Abstract
In a rotary motor FoF1-ATP synthase that couples H+ transport with ATP synthesis/hydrolysis, it is thought that an Foc subunit oligomer ring (c-ring) in the membrane rotates as protons pass through Fo and a 120° rotation produces one ATP at F1. Despite several structural studies, the copy number of Foc subunits in the c-ring has not been determined for any functional FoF1. Here, we have generated and isolated thermophilic Bacillus FoF1, each containing genetically fused 2-mer–14-mer c (c2–c14). Among them, FoF1 containing c2, c5, or c10 showed ATP-synthesis and other activities. When F1 was removed, Fo containing c10 worked as an H+ channel but Fos containing c9, c11 or c12 did not. Thus, the c-ring of functional FoF1 of this organism is a decamer. The inevitable consequence of this finding is noninteger ratios of rotation step sizes of F1/Fo (120°/36°) and of H+/ATP (10:3). This step-mismatch necessitates elastic twisting of the rotor shaft (and/or the side stalk) during rotation and permissive coupling between unit rotations by H+ transport at Fo and elementary events in catalysis at F1.
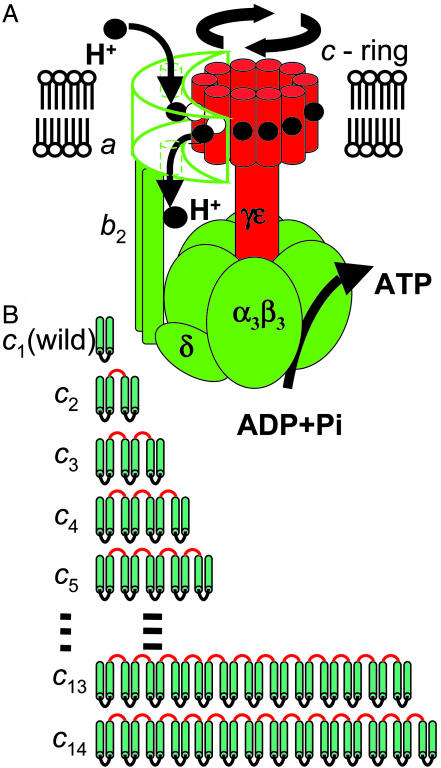
The FoF1-ATP synthase, often simply called FoF1, is composed of two portions: a water-soluble F1, which has catalytic sites for ATP synthesis and hydrolysis, and a membrane-integrated Fo, which mediates H+ (proton) transport (1, 2). When isolated, F1 has ATP-hydrolyzing activity and Fo acts as a proton channel. The bacterial FoF1 has the simplest subunit structure, α3β3γδε for F1 and ab2cn for Fo (where n is the copy number of the c subunits), as depicted schematically in Fig. 1A. F1 and Fo are motors that share a common central rotor; a down-hill proton flow through Fo drives rotation of the rotor, causing conformational changes in F1 that result in ATP synthesis. Conversely, ATP hydrolysis in F1 causes a reverse rotation of the rotor that enforces Fo to pump protons to the reverse direction. The ring of Foc subunit oligomer and the γ-ε subunits of F1 comprise the central rotor, and they rotate together as a single body (3). The side stalk made up of δ-b2 subunits connects the membrane-bound Foa subunit with the α3β3 hexamer ring of F1, which prevents the hexameric ring from rotating as γ subunit rotates. Rotary motion of F1 has been analyzed in detail, and it has been established that the γ subunit rotates with a discrete 120° step per each consumed ATP (4, 5) (three ATP molecules per revolution). However, little is known about the Fo rotation. It has been proposed that each proton is first transported to a glutamic acid of an Foc subunit of the c-ring, which is located at the middle of a transmembrane helix of Foc, through a channel in the periplasmic half of the Foa subunit, and then after one revolution of the c-ring, the proton is released to cytoplasm through another half-channel of Foa (6, 7) (Fig. 1 A). In this mechanism, the copy number of Foc in the c-ring should be equal to the number of transported protons per revolution of the c-ring that directly defines the H+/ATP ratio, which is one of the most important parameters in bioenergetics. Structural studies have suggested different copy numbers of Foc in the ring, depending on the sources. There are 10 copies of Foc in an yeast mitochondrial F1–Foc complex (crystal structure) (8), 11 copies in the c-oligomers isolated from Ilyobacter tartaricus (atomic-force microscopy and transmission cryoelectron microscopy) (9), and 14 copies of c-oligomers isolated from chloroplasts (atomic-force microscopy) (10, 11). A cross-linking study suggested 10 copies as a preferred number of Foc in Escherichia coli FoF1 (12). However, no conclusive evidence of the copy number in the functional FoF1 complex has been obtained because the results cited above were obtained for the c-ring of subcomplex lacking all other Fo subunits (8), for the c-oligomers extracted with SDS or other detergents (9–11), or by an indirect method (12). In this study, we have generated functional FoF1–ATP synthase whose proton-translocating c-ring is made from a single-polypeptide chain of 10 tandemly fused c subunits and discussed in terms of elastic coupling between Fo and F1.
Fig. 1.
Rotary model for ATP synthesis by FoF1 and diagram of the fused Foc subunits (cn) used in this study. (A) Proton transport through Fo drives rotation of c-ring made of a certain number (n) of Foc subunits. According to the current model (6, 7), each proton enters the assembly through a half-channel of Foa subunit accessible from periplasmic side and binds to the E56 carboxylate of one of the Foc subunits that is interacting with the Foa. The Foc with the protonated binding site then moves from the Foa interface region into the lipid-surrounded region in the membrane, and after n - 1 steps, the proton is released into another half-channel of Foa that is open to the cytoplasmic side. One complete revolution of the c-ring accompanies the transport of n protons across membrane and produces three ATP molecules at F1 through rotation of γ-ε subunits that are connected to c-ring. (B) Genetically fused Foc multimers used in this study. The wild-type Foc, called c1 in this article, is a hairpin-structured membrane protein consisting of 72 aa. We made 13 mutant FoF1s with c2–c14. Linker portions are shown in red.
Materials and Methods
FoF1s with Fused Multimer Foc. An AvrII restriction site was introduced to the expression plasmid pTR19-ASDS (13) for thermophilic Bacillus PS3 FoF1 by the megaprimer method (14). Appropriate oligonucleotide primers were annealed upstream of uncB (Foa) and a 3′-region of antisense strand of uncE (Foc). To introduce SpeI and NheI sites, a second PCR was carried out with the product of the first PCR as a template in the presence of the oligonucleotide primer that annealed to the downstream of uncE. The amplified DNA fragment was digested with EcoRI and SpeI and ligated to the pTR19-ASDS, digested previously with both restriction enzymes, to produce pTR19-AC1N. A plasmid pTR19-AC1 was also generated by the megaprimer method. The amplified product was digested with EcoRI and SpeI and inserted into the plasmid pTR19-ASDS, which was previously digested with both restriction enzymes. To prepare a tandemly fused dimeric uncE (c2) gene, pTR19-AC1N was digested with EcoRI and NheI, and the 1.3-kbp EcoRI–NheI fragment was ligated into an EcoRI–AvrII site of pTR19-AC1 (plasmid pTR19-AC2). To obtain a plasmid having a fused trimeric uncE (c3) gene, the EcoRI–NheI fragment of pTR19-AC1N was introduced into the EcoRI–AvrII site of pTR19-AC2 (pTR19-AC3). By using this procedure, uncE genes were fused one by one. Consequently, plasmids expressing 13 kinds of FoF1s that have tandemly fused Foc 2-mer, 3-mer, 4-mer, 5-mer, 6-mer, 7-mer, 8-mer, 9-mer, 10-mer, 11-mer, 12-mer, 13-mer, or 14-mer (c2–c14) were prepared. The multimer uncE genes of the mutants were verified by restriction mapping of the plasmids. A plasmid to express a mutant FoF1 with a substitution of FocGlu-56 by Gln (E56Q) at the N-terminal hairpin in c10 was constructed by the method of Kunkel et al. (15). Plasmids for the wild-type and mutant FoF1s obtained, as described above, were individually expressed in an Fo-deficient E. coli strain JJ001 (pyrE41, entA403, argHI, rspsL109, supE44, ΔuncBEFH, recA56, srl::Tn10) (16) (a kind gift from J. Hermolin, University of Wisconsin Medical School, Madison). Culture of the transformants, preparation of membrane vesicles, solubilization and purification of FoF1, and reconstitution of the purified FoF1 into liposomes were performed as described (13). The FoF1s used in this work has a tag of 10 His residues at the N terminus of the β subunit. Membrane vesicles and purified FoF1s were analyzed by 0.1% SDS/12–20% PAGE. Proteins (≈10 μg) in membrane vesicles, and purified preparations were precipitated with trichloroacetic acid (final concentration, 2.5%), neutralized by resuspending in 1 M Tris·HCl (pH 8.8), containing 2% SDS, and subjected to electrophoresis. Proteins were visualized with Coomassie brilliant blue R or immunoblotting. Preparation of F1-stripped membrane vesicles was carried out as follows. Membrane vesicles of E. coli cells were prepared by using PA6 buffer (10 mM Hepes·KOH/10% glycerol) instead of PA3-buffer (10 mM Hepes·KOH/5 mM MgCl2/10% glycerol). The vesicles (500 μl) were diluted 5-fold with 2 mM EDTA and incubated at 35°C for 20 min. The membrane vesicles were collected by centrifugation at 244 k × g for 20 min, resuspended in 3 ml of 0.5 mM EDTA containing 1 mM DTT, and incubated at 35°C for 20 min. The membrane vesicles were again collected by centrifugation (244 k × g for 20 min) and resuspended in 300 μl of PA3.
Analytical Procedures. F1 of thermophilic Bacillus PS3 was purified by using the procedures described in ref. 17. ATPase activity was measured with an ATP-regenerating system at 45°C in 50 mM Hepes·KOH buffer (pH 7.5), containing 100 mM KCl, 5 mM MgCl2, 1 mM ATP, 1 μg/ml p-trifluoromethoxyphenylydrazone (FCCP), 2.5 mM KCN, 2.5 mM phosphoenolpyruvate, 100 μg/ml pyruvate kinase, 100 μg/ml lactate dehydrogenase, and 0.2 mM NADH (17). Hydrolysis of 1 μmol of ATP per min is defined as one unit. The slopes of decreasing absorbance at 340 nm in the steady-state phase (400–600 sec) were used for the calculation of the activity. Sensitivity of the ATP hydrolysis activity to inactivation by N,N′-dicyclohexylcarbodiimide (DCCD) was analyzed by the same method as used in the previous study (13). ATP-driven H+-pumping activity was measured as quenching of the fluorescence (excitation, 410 nm; emission, 480 nm) of 9-amino-6-chloro-2-methoxyacridine (ACMA) in 10 mM Hepes·KOH, pH 7.5/100 mM KCl/5 mM MgCl2, supplemented with membrane vesicles (0.5 mg of protein per ml) and ACMA (0.3 μg/ml) at 45°C (17). The reaction was initiated by adding 1 mM ATP, and quenching reached a steady level after 1 min. After 5 min, FCCP (1 μg/ml) was added and reversal of fluorescence was confirmed. The magnitude of fluorescence quenching at 3 min relative to the level after addition of FCCP was taken as the proton-pumping activity. Activity of cn-Fo (n = 9–12) as a proton channel was measured for the F1-stripped membrane vesicles with attenuation of NADH-induced ACMA fluorescence quenching. The solution contained F1-stripped membrane vesicles (0.1 mg of membrane protein per ml) and ACMA (0.3 μg/ml) in 10 mM Hepes·KOH, pH 7.5/100 mM KCl/5 mM MgCl2 with or without 50 μM purified F1. The electrochemical potential of protons was generated by adding 0.3 mM NADH, and the effect of the addition of F1 on the magnitude of fluorescence quenching was monitored. The reaction was terminated by adding 1 μg/ml of FCCP. ATP synthesis activity was measured (18) as follows. The solution (200 μl) containing 30 mM Hepes·KOH, pH 7.5/50 mM KCl/5 mM MgCl2/5 mM ADP/25 mM KPi/5% glycerol were mixed with 50 μl of membrane vesicles (50 μg of protein) and incubated at 45°C for 5 min. The reaction was initiated by supplementing 2 mM NADH. After 0, 2, 6, 10, and 20 min, aliquots of the reaction mixture were transferred to another tube and mixed with trichloroacetic acid to a final concentration of 2.5% to terminate the reaction. The pH was adjusted to 7.7 by adding 125 mM Tris·acetate (pH 9.5), and the amount of ATP was determined with the CLSII ATP bioluminescence assay kit (Roche). It was confirmed that ATP synthesis did not occur when FCCP was present. Protein concentrations were determined by using the BCA protein assay kit (Pierce), with BSA as a standard.
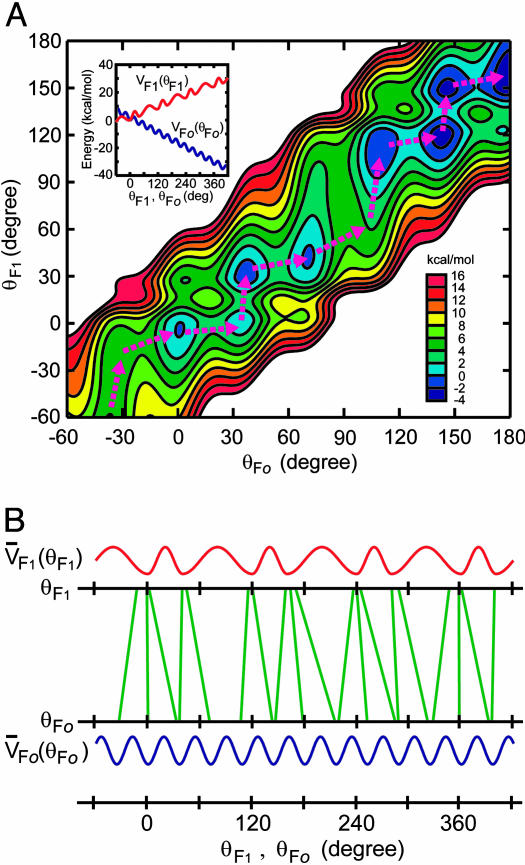
Free-Energy Functions Used in the Elastic γ Rotation Model. VFo(θFo) and VF1(θF1) are expressed by smooth cosine functions with minima at every 36° rotational step for Fo, as revealed in the present study, and every 40° and 80° rotational substeps for F1, corresponding to the substeps of the γ rotation in F1 observed by single-molecule observations (19–21), respectively. The energy-barrier heights separating the minima in VFo(θFo) and VF1(θF1), which also relates to the widths of the free-energy basins, are assumed to be 4 kcal/mol to mimic discrete stepping motions of the γ rotation observed in single-molecule experiments (19–21). It was confirmed that the essential features of the γ rotation reported here hold in a wide range of the barrier-height parameters (1–8 kcal/mol) through constructing several free-energy surfaces by changing values of the parameters. Overall, decrease by 32 kcal/mol and increase by 24 kcal/mol per revolution are then included in VFo(θFo) and VF1(θF1) to represent the proton gradient across membrane at Fo and costs of three ATP productions at F1, respectively. The elastic energy of the γ torsion is taken into account by a harmonic function, 1/2k(θFo-θF1)2, with a force constant k of 0.01 kbT, which gives rise to ≈10° thermal fluctuation of the free γ-subunit torsion.
Results
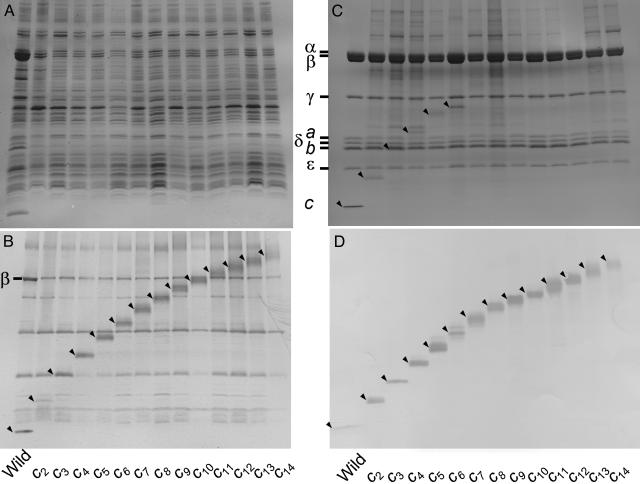
Expression and Purification of cn-FoF1s. To determine the copy number in the functional complex, we made a series of 13 kinds of thermophilic FoF1s that had multimer cn (n = 2–14) in which C termini of Foc subunits were fused genetically to N termini of adjacent Foc subunits with a linker sequence, Gly-Ser-Ala-Gly (Fig. 1B) and measured their functional activities. These cn-FoF1s were expressed in the membranes of the host E. coli cells. Membrane vesicles were prepared from the cells and analyzed with SDS/PAGE. Protein bands of the α and β subunits were seen in protein staining (Fig. 2A), and protein bands of cn were visualized by immunoblotting (Fig. 2B). Estimated from band intensities in immunoblotting with anti-β antibody, the amounts of expressed mutant FoF1s in the membranes were 20–30% of the wild-type c1-FoF1 (Fig. 2B). Apparent proteolytic degradation was not observed for cn. The cn-FoF1s were solubilized individually from the membranes and isolated by nickel–nitrilotriacetic acid (Ni-NTA) affinity chromatography. Free cn uncomplexed with other subunits, if it exists, was removed by His tags attached to the β subunits. The isolated cn-FoF1s showed clearly the bands of all subunits of the FoF1s except for the bands of cn that were poorly stained with Coomassie blue (Fig. 2C). Immunoblotting with anti-Foc antibody, however, clearly indicated the presence of cn at the expected positions (Fig. 2D). Judging from relative intensities of the bands, it appears that all of the cn-FoF1s can assemble normally despite the alterations in the c subunits.
Fig. 2.
Expression and purification of cn-FoF1s. Proteins were analyzed with SDS/PAGE and visualized with Coomassie brilliant blue (A and C) or with immunoblotting by using anti-Foc antibodies (B and D). Bands of cn were hardly stained with usual protein staining methods and were shown with immunoblotting. (A and B) Membrane vesicles prepared from E. coli cells expressing cn-FoF1s (n = 1–14). For reference, the bands of β subunit of F1 were also visualized with anti-β antibodies (B). (C and D) Purified cn-FoF1s. The positions of cn are indicated by arrowheads.
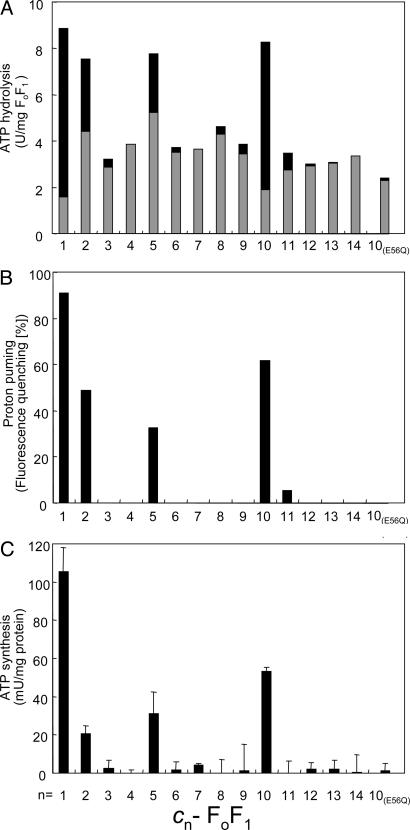
Functions of cn-FoF1s in the Membrane Vesicles. ATP hydrolysis activities derived from the expressed cn-FoF1s in the membrane vesicles were compared (Fig. 3A). To assess the amount of functional FoF1, we measured ATP hydrolysis activity with or without pretreatment by DCCD. This reagent is known to label specifically a critical glutamic acid residue in Foc, and it blocks proton translocation and rotation of the c-ring (and hence, γ-ε of F1). With rotation being prevented by DCCD, ATP hydrolysis (and ATP synthesis) cannot occur in the coupled FoF1 complex. However, when the connection between F1 and Fo is defective, the F1 component can hydrolyze ATP uncoupled to H+ translocation. Therefore, the DCCD-sensitive fraction of ATP hydrolysis activity corresponds to the functional FoF1 in which the proton translocation, ATP hydrolysis, and ATP synthesis are properly coupled by rotation of the central rotor. For the wild-type c1-FoF1, 85% of the ATP hydrolysis activity was DCCD-sensitive (Fig. 3A, black bars) and 15% was resistant (Fig. 3A, gray bars). Among cn-FoF1s, only c2-, c5-, and c10-FoF1s showed significant DCCD-sensitive ATP hydrolysis activities, with c10-FoF1 being the highest. ATP hydrolysis activities of other cn-FoF1s were low (less than half of the wild-type c1-FoF1) and not affected by DCCD.
Fig. 3.
ATP hydrolysis, proton pumping, and ATP synthesis activities of membrane vesicles containing cn-FoF1s. The rightmost bars [c10(E56Q)] show the results of c10(E56Q)-FoF1. (A) ATP hydrolysis activities of the membrane vesicles that contained cn-FoF1. Black and gray portions of the bars represent the DCCD-sensitive and -resistant fractions of activities, respectively. (B) ATP-driven proton-pumping activities measured with ACMA fluorescence quenching. The relative magnitude of quenching induced by addition of ATP is shown. (C) ATP synthesis driven by NADH oxidation. The amounts of synthesized ATP after addition of NADH were measured, and the rates of ATP increase are shown. Experimental details are described in Materials and Methods.
Next, the proton-pumping driven by ATP hydrolysis was tested with the membrane vesicles containing cn-FoF1s (Fig. 3B). Proton-pumping into vesicles was monitored as acidification of the lumen of the vesicles by fluorescence quenching of ACMA, and the degree of induced quenching was taken as the proton-pumping activity. As shown, only c2-, c5-, and c10-FoF1s, as well as the wild-type c1-FoF1, showed the proton-pumping activities in response to the addition of ATP. Purified c10-FoF1 reconstituted into liposomes also exhibited the same ATP-driven proton-pumping activity (data not shown). We also monitored protons pumped into the membrane vesicles by oxidation of NADH through the respiratory chain. Upon addition of NADH, instead of ATP, to the vesicles, similar ACMA quenching was observed for all membrane vesicles containing cn-FoF1s (data not shown), indicating that the protons pumped into the membrane vesicles were retained in the lumens of the vesicles and any cn-FoF1s did not increase proton leakage of the membranes. When NADH, ADP, and phosphate were given, membrane vesicles containing c2-, c5-, and c10-FoF1s exhibited the synthesis of ATP with 18%, 27%, and 50% yields, respectively, compared with the wild-type c1-FoF1 (Fig. 3C). Other cn-FoF1s did not exhibit ATP synthesis activity. In summary, even though all of cn-FoF1s can form stable FoF1 complexes, the capacity to couple proton transport to ATP synthesis and hydrolysis is observed only with c10-FoF1 and, to a lesser extent, c2- and c5-FoF1s. The activities of c2- and c5-FoF1s are explained by assuming that five copies of c2 and two copies of c5 can substitute, although inefficiently, the role of 10 copies of Foc in the wild-type c1-FoF1. The generation of the functional c10-FoF1 allows one to carry out experiments that are otherwise difficult. For example, when only a single E56Q mutation was introduced into the first hairpin unit of the c10, the resulting membrane vesicles containing c10-FoF1 did not catalyze ATP-driven proton pumping or ATP synthesis (Fig. 3). Therefore, the E56Q substitution in a single copy of the naturally occurring c-ring is sufficient to abolish H+ translocation coupled to ATP synthesis and hydrolysis. This result provides evidence that each of all 10 E56 in the c-ring is indispensable. Studies have suggested a similar conclusion (22, 23), but they were based on statistical introduction of the mutation or DCCD-labeling into the c-ring.
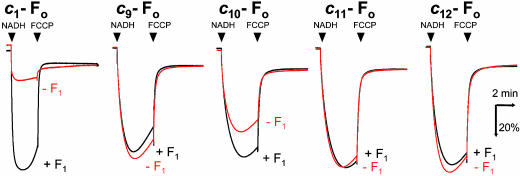
Proton-Channel Activities of c9-, c10-, c11-, and c12-Fo. Another intriguing question is whether cn fusion proteins that are incapable of participating in proton translocation during ATP synthesis and hydrolysis are capable of participating in passive proton diffusion. EDTA treatment of the membrane vesicles removed F1 portions from ≈90% of cn-FoF1s, leaving F1-stripped cn-Fo vesicles. NADH was used to generate a proton gradient across membranes that was monitored with fluorescence quenching of ACMA. If Fo acts as a proton channel and mediates passive proton diffusion, the proton gradient is dissipated and fluorescence quenching should be attenuated. The inclusion of F1 should increase the quenching because it binds to Fo to block the channel. We tested membrane vesicles containing c9-, c10-, c11-, and c12-Fos as well as the wild-type c1-Fo (Fig. 4). For c1-Fo, the quenching was very small in the absence of F1 but large in the presence of F1. Among c9-, c10-, c11-, and c12-Fos, only c10-Fo showed the attenuated quenching in the absence of F1 and the increased quenching by the inclusion of F1. Others did not show such characteristic quenching behavior but showed simple quenching in both the presence and absence of F1, implying a loss of the proton channel activity. The degree of attenuation of c10-Fo vesicles was less than that of c1-Fo vesicles, and it was partly due to the relatively low content of c10-Fo in the membranes. Thus, the proton transport through Fo requires very strict arrangement of contact surface between Foa and Foc in the Fo assembly and even a rotary displacement as tiny as 3.3° (360°/10–360°/11) seems to be enough to disable a proton transfer between them.
Fig. 4.
Activities of c9-, c10-, c11-, and c12-Fos as a proton channel. The activity of Fo as a proton channel was assessed from the ACMA fluorescence quenching in the presence (black traces) or absence (red traces) of F1. Proton leak was detected as attenuation of the quenching and restoration of the quenching by F1 ensured the specific leak through Fo. The reactions were started by addition of NADH and terminated by addition of FCCP. The results for the wild-type c1-Fo are shown as a reference. Experimental details are described in Materials and Methods.
Discussion
From the results described here, we conclude that the naturally occurring FoF1 of the thermophilic Baccilus PS3 contains 10 copies of Foc subunits in the c-ring. In the case of E. coli FoF1, even though the preferred copy number of Foc in the c-rings is believed to be 10, it has been argued that a c-ring composed of eight or nine Foc subunits can have at least partial function (12) and that the copy number of Foc can vary depending on the growth conditions (24). However, our results show that such ambiguity of the copy number of Foc would not be allowed, and the c-ring of the functional E. coli FoF1 is most likely constituted by 10 copies of Foc under any conditions. Another possibility is that the greater flexibility of the longer linker sequence (10 or 13 aa) in E. coli fused Foc (12) may allow a c-ring of nine subunits (3 × c3) to function even though the packing is not perfect as in an optimal c-ring of 10 subunits.
Given that the c-ring of our FoF1 is made of 10 Foc subunits, step sizes of unit-rotation do not fit between F1 and Fo. If the c-ring were a dodecamer, the unit-rotation angle by a single proton transport is 30° and four unit-rotations give rise to a 120° rotation of the γ-subunit that produces one ATP at F1. The unit-rotation angle of the decamer c-ring, however, must be 36°, and a 120° rotation of the γ-subunit cannot be a multiple of a 36° rotation. During rotation, the catalytic events at F1 may occur only at the moments when γ–β interfaces are aligned precisely to give the most suitable geometries of catalytic sites. These moments are realized by experiments as dwelling times of F1 rotation, and at least two kinds of them have been identified to date in a 120° rotation, ATP-waiting dwell (0°) and catalytic dwell (80°) (19–21). Therefore, rotation with a 36° unit at Fo must be adjusted to these dwelling positions at F1 by some means. Slip at the connection between γ–ε and the c-ring does not occur during rotation because a cross-linking between them does not impair the activities of FoF1 (3). Because the coiled-coil structure of the γ subunit allows some internal twisting and the Fob2 subunits of the side stalk have extra flexibility (25), they can undergo elastic twisting or bending (26, 27) to enable the proper alignment of rotor–stator contacts at both Fo and F1. Assuming that the γ subunit takes this task with its torsional spring constant being in the range allowing ≈10° thermal fluctuation, we calculated how it rotates with twisting itself (Fig. 5). The model shows that the rotations of γ at the Fo and F1 interfaces do not coincide with each other but evolve with temporal gaps between them permitted by the elasticity of γ (Fig. 5A). The twisting angle of γ at the energy minima in the course of the γ rotation is mainly distributed in the range of 0–30°, but it reaches to ≈40° at the maximum (Fig. 5B). The flexibility of γ allows both the Fo-γ and F1-γ interfaces at the free-energy minima to stay in conformations adequate for the proton transport in Fo and the catalysis in F1 despite the step-size mismatch, providing sufficient time for those events to take place.
Fig. 5.
Model of the rotation of an elastic γ in the ATP-synthetic process of c10-FoF1. (A) Contour plot of the modeled free-energy surface of the γ rotation in c10-FoF1 in terms of rotational angles of γ at the Fo and F1 interfaces, which are θFo and θF1, respectively. The free-energy surface possesses several minima, and the rotational motion of γ is regarded as transitions between the minima. Pink dashed arrows indicate energetically preferable paths of the transitions for the ATP synthetic process, indicating temporal gaps between the rotations of θFo and θF1. The free-energy surface was constructed with free-energy functions VFo(θFo) and VF1(θF1)(Inset), representing inherent free-energy profiles along the rotations at the Fo and F1 interfaces, respectively, and a harmonic term of the elastic γ torsion connecting the rotations of those interfaces. Detailed functional forms of those energy components are described in Materials and Methods. (B) Twisting of γ in the course of the γ rotation. Green lines connect values of θFo and θF1 at the minima of the free-energy surface in the γ rotation shown in A. Tilt of the green lines from the vertical, therefore, indicates the twisting of γ. Note that the θFo and θF1 values are within narrow range around the energy minima of V̄Fo(θFo) and V̄F1(θF1), representing the oscillatory energy parts along θFo and θF1, respectively.
Another important consequence of the decamer c-ring is that 10 protons are required for the synthesis of three ATPs. This noninteger H+/ATP (10:3) ratio means that one molecule of ATP is produced by transport of three protons on one occasion but four protons on another occasion. Therefore, microscopic couplings between events at Fo and those at F1 cannot be strict like a meshed gear but rather “permissive;” consecutive transports of three protons at Fo, for example, do not necessarily require to accompany three corresponding elementary catalytic steps of ATP synthesis at F1. It is easily understood that the microscopic coupling should be permissive if the central rotorshaft twists during rotation, as described in the previous paragraph. Here, we report the permissive nature of the coupling between proton transport and ATP synthesis of FoF1, but such nature of the coupling can be general among other biological motor systems to connect critical well tuned microscopic events in the large domain motions.
Finally, we have made a functional FoF1 that has a single polypeptide c-ring of genetically fused decamer of Foc. A recent genome project of an achaebacterium, Methanopyrus kandleri has revealed the presence of a single gene for c-ring consisting of 13 repeats of the hairpin domains (28). Thus, nature has already made a single-polypeptide version of the c-ring.
Acknowledgments
We thank J. Suzuki for F1 preparation and R. Iino, T. Masaike, H. Imamura, T. Ariga, H. Ueno, K. Shimabukuro, T. Hisabori, E. Muneyuki, M. Motojima, and N. Sone for helpful discussions. N.M. is supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DCCD, N,N′-dicyclohexylcarbodiimide; ACMA, 9-amino-6-chloro-2-methoxyacridine; FCCP, p-trifluoromethoxyphenylydrazone.
References
- 1.Boyer, P. D. (1997) Annu. Rev. Biochem. 66, 717-749. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida, M., Muneyuki, E. & Hisabori, T. (2001) Nat. Rev. Mol. Cell Biol. 2, 669-677. [DOI] [PubMed] [Google Scholar]
- 3.Tsunoda, S. P., Aggeler, R., Yoshida, M. & Capaldi, R. A. (2001) Proc. Natl. Acad. Sci. USA 98, 898-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. J. (1997) Nature 386, 299-302. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda, R., Noji, H., Kinosita, K. J. & Yoshida, M. (1998) Cell 93, 1117-1124. [DOI] [PubMed] [Google Scholar]
- 6.Junge, W., Lill, H. & Engelbrecht, S. (1997) Trends. Biochem. Sci. 22, 420-423. [DOI] [PubMed] [Google Scholar]
- 7.Vik, S. B., Patterson, A. R. & Antonio, B. J. (1998) J. Biol. Chem. 273, 16229-16234. [DOI] [PubMed] [Google Scholar]
- 8.Stock, D., Leslie, A. G. & Walker, J. E. (1999) Science 286, 1700-1705. [DOI] [PubMed] [Google Scholar]
- 9.Stahlberg, H., Muller, D. J., Suda, K., Fotiadis, D., Engel, A., Meier, T., Matthey, U. & Dimroth, P. (2001) EMBO Rep. 2, 229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seelert, H., Poetsch, A., Dencher, N. A., Engel, A., Stahlberg, H. & Muller, D. J. (2000) Nature 405, 418-419. [DOI] [PubMed] [Google Scholar]
- 11.Seelert, H., Dencher, N. A. & Muller, D. J. (2003) J. Mol. Biol. 333, 337-344. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, W., Hermolin, J. & Fillingame, R. H. (2001) Proc. Natl. Acad. Sci. USA 98, 4966-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki, T., Ueno, H., Mitome, N., Suzuki, J. & Yoshida, M. (2002) J. Biol. Chem. 28, 13281-13285. [DOI] [PubMed] [Google Scholar]
- 14.Landt, O., Grunert, H. P. & Hahn, U. (1990) Gene 96, 125-128. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel, T. A., Roberts, J. D. & Zakour, R. A. (1987) Methods Enzymol. 154, 367-382. [DOI] [PubMed] [Google Scholar]
- 16.Jones, P. C. & Fillingame, R. H. (1998) J. Biol. Chem. 273, 29701-29705. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki, T., Suzuki, J., Mitome, N., Ueno, H. & Yoshida, M. (2000) J. Biol. Chem. 275, 37902-37906. [DOI] [PubMed] [Google Scholar]
- 18.Schulenberg, B. & Capaldi, R. A. (1999) J. Biol. Chem. 274, 28351-28355. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda, R., Noji, H., Yoshida, M., Kinosita, K. J. & Itoh, H. (2001) Nature 410, 898-904. [DOI] [PubMed] [Google Scholar]
- 20.Shimabukuro, K., Yasuda, R., Muneyuki, E., Hara, K. Y., Kinosita, K. J. & Yoshida, M. (2003) Proc. Natl. Acad. Sci. USA 100, 14731-14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishizaka, T., Oiwa, K., Noji, H., Kimura, S., Muneyuki, E., Yoshida, M. & Kinosita, K. J. (2004) Nat. Struct. Mol. Biol. 11, 142-148. [DOI] [PubMed] [Google Scholar]
- 22.Hermolin, J. & Fillingame, R. H. (1989) J. Biol. Chem. 264, 3896-3903. [PubMed] [Google Scholar]
- 23.Dmitriev, O. Y., Altendorf, K. & Fillingame, R. H. (1995) Eur. J. Biochem. 233, 478-483. [DOI] [PubMed] [Google Scholar]
- 24.Schemidt, R. A., Qu, J., Williams, J. R. & Brusilow, W. S. (1998) J. Bacteriol. 180, 3205-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorgen, P. L., Bubb, M. R. & Cain, B. D. (1999) J. Biol. Chem. 274, 36261-36266. [DOI] [PubMed] [Google Scholar]
- 26.Oster, G. & Wang, H. (2000) Biochim. Biophys. Acta 1458, 482-510. [DOI] [PubMed] [Google Scholar]
- 27.Junge, W., Panke, O., Cherepanov, D. A., Gumbiowski, K., Muller, M. & Engelbrecht, S. (2001) FEBS Lett. 504, 152-160. [DOI] [PubMed] [Google Scholar]
- 28.Lolkema, J. S. & Boekema, E. J. (2003) FEBS Lett. 543, 47-50. [DOI] [PubMed] [Google Scholar]