Abstract
Severe anemia is an important cause of morbidity and mortality among children in resource-poor settings, but laboratory diagnostics are often limited in these locations. To address this need, we developed a simple, inexpensive, and color-based point-of-care (POC) assay to detect severe anemia. The purpose of this study was to evaluate the accuracy of this novel POC assay to detect moderate and severe anemia in a limited-resource setting. The study was a cross-sectional study conducted on children with sickle cell anemia in Luanda, Angola. The hemoglobin concentrations obtained by the POC assay were compared to reference values measured by a calibrated automated hematology analyzer. A total of 86 samples were analyzed (mean hemoglobin concentration 6.6 g/dL). There was a strong correlation between the hemoglobin concentrations obtained by the POC assay and reference values obtained from an automated hematology analyzer (r=0.88, P<0.0001). The POC assay demonstrated excellent reproducibility (r=0.93, P<0.0001) and the reagents appeared to be durable in a tropical setting (r=0.93, P<0.0001). For the detection of severe anemia that may require blood transfusion (hemoglobin <5 g/dL), the POC assay had sensitivity of 88.9% and specificity of 98.7%. These data demonstrate that an inexpensive (<$0.25 USD) POC assay accurately estimates low hemoglobin concentrations and has the potential to become a transformational diagnostic tool for severe anemia in limited-resource settings.
Introduction
Anemia is one of the most common causes of morbidity and mortality worldwide, disproportionately affecting persons from limited-resource settings. An estimated 1.62 billion people are affected by anemia globally, with a prevalence of 9% among persons in high-resource countries versus 43% prevalence in limited resource countries. Overall, the estimated global prevalence of anemia is 32.9%, causing 68.36 million years lived with disability [1,2]. The most commonly affected populations are pregnant women and children under five years of age.
Severe anemia, defined by World Health Organization (WHO) as hemoglobin <7 g/dL for pregnant women and children under five years of age, most commonly results from acute malaria or complications of hemoglobin disorders such as sickle cell anemia. Severe anemia is associated with significant morbidity and mortality, largely due to limited blood supply for transfusions in limited-resource settings. In sub-Saharan Africa, only 20–50% of blood requirements are fulfilled by the current donor blood supply [3], which results in rationing of available blood, and difficult decisions about which patients with what hemoglobin concentrations should receive a transfusion.
Anemia causing so much morbidity and mortality is often undiagnosed, misdiagnosed, or diagnosed too late to make a meaningful intervention. To diagnose and treat severe anemia appropriately, a reliable, rapid, and cost-effective means by which to measure the hemoglobin concentration accurately is needed. Many limited-resource settings do not have access to advanced laboratory equipment, such as automated hematology analyzers that are used routinely in high resource settings to obtain complete blood counts, including hemoglobin measurement, so instead rely on signs and symptoms of anemia. The WHO Integrated Management of Childhood Illness manual recommends the clinical assessment of anemia in children using simply palmar and conjunctival pallor [4,5]. However, the clinical assessment of anemia has been demonstrated to have highly variable sensitivity and specificity [6]. Despite the recognition by WHO that a low-cost, inexpensive, and reliable measure of hemoglobin is urgently needed, such an assay has yet to reach the countries where the need is the greatest [7,8]. Recently, with significant advancements in biotechnology and bioengineering, several point-of-care (POC) assays for the measurement of hemoglobin concentration have been developed [9–12], but none have become widely utilized in resource-poor settings where they are most urgently needed.
Recently, we developed a rapid and inexpensive visual, color-based POC assay to identify mild-to-moderate anemia, a common and important clinical diagnosis in developed countries. This POC assay was both sensitive and specific for detecting this degree of anemia and correlated well with hemoglobin results from a standard hematology analyzer [9]. The limits of hemoglobin detection for this prototype platform are 7.2–16.3 g/dL, representing mild-to-moderate anemia. However, in limited-resource settings where nearly half of the population may be anemic and severe, life-threatening anemia is common, it is more important to accurately identify a hemoglobin concentration less than 7 g/dL, for which a blood transfusion may be urgently required. The chemical formulation of this initial assay was thus modified to “shift” the color scale leftward, to identify clinically important degrees of severe anemia, more frequently found in limited-resource settings. In this report, we describe the accuracy and practical application of this “shifted” visual, color-based POC hemoglobin assay in the limited-resource setting of a large pediatric sickle cell clinic in Luanda, Angola.
Methods
Development of point-of-care assay
The previously described visual, color-based assay relies upon the reaction between hemoglobin, hydrogen peroxide, and 3,3′,5,5′-tetramethylbenzidine (TMB), in which hemoglobin catalyzes a redox reaction between TMB and hydrogen peroxide, leading to oxidized TMB products that can be visually differentiated based on color [9,13–16]. In the presence of a small concentration of hemoglobin, TMB reacts to form a single-electron product that is blue in color. In the presence of a slightly higher concentration of hemoglobin, the reaction results in a two-electron product with a yellow color; values between these concentrations are bluish green. In the presence of an excess concentration of hemoglobin, the yellow color of the two-electron product and the red color of hemoglobin results in an orange color; with higher hemoglobin concentrations it becomes red. The initial assay was developed to detect hemoglobin concentrations ranging from 7.2–16.3 g/dL. In order to detect more severe and clinically relevant degrees of anemia often found in limited-resource settings, the assay was modified to detect hemoglobin concentrations ranging from 2.5–9.1 g/dL by altering the reagent concentrations. As the initial prototype is seeking FDA-approval, the specific details of the chemical formulation of the assay remain proprietary at this time. Figure 1 demonstrates the colors associated with the clinical spectrum of moderate to severe anemia used for the modified assay [9].
Figure 1.
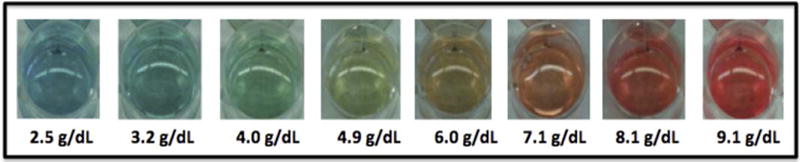
Color spectrum of the POC assay. The previously described chemical reaction between hemoglobin, hydrogen peroxide, and 3,3′,5,5′-tetramethylbenzidine was augmented to identify moderate and severe anemia with a hemoglobin range of 2.5 to 9.1 g/dL.
Study design
In order to evaluate the “real world” accuracy of this novel POC a assay in a limited-resource setting, this cross-sectional study was conducted on Angolan children with sickle cell anemia who receive care at Hospital Pediátrico David Bernardino (HPDB) in Luanda, Angola. HPDB is a large, public pediatric hospital with a high-volume outpatient sickle cell clinic that averages more than 100 outpatient visits per day. Research participants included children with sickle cell anemia having blood drawn for a routine hemoglobin determination as part of their clinic visit. The study was approved by the Ethics Committee at HPDB and by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center. For samples that were collected at the point-of-care, written, informed consent was obtained from the parent or legal guardian in Portuguese.
The primary aim of the study was to investigate the accuracy of hemoglobin determination by the POC assay, when compared to the local standard of care spectrophotometer and a reference automated hematology analyzer. At HPDB, capillary blood is routinely collected for hemoglobin determination by spectrophotometry (BTS-350, Biosystems S.A., Barcelona, Spain). Complete blood counts obtained by the Sysmex KX-21N™ hematology analyzer (Sysmex Corporation, Kobe, Japan) are reserved for acutely ill patients or when the screening hemoglobin value is below 5 g/dL. The Sysmex hemoglobin value was used as the reference value, i.e., the “gold standard” for the purposes of comparison. The study utilized both samples obtained at the point-of-care and leftover blood samples in order to analyze the accuracy of the assay on both venous and capillary blood.
For this pilot study, a single blinded reader was used to evaluate all samples. This person was not involved in the development or commercialization of the assay. In order to best simulate actual users in the field, training involved only limited hands-on practice (~10 practice tests) prior to actual use and interpretation. Although formal training is required for many POC assays, one benefit of this assay is that it can be used widely without substantial training.
Measurement of hemoglobin concentration
For samples collected at the point-of-care, capillary blood was obtained for routine spectrophotometric hemoglobin measurement and an additional 10 μL of capillary blood was collected by an end-to-end capillary tube for the POC assay. As the reaction is sensitive to the volume of blood collected, we utilized collection tubes of specific volumes and via capillary action, exactly 10 μL of blood is wicked into the system and automatically ceases upon filling of the tube. Though at times there could be excess blood on the ends of this capillary tube, this volume is insignificant compared to the sample volume and therefore would minimally affect the result. As an additional safeguard, the person performing the test was instructed to wipe off any additional blood at the ends of the tube, if present. The Supporting Information video demonstrates the collection of 10 μL of blood using the capillary tube. After collection, the capillary was placed into a 1.5-mL Eppendorf tube, which was prefilled with 0.5 mL of the POC reagents in the United States approximately one month prior to use; the tubes were covered with aluminum foil after preparation to prevent the exposure to light, which could affect the chemical reaction. For the previously collected venous blood samples, 10 μL of blood was pipetted from the EDTA tube and added to the pre-filled Eppendorf tube.
After the blood was added to the POC reagents, the tube was inverted and mixed thoroughly. The resulting color was then evaluated after 60 sec, holding the tube against a white background using a pre-printed copy of the color scale (Fig. 1). Because the reagents and the reaction are light sensitive, results are best interpreted after 1 min. However, the color of the test result appeared stable for at least 15 min after the completion of the reaction as previously determined [9]. The color scale depicted in Fig. 1 was printed and used as a visual comparison for test interpretation. For samples with colors that appeared between designated values, the scorer estimated the hemoglobin concentration using interval scores. The color spectrum of the assay goes from red (high hemoglobin concentration) to blue (low hemoglobin concentration) with orange and green colors in between these ranges as depicted in Figure 1. For example, a sample that is not quite as orange as the 7.1 g/dL color but not quite as yellow as the 6.0 g/dL color could be scored as 6.5 g/dL. Or, if the color was orange, but not quite as bright as the 7.1 g/dL color, the result could be interpreted as 6.9 g/dL. Figure 2 illustrates the individual steps of the visual, color-based POC assay and the Supporting Information Video depicts the entire procedure, with the process of color change to blue representing a sample with severe anemia and a hemoglobin concentration below 5 g/dL.
Figure 2.
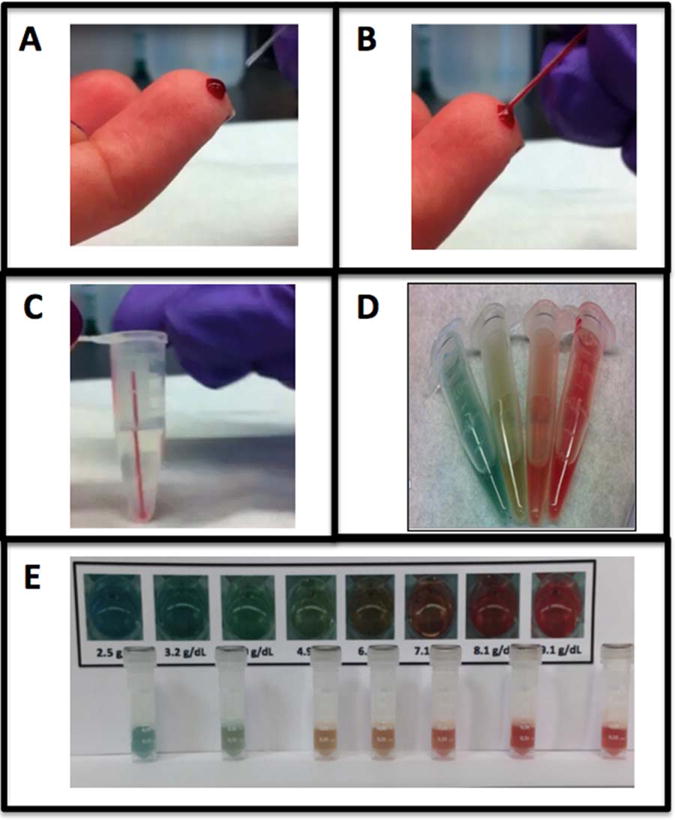
Steps of point-of-care assay. The visual, color-based assay requires 10 μL of blood, most easily collected by finger stick using an end-to-end capillary collection tube (Panel A and B). The entire 10 μL capillary tube is then placed into the 1.5mL Eppendorf tube, which is prefilled with the assay reagents (Panel C). The tube is then inverted and shaken for approximately 10 sec and the result is interpreted after 60 sec. The color changes based upon the hemoglobin concentration ranging from blue (hemoglobin ~<3 g/dL), blue/green (hemoglobin 3–5 g/dL), yellow/orange (hemoglobin ~5–7 g/dL), orange/red (hemoglobin ~7–9) to red (hemoglobin >9 g/dL). Panels D and E depict various hemoglobin concentrations across this spectrum.
Statistical analysis
All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX) except for the power calculation, which was conducted using PROC POWER in SAS version 9.3. A P value of less than 0.05 was considered significant. Pearson correlations and general linear regression models and prediction limits were used to assess associations between hemoglobin level via the color-based POC assay and the standard Sysmex hematology analyzer. The Bland-Altman method was used to calculate the mean difference in hemoglobin obtained by the POC assay and the reference Sysmex Hematology Analyzer, including the determination of the 95% limits of agreement. Pearson correlation coefficient was used for all other comparisons between the various hemoglobin measures for a single sample and also for comparisons between repeated measures of the POC assay, to assess precision of the assay and durability of the reagents, respectively. Sensitivity and specificity were calculated for the diagnosis of severe anemia using both point estimates and 95% confidence intervals using the Wilson method.
Power analysis was conducted post hoc; however, an effort was made to be more conservative than the collected data. Our hypothesis was that the two methods (POC assay and Sysmex Hematology Analyzer) would provide similar per-patient values, therefore our interest was to assess the power that our sample size of 86 subjects had to detect a clinically meaningful difference (mean absolute hemoglobin difference > 0.5 g/dL) in order to provide evidence that this hypothesis should be discarded. Further, a standard deviation of the mean difference of 1.0 g/dL (considerably higher than the observed data value) and inter-subject correlation of zero (considerably lower than the observed data value) were assumed in an effort to be conservative. Based on these assumptions a two-sided paired t-test would yield a p-value < 0.05 with 90% power. Power calculation was conducted using PROC POWER in SAS version 9.3.
Results
Accuracy of the point-of-care assay
A total of 86 independent blood samples were analyzed (40 capillary samples and 46 venous samples), comparing the visual, color-based POC assay and the reference method (Sysmex Hematology Analyzer). The average (mean ± 1 SD) hemoglobin concentration as determined by the reference method was 6.6 ± 1.3 g/dL, range 2.8–9.3 g/dL. There was a strong positive correlation between the POC hemoglobin assay and the reference values (Fig. 3A, r=0.88, P<0.0001). The mean difference between the POC assay value and the Sysmex value was 0.04 ± 0.6 g/dL and the mean absolute difference was 0.5 ± 0.4 g/dL. There were 36 samples with a POC hemoglobin value below the Sysmex value, eight with the same values, and 42 samples with a POC value greater than the Sysmex value. However, a majority of samples (54/76) were within 0.5 g/dL of the reference value and more than 90% (78/86) were within 1.0 g/dL. The correlation between the POC assay and the reference values was high for both fingerstick (r=0.75, P<0.001) and venous (r=0.93, P<0.001) blood samples. Figure 3B is a Bland-Altman plot illustrating that the correlation was consistent across the spectrum of hemoglobin values, with no bias according to the degree of anemia. Almost all values obtained by the POC assay (84/86) fell within the 95% limits of agreement, which are indicated by the dashed lines.
Figure 3.
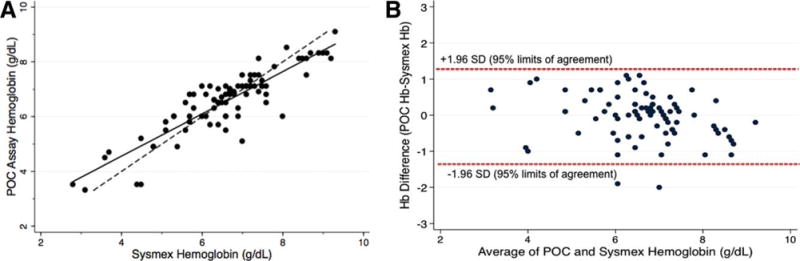
Accuracy of POC Assay. Panel A illustrates a strong positive correlation between the POC hemoglobin assay and the reference values obtained by the Sysmex automated hematology analyzer (r = 0.88, P < 0.0001). The solid line is a regression line and the dashed line represents a perfect 1: 1 correlation for comparison. Panel B shows a Bland-Altman Plot; the correlation between the hemoglobin concentration obtained by the POC assay and the reference Sysmex values are consistent across the spectrum of hemoglobin values, with no bias according to the degree of anemia. Most values obtained by the POC assay fall within the 95% limits of agreement, which are indicated by the dashed lines. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Comparison to the standard of care
For the 40 children who received fingerstick sampling to obtain capillary blood at the point-of-care, the POC assay was compared to the values obtained by spectrophotometry, which is the standard and daily method of hemoglobin determination in the Luanda sickle cell clinic. The average (mean ± 1 SD) hemoglobin concentration for these 40 samples as determined by the reference method was 6.9 ± 1.0 g/dL, range 4.8–9.2 g/dL. The mean absolute difference between the POC assay and the Sysmex value was 0.5 ± 0.5 g/dL compared to 0.7 ± 0.6 g/dL for the spectrophotometric method. Using the Sysmex values as the reference value, the hemoglobin concentrations obtained from the POC assay demonstrated a greater correlation (r=0.75, P<0.001) to the reference hemoglobin value than the routinely used spectrophotometric method (r=0.60, P<0.001).
Sensitivity and specificity for severe anemia
WHO defines severe anemia as a hemoglobin concentration less than 7.0 g/d, which is also a clinically important value, since it represents a threshold below which a blood transfusion may be required. Because the POC assay has a color representative of 7.1 g/dL (Fig. 1), this value was used as the hemoglobin concentration cutoff below which represented a clinically important degree of severe anemia. For these sensitivity and specificity analyses, hemoglobin <7.0 g/dL by Sysmex or <7.1 g/dL by the POC assay were defined as “severe anemia.” Based on the 86 reference Sysmex values, 51 patients had hemoglobin <7.0 g/dL and were designated severe anemia. For the detection of severe anemia, the POC assay had a sensitivity of 92.2% (95% Confidence Interval 80.3%–97.5%) and a specificity of 82.9% (95% Confidence Interval 65.8–92.8%). At HPDB, a hemoglobin concentration <5.0 g/dL is an indication for blood transfusion. For the detection of this degree of severe anemia, the color-based POC assay had a sensitivity of 88.9% (95% Confidence Interval 50.7%–99.4%) and a specificity of 98.7% (95% Confidence Interval 92.0–99.9%).
Reproducibility of POC assay
The POC assay was performed and interpreted in duplicate for 46 venous blood samples in order to evaluate the precision of the assay when measurements are independently obtained. For these experiments, the hemoglobin concentration was determined independently by the POC assay at two separate time points. The person performing the evaluation was blinded to sample number and any previous results. Samples were processed in a different order with the duplicate test and the evaluator was blinded to the first result. The average (mean ± 1 SD) hemoglobin concentration as determined by the Sysmex hematology analyzer for these samples was 6.4 ± 1.0 g/dL, range 2.8–9.3 g/dL. The results demonstrated excellent reproducibility (Fig. 4A, r=0.93, P<0.0001) with a mean difference of 20.1 ± 0.5 g/dL (mean absolute difference 0.4 ± 0.4 g/dL, range 0.0–1.5 g/dL).
Figure 4.
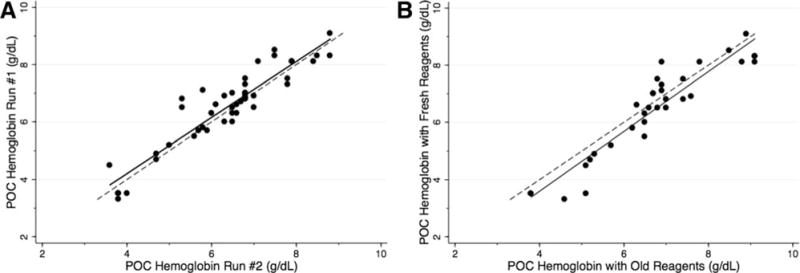
Reproducibility and Stability of the POC Assay. Panel A shows that the visual, color-based POC hemoglobin assay demonstrated excellent reproducibility when performed and interpreted in duplicate (r=0.93, P<0.0001) with a mean absolute difference of 0.4 g/dL (SD 0.4, range 0.0–1.5 g/dL). Panel B shows that the POC assay is stable with excellent reproducibility using reagents stored for 8 months in local conditions in comparison to reagents freshly prepared within one month of use (r = 0.93, P < 0.0001). The solid line is a regression line and the dashed line represents a perfect 1: 1 correlation for comparison.
Stability of POC assay reagents
In order to evaluate the stability of the POC assay reagents, results obtained from the assay using reagents freshly prepared within one month of analysis were compared to results obtained using a separate set of reagents that were stored for eight months in local humid and tropical conditions found in Luanda, Angola. The assay and reagents were stored on a shelf in a storage room at HPDB. The storage room was not primarily air conditioned, but attached rooms were intermittently air conditioned from Monday through Friday. Mean daily temperature in Luanda during the storage period was 25.0 ± 2.6°C and mean daily humidity was 78.6 ± 4.4% (www.wunderground.com). The average (mean ± 1 SD) hemoglobin concentration as determined by the Sysmex hematology analyzer for these samples was 6.3 ± 1.0 g/dL, range 2.8–9.3 g/dL. The results demonstrated excellent reproducibility despite the age and storage conditions of the reagents (Fig. 4B, r=0.93, P<0.0001) with a mean difference of 0.3 ± 0.6 g/dL (mean absolute difference 0.5 ± 0.4 g/dL, range 0–1.6 g/dL).
Discussion
WHO estimates that nearly 80% of health-care equipment in many limited-resource countries is donated by international agencies, foreign governments, or non-governmental organizations [17]. The installation, training, day-to-day utilization, and ongoing maintenance of these expensive pieces of equipment require often-overlooked financial resources. It is thus disturbing, but not surprising, that only 10–30% of donated equipment ever becomes operational in the country where it was donated, due to lack of appropriate training of laboratory personnel, insufficient long-term technical support and maintenance plans, and expensive and difficult to acquire reagents [13]. Given these challenges, the development and implementation of inexpensive and accurate point-of-care assays are urgently needed in limited-resource settings in order to rapidly, easily, and inexpensively diagnose life-threatening conditions such as severe anemia. There have been several novel, inexpensive assays for the diagnosis of anemia recently developed [9–12], including the one described here, but none has become widely available in resource-poor locations where severe anemia is prevalent and where low-cost POC technology would have the highest impact. Although the tremendous global burden of anemia has been recently documented and described through the Global Burden of Diseases Study [1], the political and philanthropic recognition of non-infectious causes of anemia (primarily inherited hemoglobinopathies and iron deficiency) remains low.
The significant morbidity and mortality associated with severe anemia, particularly among young children and pregnant women, is a long-standing problem. The need to introduce an accurate point-of-care device to detect anemia was identified by WHO as early as 1975 [7]. The requirements for such a device were reliability as a screening test, durability, not using electricity, and easy to use by local staff. In response to this calling, WHO developed the Hemoglobin Colour Scale (HbCS) in 1995. The HbCS is an inexpensive test (approximately $0.12 per test) [18] that relies on comparing the color of a drop of blood absorbed on chromatography paper with a standard red-based color scale, which is provided on a laminated card. This scale has standard colors in increments of 2 g/dL [7,8]. Despite its simplicity, the utility and reliability of the HbCS as a clinical tool to detect anemia has been questioned. The ability of the HbCS to detect any degree of anemia has ranged from 23–97% with a specificity of 41–98% [7,8,19]. Two studies even demonstrated that the clinical diagnosis of anemia was more reliable than the HbCS [20,21]. The benefits of the HbCS are that it is rapid, relatively easy to perform, and inexpensive, but there are a number of limitations surrounding its sensitivity and specificity. Although the HbCS may be useful as a first-line screening tool, its inaccuracy and low sensitivity limit more widespread use as a measure of hemoglobin concentration in a clinical setting.
The Hemocue® device (Hemocue AB, Angelholm, Sweden) is a portable, battery-operated, point-of-care photometer that measures the hemoglobin concentration based on a modified azide methemoglobin reaction [22]. The machine costs approximately $700 USD, and the per-test cost for the disposable cuvettes (bill of materials) is approximately $1 USD. The Hemocue system has been widely used for many population-based surveys of anemia and produces reliable results, usually within 1 g/dL of reference methods [23–25]. Although most reports are favorable, some studies have questioned the reliability of the Hemocue system particularly for capillary blood, and one report suggested that the microcuvette function may be decreased in high humidity environments [26–28]. Overall, the Hemocue system is rapid, easy-to-use, and accurate means to measure hemoglobin, but the cost of the disposable cuvettes, although less expensive than more advanced hematology analyzers, is likely still too expensive for widespread use in the clinical setting of limited-resource-poor countries. The currently described assay is one among several other recently developed POC assays [9–12] that have the potential to improve the diagnosis of severe anemia in resource-limited settings where anemia is common and life-threatening.
The pilot data described here demonstrate the accuracy and reproducibility of a novel, rapid and inexpensive point-of-care hemoglobin assay. The actual bill of materials for the test results in a cost of $0.26 USD ($0.08 for capillary tube, $0.08 reagent tube, and $0.09 for chemical reagents), but these costs represent retail costs for these supplies. With scale-up of the assay, supplies can be purchased at wholesale prices and it is estimated that the cost of this device will be less than $0.25. In addition to the low cost, the device does not require electricity, is inexpensive, rapid, and easy-to-use, fulfilling all of the requirements previously described by the WHO for such an assay. The ability of this particular assay to detect severe and clinically relevant degrees of anemia is critical, as these are the levels of anemia that will require clinical intervention. In settings where blood products are extremely limited, it is essential to accurately and rapidly determine which patients need to be transfused. We found that the POC assay was reproducible, reliable, and simple to use (Fig. 4). The hemoglobin concentrations obtained using the POC assay were accurate using either capillary or venous blood. There was a tendency toward greater accuracy using venous blood, but this should be further investigated with larger sample sizes in future studies. The assay reagents were stable for eight months in a humid environment without apparent loss of accuracy. The POC assay gave results that were accurate compared to both a Sysmex reference (Fig. 3) and the routinely used spectrophotometer. The sensitivity and specificity to detect severe and very severe anemia were better than those previously reported using the WHO color scale [7,8,19], although a side-by-side comparison will be necessary to make a more definitive comparison of these two POC assays. The initial report of this POC assay demonstrated that it could accurately measure hemoglobin for anemia of various etiologies [9]. This study demonstrates that the POC assay is also able to accurately determine hemoglobin concentrations in the setting of untreated sickle cell anemia (i.e. patients who have not received blood transfusions or hydroxyurea therapy), which represents a severe hemolytic anemia with significant plasma free hemoglobin. Future studies in Angola and other resource-limited settings with a high prevalence of severe anemia will further investigate the accuracy and utility of the assay for the detection of anemia due to a variety of causes (e.g. blood loss, malaria, nutritional deficiencies, etc.).
There are several limitations in this current study. First, a single person interpreted all of the POC results, which provided internal reliability with excellent intra-observer variation, but no data regarding the inter-observer validity of the assay. The “real-world” applicability of this test will require measurements with multiple different people performing and interpreting the test. Second, the color scale used for result interpretation included examples in intervals of approximately 1.0 g/dL hemoglobin (Fig. 2), which made it difficult to estimate the value to 0.1 or 0.2 g/dL precision. The earlier prototype developed to detect mild to moderate anemia had an associated smart phone application that analyzed the color automatically, which allowed more automated and precise hemoglobin determination [9]. One critique of the HbCS is that it is highly sensitive to lighting conditions and readout angle. Because the entire visual spectrum of colors (blue to green to orange to red) is used for the currently reported POC assay, there is less dependence on the ambient lighting as opposed to the WHO HbCS, which relies on only various shades of red. In addition, when interpreted against a white background, this test does not appear to be influenced by ambient lighting or readout angle, but as with the initially described assay, an objective electronic reading device such as a smartphone app would improve the variability and reduce the subjectivity of result interpretation. For more reliable and broad implementation of this POC assay, a more precise means by which to determine the hemoglobin concentration, such as an application software program with automatic scoring, will be necessary. The POC assay is also dependent upon specific volumes of both assay reagents and added blood. In this study and for this assay, we utilize collection tubes relying upon capillary action to allow for the accurate collection of 10 μL of capillary blood, but further studies with multiple perhaps less fastidious users will evaluate the likelihood of the effect of blood volumes that are not as precise. Finally, real-world applicability of this test will be important in future studies to determine if this assay favorably affects the timing and provision of certain health interventions for severe anemia, such as hospitalization or blood transfusion.
The results of this study are encouraging, but their meaning must be considered in the context of real-world application. The potential applicability of this assay is to quickly and accurately identify patients with severe anemia, who may need additional testing or urgent blood transfusion, particularly in areas where blood products are limited. The hemoglobin values obtained using the POC assay were mostly within 0.5 g/dL and almost always within 1.0 g/dL of the reference value, which is excellent compared to other POC assays and indicate that the assay could serve as a reliable screening test to identify clinically significant anemia. Transfusion thresholds vary, but many resource-limited settings, including Angola, recommend transfusion for patients with a hemoglobin concentration less than 5.0 g/dL. In this study, there were nine patients below this threshold (as determined by the reference Sysmex method) and the POC assay correctly identified eight of these nine patients. (The one that was “missed” had a POC hemoglobin value of 5.2 g/dL.) As visualized in the Supporting Information video and Fig. 1, the distinct color change to green/blue that occurs when the hemoglobin is below 5.0 g/dL is relatively easy to identify and perhaps less subjective than variations at higher concentrations of hemoglobin in the orange/red spectrum. Future studies will focus upon the utility of this assay in emergency settings and should investigate the ability of this assay to accurately identify patients with severe anemia who need more invasive testing or transfusion.
In summary, this novel, rapid and inexpensive visual, color-based POC assay designed specifically to detect more severe degrees of anemia in limited-resource settings appears to be an accurate and reproducible measure of the hemoglobin concentration. Future research using this device will focus on practical implementation, optimization of shelf-life of reagents, inter-observer variability, further evaluation of effects of environmental conditions, and evaluation of possible test interference by oxidative substances such as bacteria, antibiotics, or antimalarial. Most importantly, this assay has all of the characteristics of a POC anemia assay as recommended by WHO, and thus has the potential to be a transformational device for the quick and accurate assessment of severe anemia in limited-resource settings.
Supplementary Material
Acknowledgments
We thank the entire laboratory staff at Hospital Pediátrico David Bernardino and the patients and families for participating in this study. The funding sources did not have any role in the design of the study, the collection, analysis, or interpretation of results, or in the writing the manuscript or the decision to submit the manuscript for publication.
Contract grant sponsors: Cincinnati Children’s Research Foundation, FDA-funded Atlantic Pediatric Device Consortium, the Georgia Research Alliance, Children’s Healthcare of Atlanta, the Georgia Center of Innovation for Manufacturing, and the InVenture Prize and Ideas to Serve competitions at the Georgia Institute of Technology.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: W. A. Lam and E. A. Tyburski hold equity in the company that has licensed the technology being evaluated in the study. They are eligible to receive royalties and milestone payments under the Emory University Patent Policy. Neither Dr. Lam nor Ms. Tyburski were involved in the collection or analysis of any data described in this article. The remaining authors do not have any conflicts of interest.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 3.Lund TC, Hume H, Allain JP, et al. The blood supply in Sub-Saharan Africa: Needs, challenges, and solutions. Transfus Apher Sci. 2013;49:416–421. doi: 10.1016/j.transci.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Costello A. Integrated management of childhood illness. Lancet. 1997;350:1266. doi: 10.1016/S0140-6736(05)62471-3. [DOI] [PubMed] [Google Scholar]
- 5.Integrated Management of Childhood Illness: Conclusions WHO Division of Child Health and Development. Bull World Health Organ. 1997;75:119–128. [PMC free article] [PubMed] [Google Scholar]
- 6.Chalco JP, Huicho L, Alamo C, et al. Accuracy of clinical pallor in the diagnosis of anemia in children: A meta-analysis. BMC Pediatr. 2005;5:46. doi: 10.1186/1471-2431-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Review of the Hemoglobin Colour Scale: Report of an informal consultation. Geneva, Switzerland: World Health Organization; 2004. Available at: http://www.who.int/medical_devices/publications/en/HbCS_Research_AgendaStudy-Design.pdf (Last accessed September 5, 2015) [Google Scholar]
- 8.Hemoglobin Colour Scale: Operational Research Agenda and Study Design. Geneva, Switzerland: World Health Organization; 2004. Available at: http://www.who.int/medical_devices/publications/en/HbCS_Research_AgendaStudyDesign.pdf (Last accessed September 5, 2015) [Google Scholar]
- 9.Tyburski EA, Gillespie SE, Stoy WA, et al. Disposable platform provides visual and color-based point-of-care anemia self-testing. J Clin Invest. 2014;124:4387–4394. doi: 10.1172/JCI76666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Piety NZ, Vignes SM, et al. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin Chem. 2013;59:1506–1513. doi: 10.1373/clinchem.2013.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Koul V, Dwivedi SN, et al. Performance analysis of newly developed point-of-care hemoglobinometer (TrueHb) against an automated hematology analyzer (Sysmex XT 1800i) in terms of precision in hemoglobin measurement. Int J Lab Hematol. 2014;37:483–485. doi: 10.1111/ijlh.12314. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad NA, Awaluddin SM, Samad R, et al. Validity of point-of-care testing mission plus in detecting anemia. Int J Biomed. 2015;5:91–94. [Google Scholar]
- 13.Josephy PD, Eling T, Mason RP. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J Biol Chem. 1982;257:3669–3675. [PubMed] [Google Scholar]
- 14.Reynolds M, Lawlor E, McCann SR, Temperley U. Use of 3,3′,5,5′-tetramethylbenzidine (TMB) in the identification of erythroid colonies. J Clin Pathol. 1981;34:448–449. doi: 10.1136/jcp.34.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levinson SS, Goldman JJ. Measuring hemoglobin in plasma by reaction with tetramethylbenzidine. Clin Chem. 1982;28:471–474. [PubMed] [Google Scholar]
- 16.Zierdt WS, Zierdt CH. Occult blood testing using tetramethylbenzidine in an extraction procedure for patients on unrestricted diets. Am J Clin Pathol. 1985;83:486–488. doi: 10.1093/ajcp/83.4.486. [DOI] [PubMed] [Google Scholar]
- 17.Medical device donations: Considerations for solicitation and provision: WHO Medical device technical series. Geneva, Switzerland: World Health Organization; 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241501408_eng.pdf (Last accessed September 5, 2015) [Google Scholar]
- 18.M’Baya B, Mbingwani I, Mgawi L, et al. Validation of the hemoglobin colour scale for screening blood donors in Malawi. Malawi Med J. 2014;26:30–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Critchley J, Bates I. Hemoglobin colour scale for anemia diagnosis where there is no laboratory: A systematic review. Int J Epidemiol. 2005;34:1425–1434. doi: 10.1093/ije/dyi195. [DOI] [PubMed] [Google Scholar]
- 20.Aldridge C, Foster HM, Albonico M, et al. Evaluation of the diagnostic accuracy of the hemoglobin colour scale to detect anemia in young children attending primary healthcare clinics in Zanzibar. Trop Med Int Health. 2012;17:423–429. doi: 10.1111/j.1365-3156.2011.02944.x. [DOI] [PubMed] [Google Scholar]
- 21.Gies S, Brabin BJ, Yassin MA, Cuevas LE. Comparison of screening methods for anemia in pregnant women in Awassa, Ethiopia. Trop Med Int Health. 2003;8:301–309. doi: 10.1046/j.1365-3156.2003.01037.x. [DOI] [PubMed] [Google Scholar]
- 22.von Schenck H, Falkensson M, Lundberg B. Evaluation of “HemoCue,” a new device for determining hemoglobin. Clin Chem. 1986;32:526–529. [PubMed] [Google Scholar]
- 23.Paddle JJ. Evaluation of the hemoglobin colour scale and comparison with the HemoCue hemoglobin assay. Bull World Health Organ. 2002;80:813–816. [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, et al. Hemoglobin point-of-care testing: The HemoCue system. J Lab Autom. 2013;18:198–205. doi: 10.1177/2211068212457560. [DOI] [PubMed] [Google Scholar]
- 25.Nkrumah B, Blay Nguah S, Sarpong N, et al. Hemoglobin estimation by the HemoCue(R) portable hemoglobin photometer in a resource poor setting. BMC Clin Pathol. 2011;11:5. doi: 10.1186/1472-6890-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen HT. High humidity affects HemoCue cuvette function and HemoCue hemoglobin estimation in tropical Australia. J Paediatr Child Health. 2002;38:427–428. doi: 10.1046/j.1440-1754.2002.t01-4-00029.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen PP, Short TG, Leung DH, Oh TE. A clinical evaluation of the Hemocue hemoglobinometer using capillary, venous, and arterial samples. Anaesth Intensive Care. 1992;20:497–500. doi: 10.1177/0310057X9202000419. [DOI] [PubMed] [Google Scholar]
- 28.Neufeld L, García-Guerra A, Sánchez-Francia D, et al. Hemoglobin measured by Hemocue and a reference method in venous and capillary blood: A validation study. Salud Publica Mex. 2002;44:219–227. doi: 10.1590/s0036-36342002000300005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


