3 months and 13 at 6 months. The children who achieved >50% seizure reduction were 17 (54.8%) at 3 months and 9 (29%) at 6 months. Refusal to eat was a significant problem seen in eight children. Three children discontinued the diet due to adverse effects.
Conclusion:
The MAD was found to be feasible, effective, and well-tolerated.
Keywords: Dietary therapy, infantile spasms, West syndrome
Introduction
The ketogenic diet is an effective treatment for refractory epilepsy which is not surgically remediable. However, it is very restrictive and has drawbacks such as a requirement for the strict weighing of foods and hospitalization for initiation. Side effects of the diet include kidney stones, constipation, acidosis, diminished growth, weight loss, and hyperlipidemia.[1] The modified Atkins diet (MAD) is a less restrictive alternative, which is started on an outpatient basis and allows unlimited protein and fat.[2] The MAD has been shown to be effective in multiple uncontrolled studies and a recent randomized controlled trial.[3,4,5]
The MAD has been used predominantly in older children, adolescents, and adults. There is paucity of data on the use of the MAD in refractory epilepsy in young children. This diet may be of special importance in young children as proteins are not restricted hence fewer problems with growth are expected. A preliminary study showed good efficacy and tolerability of the MAD in children with refractory infantile spasms.[6] Hence, this study was planned to evaluate the efficacy and tolerability of the MAD in refractory epilepsy in young children.
Methods
This observational interventional, uncontrolled study was conducted in a Tertiary Care Pediatric Hospital in New Delhi from November 2012 to March 2014. Ethical approval from the Institutional Ethics Committee was obtained. Written informed consent was taken from the parents. Children aged 9 months to 3 years who had at least three seizures/week for >1 month despite the appropriate use of at least two antiepileptic drugs were enrolled. Children with known or suspected inborn error of metabolism, systemic illness, and severe acute malnutrition were excluded. The trial was registered in clinicaltrials.gov (NCT01880333).
A detailed history and examination were performed. Age at onset, seizure type, frequency, perinatal details, family history, developmental status, and treatment history were noted. Appropriateness and dosing of the anti-epileptic drugs were checked. The results of investigations such as electroencephalography (EEG) and neuroimaging were noted. All the patients had undergone a high resolution (1.5 or 3 Tesla) magnetic resonance imaging (MRI) of the brain as a part of the refractory epilepsy work up.
Eligible children were started on the MAD with help of a trained dietician. Carbohydrates intake was restricted to 5 g/day in children <18 months of age and 10 g/day in children 18 months to 3 years of age. Carbohydrate values of various food items were explained in detail. Fats (e.g. cream, butter, oils, and ghee) were encouraged. Parents were advised to give proteins (cheese, fish, eggs, chicken, and soya products) in unrestricted amount. Calcium (50–80 mg/kg/day) and multivitamin supplementation were added. Syrups were restricted. On-going medications were changed to carbohydrate-free preparations, wherever available. Corticosteroids and adrenocorticotropic hormone were tapered off and stopped 2 weeks before diet initiation. Parents were asked to maintain a seizure diary recording the seizure frequency type and duration and any adverse effects. Parents were taught to measure and document urine ketones at home. Anti-epileptic medications were unchanged during the first 3-month trial, unless otherwise indicated, for example, development of status epileptics or evidence of drug toxicity. After 3 months, the anticonvulsants were tapered gradually in case the patient became seizure free. Patients were followed up at 1, 2, 3, and 6 months after the diet initiation. Seizure frequency was checked according to parental reports and seizure diaries. The seizure control was categorized as seizure free, >50% seizure reduction, <50% seizure reduction or increase in seizures. Compliance with the diet was checked by parental records of daily urine ketones chart. Weight of the child, adverse effects of the diet, any other parental concerns were noted at each visit. Biochemical profile (blood urea, serum creatinine, liver function tests, serum albumin, and fasting lipid profile [triglycerides, total cholesterol]) was obtained at 3 and 6 months. Repeat EEG was performed in patients with seizure freedom.
Parents were asked to rate the effects of MAD on the following nonseizure domains at 3 months and after starting the diet: Alertness, activity level, speech/communication, comprehension/understanding, sleeping, motor skills, social interaction, and behavior. These characteristics were rated on a Likert scale ranging from much worse (1), somewhat worse (2), same (3), somewhat better (4), or much better (5).
STATA 9.0 was used to analyze the data (STATA 9.0, StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA). Summary statistics were used for the various study observations. Qualitative variables were compared using Chi-square test or Fisher exact tests. Quantitative variables were compared using Student's t-test and paired observations using paired t-test or sign rank test.
Results
During the study, 42 patients met the inclusion criteria. Out of these, parents of 31 children were willing to initiate the MAD [Figure 1]. Out of 31 patients included in trial, 19 were vegetarian. The median age was 18 months (range 9–30 months) at the start of the diet. Multiple antiepileptic drugs had been tried (median 5, range 3–11). All the patients suffered from daily seizures. Most frequent seizure type was infantile spasms. Other baseline characteristics are summarized in Table 1. The MRI and EEG findings of the study population are detailed in Tables 2 and 3.
Figure 1.
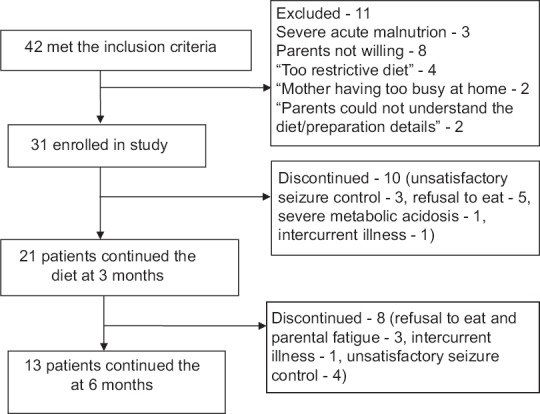
Flow of study participants
Table 1.
Baseline characteristics of the study participants (n=31)
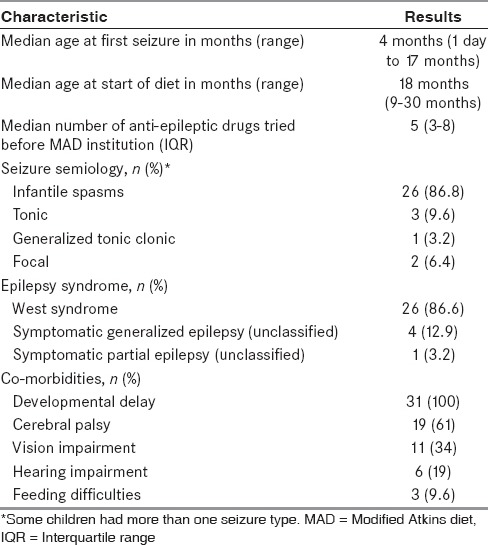
Table 2.
Magnetic resonance imaging abnormalities in the study population
Table 3.
Electroencephalography abnormalities in the study population (n=31)

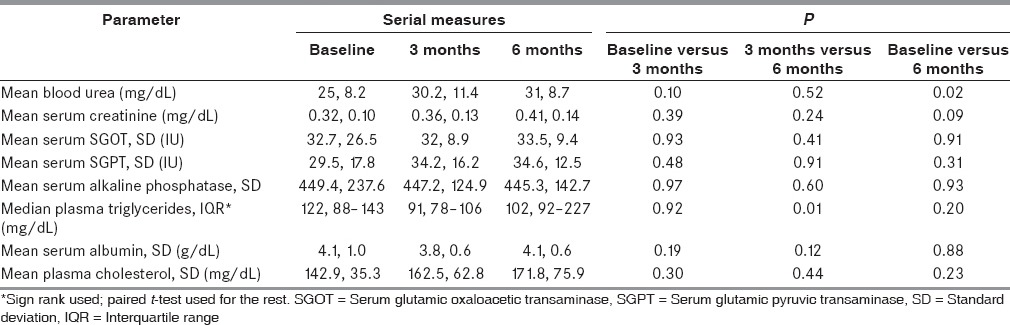
Twenty-one children remained on diet at 3 months and 13 at 6 months. The children who achieved >50% seizure reduction were 17 (54.8%) at 3 months and 9 (29%) at 6 months. Other relevant seizure outcomes are shown in Table 4. The two children who were seizure free had spasms earlier, and their repeat EEG showed resolution of hypsarrhythmia. The biochemical profile of patients at baseline and follow-up is detailed in Table 5. There was a statistically significant rise in blood urea at 6 months as compared to baseline. A similar increase was seen in plasma triglycerides at 6 months as compared to 3 months. However, these increases were not clinically relevant. Other parameters did not show significant change over time [Table 5].
Table 4.
Seizure outcomes of the study participants on modified Atkins diet on follow-up (n=31)
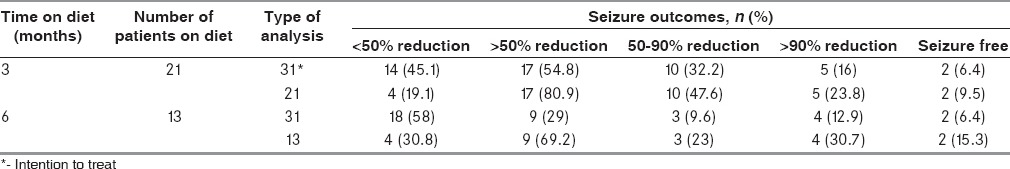
Table 5.
Biochemical profile of study participants on modified Atkins diet
Constipation was most common adverse effect, noted in ten children (32%). Vomiting was the next most frequent side effect noted in six children, especially in the initial 1–2 weeks of diet initiation. Other adverse effects included weight loss (5), anorexia (6), lethargy (2), and intercurrent illness warranting hospitalization (2). One child developed severe metabolic acidosis. This child had refractory seizures secondary to perinatal asphyxia sequelae. The metabolic screen (blood ammonia, arterial blood gas, blood lactates, blood tandem mass spectrometry, and urine gas chromatography mass spectrometry) before starting the diet was normal. The metabolic testing was repeated at the time of developing acidosis; however, no abnormalities were detected. The on-going anticonvulsant medications at the time of the diet included sodium valproate, levetiracetam, and clobazam.
The positive changes on nonseizure domains included improvement in alertness, activity level, speech/communication, comprehension/understanding, sleeping, motor skills, social interaction, and behavior at 3 months after starting MAD. Eighty-five percent of parents at reported improvement in sleep pattern. A majority of parents reported improvement in alertness (85%) and activity level (42.8%). Only one child had reduced alertness. Many parents also reported improvement in comprehension/understanding (33.3%), social interaction (66.6%), speech/communication (33.3%), and behavior (38%).
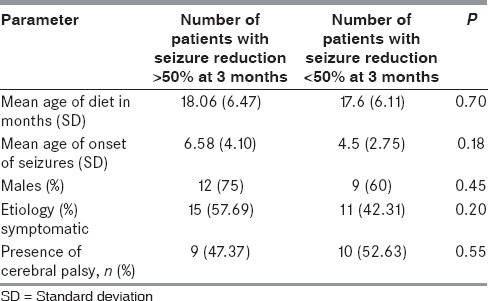
Table 6 describes the effects of age of onset of seizures, age of onset of diet, sex, etiology, comorbidities, and on seizure control. There were no statistically significant differences in seizure control based on age, sex, etiology, or the presence or absence of comorbid cerebral palsy. The urine ketones (mild to moderate ketosis vs. adequate ketosis) were not statistically significant in determining seizure control.
Table 6.
Possible factors associated with the antiepileptic efficacy of modified Atkins diet
Discussion
In this study, we evaluated the use of the MAD in young children with refractory epilepsy in a low-resource setting. The MAD is a much more feasible option in such settings. As has been noted in earlier studies, the MAD has the advantages of outpatient initiation, ease of administration, and reduced counseling time (30–45 min).[5] Hence, this is an ideal treatment option for resource constraint settings with a paucity of trained dieticians. Furthermore, the commercially available liquid ketogenic formula is prohibitively expensive and out of reach for most patients. Although in the West, the MAD is preferred mainly in older children, adolescents and adults, in India, it may be a much more feasible option as compared to the ketogenic diet in young children with refractory epilepsy.
In this study, 6.4% children were seizure free, and overall 54.8% had a 50% or greater reduction in seizures at 3 months on the diet. A recent review analyzed all studies of the MAD published after 2003.[7] Fourteen studies included in this review have revealed an efficacy of around 50%. The findings of the present study are comparable to the studies in this review. Among the three nonresponders at 3 months, two had spasms, and one had focal seizures. Among the four nonresponders at 6 months, three had spasms, and one had focal seizures. Therefore, in this small sample, we cannot comment if any particular seizure type or epilepsy syndrome was nonresponsive to the diet.
The proportion of patients continuing the diet at 6 months was 41.9% in this study. The retention rate of this study is comparable to the previous study of the diet in young children with infantile spasms from India.[6] Authors from the West have reported a higher retention rates. As the administration of the diet requires a lot of effort on the part of the family, the parents generally wish to continue the diet only in children who are seizure-free or have > 90% reduction in seizures.
In a recent randomized open-label study, Kim et al. compared the efficacy of the classic ketogenic and the MADs in refractory childhood epilepsy.[8] The authors reported that overall the efficacy of the two diets was comparable but for patients aged 1–2 years, seizure outcomes were consistently much more favorable in patients consuming the ketogenic diet as compared with those on the MAD.
Refusal to eat was a significant problem noted in this study, seen in eight children. As the usual Indian diet is very carbohydrate rich, the severe carbohydrate restriction in the MAD could have been unpalatable and difficult to consume for some children. In such children, the low glycemic index diet may be a better option. MAD has been reported to be well-accepted, in general. Sharma et al.[5] and Kossoff et al.[2] had reported one child each who found this diet “too restrictive.”
Common adverse effects noted in this study included constipation, lethargy, and vomiting, which have been reported in earlier studies of the MAD as well.[2,3,4,5,6] These were generally manageable by counseling and medical management. Three children discontinued the diet due to adverse effects. Similar to previous reported literature,[7] there was no significant clinically relevant change in the biochemical parameters over 6 months.
The limitations of the study include the absence of a control group and unblended assessment of the outcome measures. Furthermore, the use of parental reports of seizures are subjective and error prone, especially in children who have multiple and nocturnal seizures.
Conclusion
The MAD was found to be feasible, effective, and well-tolerated in young children with refractory epilepsy. However, side effects are common and need medical supervision. The high drop-out rates are a concern, and we may need more palatable and easier versions of the diet for long-term use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to the EEG technicians Mr. M. P. Sharma and Mr. Manas Guchait.
References
- 1.Sharma S, Jain P. The ketogenic diet and other dietary treatments for refractory epilepsy in children. Ann Indian Acad Neurol. 2014;17:253–8. doi: 10.4103/0972-2327.138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the modified Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–91. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 3.Kossoff EH, McGrogan JR, Bluml RM, Pillas DJ, Rubenstein JE, Vining EP. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia. 2006;47:421–4. doi: 10.1111/j.1528-1167.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 4.Kang HC, Lee HS, You SJ, Kang du C, Ko TS, Kim HD. Use of a modified Atkins diet in intractable childhood epilepsy. Epilepsia. 2007;48:182–6. doi: 10.1111/j.1528-1167.2006.00910.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet for treatment of refractory childhood epilepsy: A randomized controlled trial. Epilepsia. 2013;54:481–6. doi: 10.1111/epi.12069. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Sankhyan N, Gulati S, Agarwala A. Use of the modified Atkins diet in infantile spasms refractory to first-line treatment. Seizure. 2012;21:45–8. doi: 10.1016/j.seizure.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Jain P. The modified Atkins diet in refractory epilepsy. Epilepsy Res Treat 2014. 2014:404202. doi: 10.1155/2014/404202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JA, Yoon JR, Lee EJ, Lee JS, Kim JT, Kim HD, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016;57:51–8. doi: 10.1111/epi.13256. [DOI] [PubMed] [Google Scholar]


