Abstract
Purpose of Review
Infection with the Gram-negative, microaerophilic pathogen Helicobacter pylori results in gastric cancer (GC) in a subset of infected individuals. As such, H. pylori is the only World Health Organization classified bacterial class I carcinogen. Numerous studies have identified mechanisms by which H. pylori alters host cell signaling pathways to cause disease. The purpose of this review is to highlight recent studies that explore mechanisms associated with induction of GC.
Recent Findings
Over the last year and a half, new mechanisms contributing to the etiology of H. pylori associated GC development have been discovered. In addition to utilizing the oncogenic CagA toxin to alter host cell signaling pathways, H. pylori also induces host DNA damage and alters DNA methylation to perturb downstream signaling. Furthermore, H. pylori activates numerous host cell pathways and proteins that result in epithelial-to-mesenchymal transition (EMT) and induction of cell survival and proliferation.
Summary
Mounting evidence suggests that H. pylori promotes gastric carcinogenesis using a multifactorial approach. Intriguingly, many of the targeted pathways and mechanism show commonality with diverse forms of cancer.
Keywords: Helicobacter pylori, gastric carcinogenesis, PI3K/Akt, DNA damage and methylation, epithelial-to-mesenchymal transition
INTRODUCTION
Given its association with gastric cancer (GC), the World Health Organization classified Helicobacter pylori as a class I carcinogen in 1994; to date, H. pylori remains the only bacterium with this distinction [1, 2]. This Gram-negative, microaerophilic bacterium persistently colonizes the gastric mucosa of the majority of the population. While, infections with H. pylori are frequently asymptomatic, they are also associated with gastritis, peptic ulcers, and in the most severe form, GC. Currently, GC is the fifth most common cause of cancer worldwide with a case fatality rate of 75%; these statistics make GC the third most common cause of cancer-related death [3]. The most recent reports from the International Agency for Research on Cancer (IARC) estimates that 78% of all gastric cancers are attributable to H. pylori infection [2]. Furthermore, seroprevalence studies estimate that 90% of GC patients have encountered H. pylori [4]. Given the striking association between H. pylori and GC, decades of research has sought to determine the molecular mechanisms underlying H. pylori-associated cancer development. Indeed, numerous molecular pathways have been identified as targets of this pathogen (reviewed in [5, 6]). Recent reports have revealed several H. pylori-targeted pathways that are also altered in various forms of cancer. Thus, a greater understanding of H. pylori-associated cancer development may shed insight into other cancer types. Herein, we highlight recent studies that explore H. pylori’s ability to induce DNA damage and aberrant methylation patterns that effect downstream host cell signaling. Additionally, we highlight recent discoveries that demonstrate how H. pylori initiates epithelial-to-mesenchymal transition (EMT) and potentiates pro-survival and proliferation signals that contribute to the cancer phenotype.
H. pylori induces DNA Damage and Aberrant DNA Methylation
Genome instability and mutation are emerging hallmarks of cancer development [7]. These genomic changes result from direct DNA damage, DNA repair failure, or via epigenetic mechanisms such as DNA methylation [7]. H. pylori targets each of these pathways: aberrant methylation [8], decreased expression of mismatch repair genes [9], increased expression of activation-induced cytidine deaminase (AID) [10], and induction of double strand DNA breaks (DSBs) [11**].
While the concept of H. pylori-induced DNA damage is not new, the mechanisms underlying these processes remain poorly defined [11**]. Two recent studies further characterize the molecular mechanisms behind H. pylori associated DNA damage [11**, 12*]. From a global perspective, distinct DNA damage patterns, which involve accumulation of DSBs in regions of active transcription and near telomeres, occur in response to H. pylori infection [12*]. Moreover, H. pylori-induced damage patterns are similar to structural variations found in gastric cancers [12*, 13]. Strains lacking the cag-pathogenicity island (cag-PAI) are impaired in their ability to induce DSBs [12*], suggesting a role for the type IV secretion system (T4SS) in this process. This suggestion is supported by additional work which shows that the DSBs occur in an NF-κB dependent manner [11**]. A molecular model has been proposed that suggests that binding of the T4SS pili to β1 integrin triggers activation and translocation of NF-κB to the nucleus where it serves as a chromatin binding platform for RelA, the NER endonucleases XPG and XPF, and recruitment factor XPA [11**]. XPA and XPF then induce DSBs that result in enhanced expression of NF-κB-dependent anti-apoptotic genes that promote cell survival [11**]. Taken together, these studies suggest that the DSBs induced by H. pylori are unique and perhaps directed by this pathogen.
Another mechanism by which H. pylori contributes to genomic instability is via manipulation of host gene methylation [8]. In this regard, DNA methylation is increased in the gastric antrum of H. pylori infected patients as compared to uninfected patients [14]. DNA methylation is also increased in the “normal tissue” surrounding the lesions [15**]. The role of aberrant DNA methylation in adverse disease outcome is supported by a 16-year cohort study that showed that changes in specific gene methylation can serve as predictors for gastric cancer progression [16]. Additionally, several studies have delineated the downstream consequences of altered gene methylation. For example, H. pylori has a profound effect on N-Myc downstream-regulated gene 2 (NDRG2) promoter methylation; methylation was seen in 54% of primary gastric cancer specimens [17*]. NDRG2 is a potential tumor suppressor that aids in dephosphorylation of the phosphatase and tensin homolog (PTEN) [18]; PTEN is frequently found inactivated in gastric cancers due to increased levels of C-terminal phosphorylation [19]. In the active, unphosphorylated form, PTEN dephosphorylates PIP3, which results in decreased PI3K/Akt activity [18, 20]. Thus, as methylation increases, expression of NDRG2 decreases, PTEN C-terminal phosphorylation increases, and suppression of the PI3K/Akt pathway is relieved [17*, 20]. It is currently thought that the up-regulation of DNA methyltransferase 3b (DNMT3B), which is correlated with H. pylori infection, is responsible for the increase in NDRG2 promoter methylation [17*].
DNA methylation also impacts micro-RNA (miRNA) regulation in chronic inflammatory diseases and in cancers [15**, 21]. In H. pylori positive patients with gastric disease, aberrant methylation of the CpG island in the miR-210 promoter occurs. This methylation results in a decrease in miR-210, which leads to an increase in expression of miR-210 pro-proliferation targets such as stathmin1 (STMN1) and demethyladensosine transferase 1 (DIMT1) [15**]. H. pylori also stimulates miRNA promoter hypermethylation via cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) activation [21]. PGE2 promotes methylation in tumor cells, and in the case of H. pylori infection, miR-149 is a target of PGE2 induced DNA methylation. miR-149 targets and suppresses IL-6 and the PGE2 receptor genes. Thus, methylation of miR-149 results in a decrease in miR-149 and an increase in IL-6. Downstream consequences of IL-6 induction include EMT, stem-like traits, and invasive properties [21]. Interestingly, IL-6 regulates the cancer cell environment by activating the COX-2/PGE2 pathway and by regulating miRNAs [21]. En masse the data suggest that H. pylori regulates DNA damage and DNA methylation in a manner that promotes tumorigenesis via enhanced genome instability, and via the activation of cell signaling pathways that promote cellular proliferation, EMT, and stemness, [15**–17*].
Role of H. pylori in EMT
One process that has taken a prominent role in cancer etiology research is EMT. EMT is critical to the developmental process; however, EMT also plays a physiopathological role in epithelial tumorigenesis [22]. During EMT, epithelial cells loose properties required to maintain a healthy cellular barrier and instead acquire mesenchymal cell like properties: loss of cellular interactions, loss of polarization, and increased mobility [22]. Several recent studies link infection with H. pylori to EMT-like changes in gastric epithelial cells [23–26]. Indeed, molecular classification of GC tumors identified a cancer subtype associated with EMT [27]. This EMT subtype occurs at a younger age than the other subtypes, is associated with poor prognosis, and has a higher chance of recurrence [27]. Another clinical study found decreased expression of the epithelial marker E-cadherin and increased expression of the mesenchymal markers SNAIL, TWIST, SLUG, and vimentin in tissue biopsies from H. pylori positive GC patients [24*]. Eradication of H. pylori results in a reversal of marker expression; E-cadherin increases while SNAIL, TWIST, SLUG, and vimentin decrease [24*]. Other studies also document EMT associated changes in gastric epithelial cells after H. pylori infection and identify associated key players in the process [23, 25*, 26]. For example, an association between CD44 and H. pylori induced EMT has been shown; increased CD44 is associated with a corresponding increase in mesenchymal markers [23, 24*]. While neither of these studies show a direct interaction between H. pylori and CD44, H. pylori may affect CD44 via suppression of miR-328, which is a known negative regulator of CD44 expression [28]. Interestingly, mir-328 expression is suppressed in response to H2O2 [28], suggesting that the gastric mucosal inflammation associated with H. pylori infection may increase host cell changes that further promote EMT.
The multipotent virulence factor, CagA, is also directly linked to H. pylori’s induction of EMT and cell proliferation via CD44 [29, 30]. Both in vivo and in vitro models demonstrate that CD44, c-Met, and CagA form a complex, which leads to increased cellular proliferation, an increase in mesenchymal markers (α-SMA, SNAIL2, TWIST1, N-cadherin, and ZEB1), and disruption of membrane associated E-cadherin [29]. The increase in mesenchymal markers, CD44, and matrix metalloproteinases occurs in a CagA phosphorylation-dependent manner [30]. Similarly, transfection with CagA leads to an increase in TWIST and vimentin with a corresponding decrease in E-cadherin [23]. This later decrease in E-cadherin appears to be tied to the downregulation of programmed cell death factor 4 (PDCD4), suggesting that CagA influences EMT through multiple pathways [23].
The factors that that are commonly used as mesenchymal or epithelial markers have diverse mechanistic roles during EMT. For instance, the aforementioned decrease and disruption of E-cadherin contributes to the weakening of cell-to-cell junctions, a hallmark of EMT. Interestingly, the histone demethylase RBP2 also plays a role in regulating E-cadherin; RBP2 forms a complex with p-SMAD3 on the E-cadherin promoter to suppress E-cadherin expression [26]. These types of junctional effects are a common theme of GC and H. pylori infection. The gap junction protein, connexin-43 (Cx43), is perturbed during H. pylori infection; H. pylori induces a GATA3 dependent decrease in Cx43, which leads to weakening of gastric epithelial integrity [31]. An additional study suggests that expression of Cx43 renders cells more susceptible to VacA induced cell death [32]. Combined, these data suggest a duel mechanism by which H. pylori generates a cell population with reduced levels of Cx43; H. pylori causes Cx43 down regulation, while VacA induces cell death in any cells that maintain Cx43. The end result is a defective gastric epithelial maintenance program and perhaps an increased propensity towards EMT.
Unlike the structural proteins, Cx43 and E-cadherin, SNAIL and TWIST function as transcription factors that control downstream effectors that contribute to EMT. For example, SNAIL acts as a transcriptional repressor of the gene encoding the junctional protein claudin-7 [25*]. Addition of H. pylori to AGS cells or a gastroid model leads to an increase in SNAIL with a corresponding decrease in claudin-7; this interaction is β-catenin dependent [25*]. Mechanistically, CagA binding to GSK-3 decreases GSK-3 activity, subsequently increasing SNAIL stability and the active, cytoplasmic form of β-catenin [33]. En masse, current data suggest that H. pylori utilizes multiple mechanisms to disrupt epithelial adhesins as a means to contribute to EMT.
Role of cell proliferation in gastric carcinogenesis
As mentioned, the interaction between CagA and β-catenin plays a role in disruption of cell-to-cell junctions, which strongly suggests that β-catenin directly or indirectly contributes to EMT. Additional evidence supports CagA-dependent, nuclear accumulation of β-catenin, which results in increased pro-proliferation gene expression; several mechanisms have been implicated in orchestrating this outcome [34–36]. Recently, the multi-functional glycol-phosphoprotein, osteopontin (OPN), has emerged as another intermediary in the CagA-dependent activation of β-catenin. Intracellular OPN increases in a CagA and T4SS-dependent manner in response to H. pylori infection [37*]. Furthermore, siRNA knockdown of OPN abolishes the H. pylori-dependent nuclear accumulation of β-catenin [37*]. The downstream effects of the OPN increase and subsequent β-catenin nuclear localization include increases in c-Myc and cyclin D1 (CCDN1), both of which are known cellular proliferation contributors. These in vitro studies are supported by mouse models that show that the loss of OPN suppresses oncogenic changes such as inflammation, epithelial proliferation, and decreased apoptosis [38, 39].
Another major cell proliferation pathway activated during H. pylori infection is PI3K/Akt. The importance of this pathway is evidenced by the dual mechanisms of regulation by H. pylori; infection enhances cell proliferation through PI3K/Akt and suppresses proteins that inhibit PI3K/Akt activation. As mentioned above, PTEN is maintained in an inactive state following suppression of NDRG2 [17*, 18]. As an additional control mechanism, H. pylori infection also leads to an increase in miR-221/222 expression; both of these miRNAs target PTEN as well as the tumor suppressor, RECK [40]. A third component altered in the PI3K/Akt pathway is PDK-1. Early in infection, H. pylori dephosphorylates PDK-1, which results in dephosphorylation of Akt and induction of apoptosis; apoptosis is followed by an increase in cellular proliferation of the surviving cells [41]. Lastly, CagA induces activation of SP1, a downstream protein in the PI3K/Akt pathway, via Akt [42]. SP1 serves as a transcription factor for RBP2; RBP2 binds to the CCDN1 promoter, which results in upregulation of cyclin D1 [42]. Thus, the role of RBP2 appears two-fold; RBP2 induces EMT and stemness, as well as cellular proliferation [26, 42].
H. pylori exploits numerous targets to foster an environment geared for enhanced cellular proliferation and survival. In addition to activating pathways that promote these features, H. pylori also suppresses mechanisms meant to keep these pathways in check. For instance, H. pylori modulates several miRNAs to affect cellular proliferation. Among these, miR-203 is downregulated in H. pylori positive tissue samples [43]. This decrease results in a corresponding increase in the PIK3CA and CASK targets, which in turn leads to enhanced cellular proliferation and invasion [43, 44]. Similarly, miR-101 is downregulated in H. pylori infected GC tissues [45]. Decreased levels of miR-101 results in the upregulation of SOCS2 and subsequent deregulation of the cell cycle, promoting tumorigenesis [45]. Other miRNAs downregulated during H. pylori infection, including miR-375 and miR101b, further potentiate carcinogenic signals [46]. Thus, alteration of miRNA targets as a mechanism to promote cell survival and proliferation appears to be a broad theme employed by H. pylori.
Finally, H. pylori also has the ability to negatively influence numerous tumor suppressors. For example, gastrokine 1 has the ability to inhibit CagA-induced cell proliferation and EMT by directly binding to CagA; this interaction prevents CagA interaction with SHP2 and E-cadherin [47]. However, H. pylori overcomes this effect via downregulation of gastrokine 1 in GC tissues. This is a CagA-mediated event and the resulting decreased gastrokine 1 levels result in activation of NF-κB and PI3K/Akt [47]. An additional tumor suppressor example can be found with trefoil factor 1 (TFF1), which inhibits β-catenin and NF-κB-p65 nuclear translocation via negative regulation of Akt/GSK-3β. This in turn prevents an increase in H. pylori-induced inflammation and pro-proliferation signals [48–50]. While TFF1 can be protective, it is downregulated or lost in almost half of all adenocarcinomas [48]. Therefore, loss of TFF1 tumor suppressor activity allows for unchecked activation of β-catenin and cell proliferation through activation of c-Myc and cyclin D1 [49]. Clearly, H. pylori utilizes a multifaceted approach to induce host cell proliferation.
CONCLUSIONS
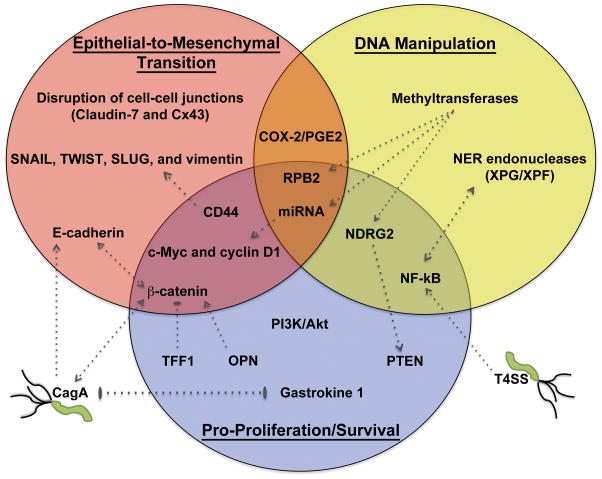
While H. pylori was identified as a carcinogen over two decades ago, researchers are still elucidating novel oncogenic mechanisms used by this fascinating pathogen. Indeed, increasing evidence indicates that H. pylori can influence numerous host pathways to promote cancer development. Though all of the mechanisms are unclear, evidence suggests that as H. pylori establishes infection, the subsequent host response creates an environment conducive to tumorigenesis. Remarkably, the host cell changes undergone in response to H. pylori infection (Figure 1) involve several hallmarks of traditional cancer progression. Included among these are DNA damage and methylation, activation of EMT pathways, activation of pro-proliferative/anti-apoptotic effectors, and suppression of tumor suppressors. H. pylori is a clearly a cunning pathogen that has the ability to directly target universal cancer pathways as a means to induce gastric carcinogenesis.
Figure 1. H. pylori activation of cancer pathways.
Recent literature highlights H. pylori’s ability to activate EMT (top left), manipulate host DNA (top right), and initiate cell pro-survival/proliferation signals (bottom center). Key components in each pathway are indicated within each circle. Stimulation of these pathways occurs primarily through interactions with proteins that serve overlapping functions in multiple pathways (overlap regions). Where appropriate, arrows indicate activation signals, blunt ended lines indicate inhibitory signals, and double-ended arrows or lines indicate reciprocating events between two proteins. Also indicated are direct interactions between host cell and bacterial factors.
KEY POINTS.
Known oncogenic pathways are targeted by H. pylori through the activation of upstream signaling components or through inhibition of known tumor suppressors.
Recent work highlights the importance of activation of the PI3K/Akt axis in H. pylori induced cancer development.
H. pylori has the ability to alter the human genome through DNA damage, methylation, and regulation of micro-RNAs.
H. pylori initiates epithelial-to-mesenchymal transition leading to loss of cell-cell junctions and induction of cellular proliferation.
Acknowledgments
The contents of this manuscript are solely those of the authors and do not reflect the views of the National Institutes of Health or the Department of Defense. Due to the limited length of this review, we apologize in advance for references that we were unable to cite.
FINANCIAL SUPPORT AND SPONSORSHIP: This work was supported by National Institutes of Health R21AI109405 to DSM, Uniformed Services University of the Health Sciences award to DRB, and a Henry M. Jackson Foundation for the Advancement of Science fellowship to SLS.
Footnotes
CONFLICTS OF INTEREST: The authors declare that there are no competing conflicts of interest.
References
- 1.Infection with Helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International gency for Research on Cancer. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 2.Helicobacter pylori eradication as a strategy for preventing gastric cancer. International Agency for Research on Cancer; 2014. IARC Working Group Reports, No. 8. [Google Scholar]
- 3.Globocan 2012 v1.0, Cancer incidence and mortality worldwide: IARC Cancerbase no. 11. International Agency for Research on Cancer; 2013. [Google Scholar]
- 4.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5.Yong X, Tang B, Li BS, et al. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Commun Signal. 2015;13:30. doi: 10.1186/s12964-015-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pachathundikandi SK, Tegtmeyer N, Backert S. Signal transduction of Helicobacter pylori during interaction with host cell protein receptors of epithelial and immune cells. Gut Microbes. 2013;4:454–74. doi: 10.4161/gmic.27001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Maekita T, Nakazawa K, Mihara M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–95. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Tao H, Carloni E, et al. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542–53. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- **11.Hartung ML, Gruber DC, Koch KN, et al. H. pylori -induced DNA strand breaks are introduced by nucleotide excision repair endonucleases and promote NK-κB target gene expression. Cell Rep. 2015;13:70–9. doi: 10.1016/j.celrep.2015.08.074. This study illustrates that H. pylori-induced translocation of NF-κB to the nucleus promotes formation of a binding platform for nucleotide excision repair endonucleases. The resulting DNA double strand breaks amplify NF-κB target gene expression to promote survival of the infected cells. [DOI] [PubMed] [Google Scholar]
- *12.Koeppel M, Garcia-Alcalde F, Glowinski F, et al. Helicobacter pylori infection causes characteristic DNA damage patterns in human cells. Cell Rep. 2015;11:1703–13. doi: 10.1016/j.celrep.2015.05.030. This study reveals a specific DNA damage pattern associated with H. pylori infection that overlaps with damage profiles found in gastric carcinoma. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazu T, Asada K, Charvat H, et al. Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: A cross-sectional study. Carcinogenesis. 2015;36:1291–8. doi: 10.1093/carcin/bgv125. [DOI] [PubMed] [Google Scholar]
- **15.Kiga K, Mimuro H, Suzuki M, et al. Epigenetic silencing of miR-210 increases the proliferation of gastric epithelium during chronic Helicobacter pylori infection. Nat Commun. 2014;5:4497. doi: 10.1038/ncomms5497. This study correlates aberrant DNA methylation with silencing of miRNA. miR-210 was shown to be epigenetically silenced by H. pylori infection; downstream effects include promotion of cell proliferation, which increases the risk of GC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider BG, Mera R, Piazuelo MB, et al. DNA methylation predicts progression of human gastric lesions. Cancer Epidemiol Biomarkers Prev. 2015;24:1607–13. doi: 10.1158/1055-9965.EPI-15-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Ling ZQ, Ge MH, Lu XX, et al. Ndrg2 promoter hypermethylation triggered by infection correlates with poor patients survival in human gastric carcinomaHelicobacter pylori. Oncotarget. 2015;6:8210–25. doi: 10.18632/oncotarget.3601. This study revealed that H. pylori induced NF-κB activation of DNMT3b leads to hypermethylation and loss of expression of the NDRG2 tumor suppressor. This work directly links H. pylori infection with aberrant methylation, gene silencing, and carcinogenic signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakahata S, Ichikawa T, Maneesaay P, et al. Loss of NDGR2 expression activates PI3K-Akt signalling via PTEN phosphorylation in ATLL and other cancers. Nat Commun. 2014;5:3393. doi: 10.1038/ncomms4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Xie C, Xu W, et al. Phosphorylation and inactivation of PTEN at residues Ser380/Thr382/383 induced by Helicobacter pylori promotes gastric epithelial cell survival through PI3K/Akt pathway. Oncotarget. 2015;6:31916–26. doi: 10.18632/oncotarget.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichikawa T, Nakahata S, Fujii M, et al. Loss of NDRG2 enhanced activation of the NF-κB pathway by PTEN and NIK phosphorylation for ATL and other cancer development. Sci Rep. 2015;5:12841. doi: 10.1038/srep12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Shan JX, Chen XH, et al. Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res. 2015;25:588–603. doi: 10.1038/cr.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/Akt pathways. Oncogene. 2005;24:7443–54. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Zeng J, Liang X, et al. Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4) PLoS One. 2014;9:e105306. doi: 10.1371/journal.pone.0105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Choi YJ, Kim N, Chang H, et al. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis. 2015;36:553–63. doi: 10.1093/carcin/bgv022. This study uses clinical samples to show that EMT markers are increased in response to H. pylori infection; these effects are further exacerbated in gastric cancer samples, but can be reversed upon H. pylori eradication. [DOI] [PubMed] [Google Scholar]
- *25.Wroblewski LE, Piazuelo MB, Chaturvedi R, et al. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut. 2015;64:720–30. doi: 10.1136/gutjnl-2014-307650. In addition to showcasing the utility of the novel gastroid model, this work details the mechanism by which H. pylori disrupts claudin-7 in a β-catenin and SNAIL dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang X, Zeng J, Wang L, et al. Histone demethylase RBP2 promotes malignant progression of gastric cancer through TGF-β1-(p-Smad3)-RBP2-E-cadherin-Smad3 feedback circuit. Oncotarget. 2015;6:17661–74. doi: 10.18632/oncotarget.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56. doi: 10.1038/nm.3850. [DOI] [PubMed] [Google Scholar]
- 28.Ishimoto T, Izumi D, Watanabe M, et al. Chronic inflammation with Helicobacter pylori infection is implicated in CD44 overexpression through miR-328 suppression in the gastric mucosa. J Gastroenterol. 2015;50:751–7. doi: 10.1007/s00535-014-1019-y. [DOI] [PubMed] [Google Scholar]
- 29.Bertaux-Skeirik N, Feng R, Schumacher MA, et al. CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 2015;11:e1004663. doi: 10.1371/journal.ppat.1004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sougleri IS, Papadakos KS, Zadik MP, et al. Helicobacter pylori CagA protein induces factors involved in the epithelial-to-mesenchymal-transition (EMT) in infected gastric epithelial cells in an EPIYA-phosphorylation-dependent manner. FEBS J. doi: 10.1111/febs.13592. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Cao K, Xu C, et al. GATA-3 augmentation down-regulates Connexin 43 in Helicobacter pylori associated gastric carcinogenesis. Cancer Biol Ther. 2015;16:987–96. doi: 10.1080/15384047.2015.1030552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radin JN, Gonzalez-Rivera C, Frick-Cheng AE, et al. Role of Connexin 43 in Helicobacter pylori VacA-induced cell death. Infect Immun. 2014;82:423–32. doi: 10.1128/IAI.00827-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DG, Kim HS, Lee YS, et al. Helicobacter pylori CagA promotes Snail-mediated epithelial-mesenchymal transition by reducing GSK-3 activity. Nat Commun. 2014;5:4423. doi: 10.1038/ncomms5423. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M, Mimuro H, Kiga K, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Neal JT, Peterson TS, Kent ML, et al. H. pylori virulence factor CagA increases intestinal cell proliferation by Wnt pathway activation in a transgenic zebrafish model. Dis Model Mech. 2013;6:802–10. doi: 10.1242/dmm.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata-Kamiya N, Kurashima Y, Teishikata Y, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–26. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- *37.Chang WL, Yang HB, Cheng HC, et al. Intracellular osteopontin induced by CagA-positive Helicobacter pylori promotes β-catenin accumulation and interleukin-8 secretion in gastric epithelial cells. Helicobacter. 2015 doi: 10.1111/hel.12225. This study identifies iOPN as a novel intermediary between CagA and activation of β-catenin, which promotes several oncogenic changes. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Park JW, Go DM, et al. Ablation of osteopontin suppresses n-methyl-n-nitrosourea and Helicobacter pylori-induced gastric cancer development in mice. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv144. [DOI] [PubMed] [Google Scholar]
- 39.Park JW, Lee SH, Go du M, et al. Osteopontin depletion decreases inflammation and gastric epithelial proliferation during Helicobacter pylori infection in mice. Lab Invest. 2015;95:660–71. doi: 10.1038/labinvest.2015.47. [DOI] [PubMed] [Google Scholar]
- 40.Liu W, Song N, Yao H, et al. miR-221 and miR-222 simultaneously target reck and regulate growth and invasion of gastric cancer cells. Med Sci Monit. 2015;21:2718–25. doi: 10.12659/MSM.894324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King CC, Obonyo M. Helicobacter pylori modulates host cell survival regulation through the serine-threonine kinase, 3-phosphoinositide dependent kinase 1 (PDK-1) BMC Microbiol. 2015;15:222. doi: 10.1186/s12866-015-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X, Zeng J, Wang L, et al. Histone demethylase RBP2 induced by Helicobactor pylori CagA participates in the malignant transformation of gastric epithelial cells. Oncotarget. 2014;5:5798–807. doi: 10.18632/oncotarget.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Xu G, Yin C, et al. Down-regulation of miR-203 induced by Helicobacter pylori infection promotes the proliferation and invasion of gastric cancer by targeting CASK. Oncotarget. 2014;5:11631–40. doi: 10.18632/oncotarget.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang M, Shi B, Liu J, et al. Downregulation of miR203 induces overexpression of PIK3CA and predicts poor prognosis of gastric cancer patients. Drug Des Devel Ther. 2015;9:3607–16. doi: 10.2147/DDDT.S85525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X, Xia Y, Li L, et al. miR-101 inhibits cell growth and tumorigenesis of Helicobacter pylori related gastric cancer by repression of SOCS2. Cancer Biol Ther. 2015;16:160–9. doi: 10.4161/15384047.2014.987523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye F, Tang C, Shi W, et al. A MDM2-dependent positive-feedback loop is involved in inhibition of miR-375 and miR-106b induced by Helicobacter pylori lipopolysaccharide. Int J Cancer. 2015;136:2120–31. doi: 10.1002/ijc.29268. [DOI] [PubMed] [Google Scholar]
- 47.Yoon JH, Seo HS, Choi SS, et al. Gastrokine 1 inhibits the carcinogenic potentials of Helicobacter pylori CagA. Carcinogenesis. 2014;35:2619–29. doi: 10.1093/carcin/bgu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soutto M, Chen Z, Katsha AM, et al. Trefoil factor 1 expression suppresses Helicobacter pylori-induced inflammation in gastric carcinogenesis. Cancer. 2015 doi: 10.1002/cncr.29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soutto M, Peng D, Katsha A, et al. Activation of beta-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut. 2015;64:1028–39. doi: 10.1136/gutjnl-2014-307191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soutto M, Romero-Gallo J, Krishna U, et al. Loss of TFF1 promotes Helicobacter pylori-induced β-catenin activation and gastric tumorigenesis. Oncotarget. 2015;6:17911–22. doi: 10.18632/oncotarget.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]