Abstract
Background
Inflow and outflow patency of the liver parenchyma is required to maximize the metabolic function of the liver. However, the definition and distribution of hepatic venous drainage regions has yet to be reported. The aim of this study was to define major hepatic venous tributaries and investigate the mean drainage volume of each territory.
Methods
Three-dimensional (3D) simulations from the livers of 100 healthy donors were reviewed for living donor liver transplantation to determine the distribution of the significant hepatic venous tributaries and the drainage patterns of each segment.
Results
The left hepatic vein (LHV), middle hepatic vein (MHV), and right hepatic vein (RHV) contributed a mean drainage of 20.7%, 32.7%, and 39.6% of the entire liver, respectively. Accessory hepatic veins accounted for remaining 7.0%. The middle right hepatic vein (MRHV) and inferior right hepatic vein (IRHV) accounted for a mean total drainage of 8.0% and 10.6%, respectively, when they present. In addition, major tributaries of hepatic veins were clearly detected, and their typical distributions were described.
Conclusions
Knowledge of hepatic venous territories is necessary for complex hepatobiliary surgery. This “venous drainage map” may provide useful information for complex liver surgery and transplantation.
Introduction
Knowledge of the vascular anatomy is important for safe liver resection and transplantation. It has been widely accepted that patency of the three major hepatic vascular structures, the hepatic artery, portal vein and hepatic vein, and biliary tree is required for maximum function of the corresponding liver territories.
Progress in liver surgery has highlighted the substantial deleterious effects of venous congestion on remnant liver function and/or regeneration.1, 2 A recent study reported that veno-occluded regions have approximately 40% of the maximum function of corresponding regions.3 As the volume of future liver remnant (FLR) is not always equal to the residual function of the liver, venous reconstruction is recommended in cases where congested regions in FLR develop because of excessive venous deprivation after hepatectomy and insufficient net functional reserve of FLR.4
Recent developments in computer simulation techniques have enabled visualization of the intrahepatic structures in three-dimensional (3D) formats from any angle preoperatively. This precise visualization of the hepatic vascular territories has provided new insights into the surgical anatomy of the liver.5 The 3D liver analysis is widely used as a component of preoperative work-up for liver resection and transplantation. However, despite recent technical innovations in imaging studies, unexpected intraoperative decision-making is frequently required during complex hepatobiliary surgery and knowledge of the surgical anatomy of the liver remains crucial in such situations.
In the history of liver surgery, the segmental anatomy of the liver has been actively studied for more than 100 years according to the patterns of portal or arterial/biliary ramification. However, because of the technical difficulty of precisely evaluating the distribution of venous drainage areas, only a few studies have described the venous ramification patterns of the liver, particularly regarding the peripheral distribution of venous tributaries. In the present study, we describe the drainage patterns of major venous tributaries of the liver based on the results of 3D vascular analysis of 100 living donors for liver transplantation.
Methods
Subjects and 3D image reconstruction
The present study comprised 100 healthy donor candidates (median age, 40 years; range, 20–63 years; male:female ratio, 52:48) who underwent 3D-CT analysis as part of preoperative assessment for living donor liver transplantation (LDLT) at the University of Tokyo Hospital between February, 2004 and May, 2009. All donor candidates underwent protocol contrast-enhanced scanning and 3D reconstruction of intrahepatic structures using liver simulation software (Organs Volume Analysis; Hitachi Medico Inc, Tokyo, Japan), as reported previously.6
Individual extraction of the portal and hepatic veins allowed free compounding with each other or with liver parenchymal data on a 3D workstation in order to determine intrahepatic vascular structures and corresponding interrelations. The use of this system allowed the vascular territories of the portal or hepatic venous tributaries to be delineated in a 3D manner, and semi-automatic estimation of the respective absolute and relative volumes.
Definition of segment and venous tributaries
Liver segments were classified according to Couinaud's classification and nomenclature used in the present study was in accordance with the Brisbane 2000 terminology.7 Segment VIII was subdivided into ventral and dorsal parts according to the anatomical findings we reported previously.8 Definitions of venous tributaries are summarized in Table 1, which presents an extension of our previous work on the venous drainage pattern of the left liver.6
Table 1.
Definition of major hepatic venous tributaries
| Tributaries of the LHV |
| Left superficial vein (LSV) |
| A tributary of the LHV or an isolated vein draining into IVC, not observed on the left portal fissure, running beneath the diaphragmatic surface of Segment II, and draining cranial portion of Segment II |
| Umbilical fissure vein (UFV) |
| A tributary arising from the LHV, MHV, or their confluence, not observed on the left portal fissure, running toward the umbilical fissure, and draining both Segment III and Segment IV |
| Tributaries of the MHV |
| Superior vein for Segment IV (V4sup) |
| A tributary of the LHV, MHV, or their confluence, not observed on the umbilical fissure, draining superior part of Segment IV, and never draining left side of umbilical fissure |
| Inferior vein for Segment IV (V4inf) |
| Tributaries of left peripheral portion of the MHV, draining inferior half of Segment IV, and usually forming the MHV trunk meeting with the drainage veins of Segment V |
| Ventral vein for Segment VIII (V8v) |
| A tributary of MHV, draining ventral part of Segment VIII and not draining dorsal part of Segment VIII |
| Intermediate vein for Segment VIII (V8i) |
| A tributary of the MHV, not observe on the main portal fissure, running between ventral and dorsal part of Segment VIII, and draining both ventral and dorsal part of Segment VIII |
| Veins for Segment V (V5) |
| Tributaries of right peripheral portion of the MHV, draining Segment V, and forming the MHV trunk meeting with the inferior veins for Segment IV |
| Tributaries of the RHV |
| Right superficial vein (RSV) |
| A tributary of the RHV or an isolated vein draining into IVC, running beneath the diaphragmatic surface of Segment VII, and draining cranial portion of Segment VII |
| Dorsal vein for Segment VIII (V8d) |
| A tributary of RHV, draining dorsal part of Segment VIII and not draining ventral part of Segment VIII |
| Accessory veins |
| Inferior right hepatic vein (IRHV) |
| An isolated vein draining into IVC, usually running along P6 and draining entire or caudal part of Segment VI |
| Middle right hepatic vein (MRHV) |
| Isolated veins draining into IVC, draining Segment VII, never draining Segment VI |
Data analysis
Clinical data were recorded using an Excel 2010 (Microsoft) spreadsheet and analyzed using the statistical software, JMP 9 (SAS Institute Japan, Tokyo, Japan). Non-parametric methods were used for all statistical analyses. P-values < 0.05 were considered statistically significant. All analyses in the present study were performed in accordance with the ethical guidelines for clinical studies of the University of Tokyo Hospital.
Results
Volumetric parameters for individual Counaud's segments and venous drainage areas of major hepatic venous tributaries are summarized in Table 2. The sum of the mean drainage areas of the left hepatic vein (LHV), the middle hepatic vein (MHV), and the right hepatic vein (RHV) accounted for a mean proportion of 93.0%. The remaining 7.0% of the liver is drained by short hepatic veins and accessory veins including the middle right hepatic vein (MRHV)9 and inferior right hepatic vein (IRHV).10
Table 2.
Volumetric data for Couinaud's segments and venous drainage areas
| Volume (mL) | vs. TLV (%) | |
|---|---|---|
| Total Liver Volume | 1138.0 ± 208.8 | 100 |
| Left liver | 377.0 ± 81.6 | 33.2 ± 4.7 |
| Right liver | 717.1 ± 144.4 | 63.0 ± 5.1 |
| Segmental volume | ||
| Segment I | 89.3 ± 46.5 | 7.6 ± 3.3 |
| Segment II | 94.1 ± 35.0 | 8.3 ± 3.0 |
| Segment III | 112.6 ± 38.8 | 10.0 ± 3.2 |
| Segment IV | 128.6 ± 37.7 | 11.3 ± 2.8 |
| Segment V | 130.2 ± 53.1 | 11.5 ± 4.7 |
| Segment VI | 137.9 ± 63.7 | 12.1 ± 4.8 |
| Segment VII | 167.7 ± 75.0 | 14.6 ± 5.6 |
| Segment VIII | 269.3 ± 78.8 | 23.8 ± 5.6 |
| Venous drainage area | ||
| Left hepatic vein | 234.2 ± 61.6 | 20.7 ± 4.4 |
| Middle hepatic vein | 369.3 ± 96.6 | 32.7 ± 7.7 |
| Right hepatic vein | 456.7 ± 188.1 | 39.6 ± 12.5 |
| Accessory veins | ||
| Middle right hepatic veina | 88.4 ± 53.7 | 8.0 ± 4.8 |
| Inferior right hepatic veinb | 117.3 ± 75.6 | 10.6 ± 6.3 |
Figures represent mean ± SD. TLV: total liver volume.
Average of 20 donors.
Average of 34 donors.
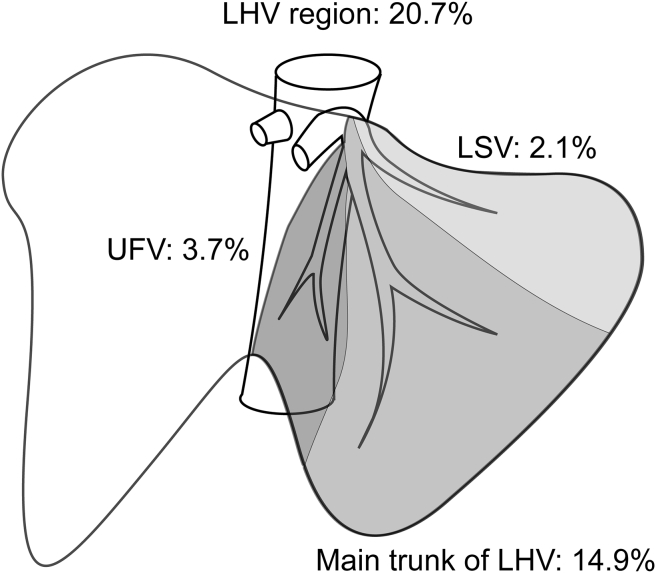
Left hepatic vein region
LHV accounted for a mean proportion of 20.7% of the entire hepatic venous drainage. Fig. 1 presents the typical distribution and volumetric data for major venous tributaries of LHV. The left superficial vein (LSV) and the umbilical fissure vein (UFV) were observed in all subjects, with a significant diameter (>3 mm) for each venous tributary observed on CT imaging in 46 and 96 of the 100 patients, respectively. The UFV was found to drain into LHV in the majority of cases (87.2%). However, the UFV was occasionally seen draining into MHV (7.3%). LSV drains segment II, and the UFV drains both segments III and IV. LSV accounted for a mean proportion of 22.9% of the drainage of segment II, and the UFV accounted for a mean proportion of 18.5% of the drainage of the medial part of segment III and 23.9% of the lateral part of segment IV. The majority of the volume of segments II and III were drained by the main trunk of LHV. Of note, a mean proportion of 76.1% of segment IV was observed draining into the MHV, with the remaining 23.9% of segment IV draining into LHV.
Figure 1.
Drainage area of left hepatic vein (LHV) tributaries
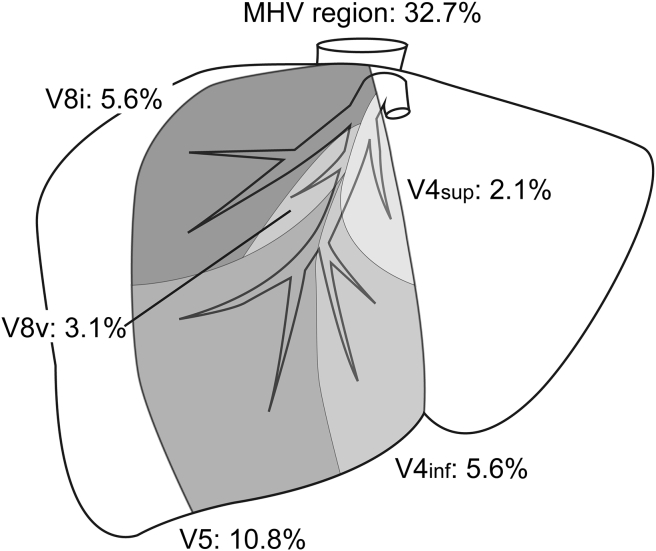
Middle hepatic vein region
MHV accounted for a mean proportion of 32.7% of the entire hepatic venous drainage. MHV has four major venous branches on both sides (Fig. 2); the superior vein for segment IV (V4sup) draining the upper part of segment IV and meeting with MHV near its root; the inferior vein for segment IV (V4inf) creating the left junction of MHV; the intermediate vein for segment VIII (V8i) running between the ventral and dorsal portal branches of segment VIII; and the drainage vein for segment V (V5) creating the right junction of MHV. The other major tributaries from segment VIII are named as ventral branches of segment VIII (V8v). Among these, V8i, V4inf, and V5 were observed in all individuals in the present study. V4sup is typically thin and was identifiable on CT imaging in 64% of study subjects. Several tributaries classified as V8v were observed in 52% of individuals. Among them, V8v joining MHV below V8i was observed in 67% of subjects and above V8i in 29% of subjects. The remaining 5.5% of the entire liver volume in the MHV region was found to be drained by small branches directly joining the main trunk of MHV. The main trunk of MHV typically drains into the ventral part of the right paramedian sector, with the dorsal part drained by the RHV, thereby creating a clear watershed at the plane of the right paramedian portal pedicle and V8i. This watershed zone is always clearly observable in segment VIII, as described in our previous work.5 However, the drainage pattern of the caudal part of the right paramedian sector (i.e., segment V region) varies among individuals, with V5 occasionally extending to the right lateral sector and draining a proportion of segment VI (n = 8, 8%). Approximately half of the right paramedian sector is drained by MHV (mean proportion, 56.1%), including the drainage region of V8i (mean proportion, 15.6%), with the remaining part drained by RHV (mean proportion, 43.9%). Patients with a MHV drainage area greater than RHV drainage area are considered at greater risk of venous congestion following living donor liver transplantation.11 In the present study, 19 cases (19.0%) had a MHV drainage area greater than RHV drainage area.
Figure 2.
Drainage area of middle hepatic vein (MHV) tributaries
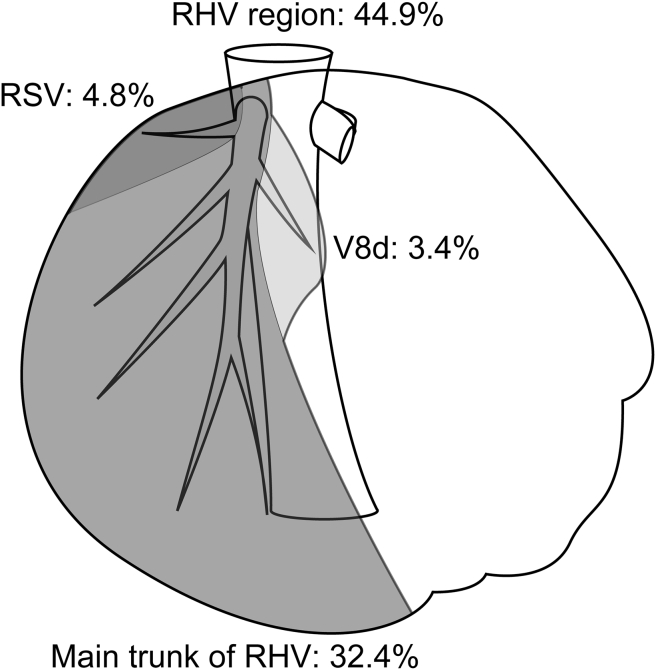
Right hepatic vein region
RHV (also termed as “superior” right hepatic vein in some papers) was found to account for a mean proportion of 39.6% of the entire hepatic venous drainage in the present, and is known to have two major branches (Fig. 3); the right superficial vein (RSV), and the dorsal vein for segment VIII (V8d). RSV, which is a counterpart of LSV, was observed in 98% subjects and typically has moderate thickness. In the present study, RSV accounted for a mean proportion of 4.8% of the entire hepatic venous drainage, and accounted for a mean proportion of 10.9% of RHV region. RSV is typically located directly inferior to the caval ligament and is occasionally as thick as the main trunk of RHV and was found to account for a mean proportion of >20% of the right hepatic venous territory (n = 20, 20%). Another major branch of RHV is the V8d, which drains a major proportion of the dorsal area of the right paramedian sector. The V8d was observed joining RHV in all cases and was found to account for a mean proportion of 5.0% of the entire hepatic venous drainage. In addition, V8i occasionally drains into RHV instead of the ordinal ramification of MHV.12 In the present study population, six (6%) individuals were found to have aberrant ramifications of the V8i draining into RHV.
Figure 3.
Drainage area of right hepatic vein (RHV) tributaries
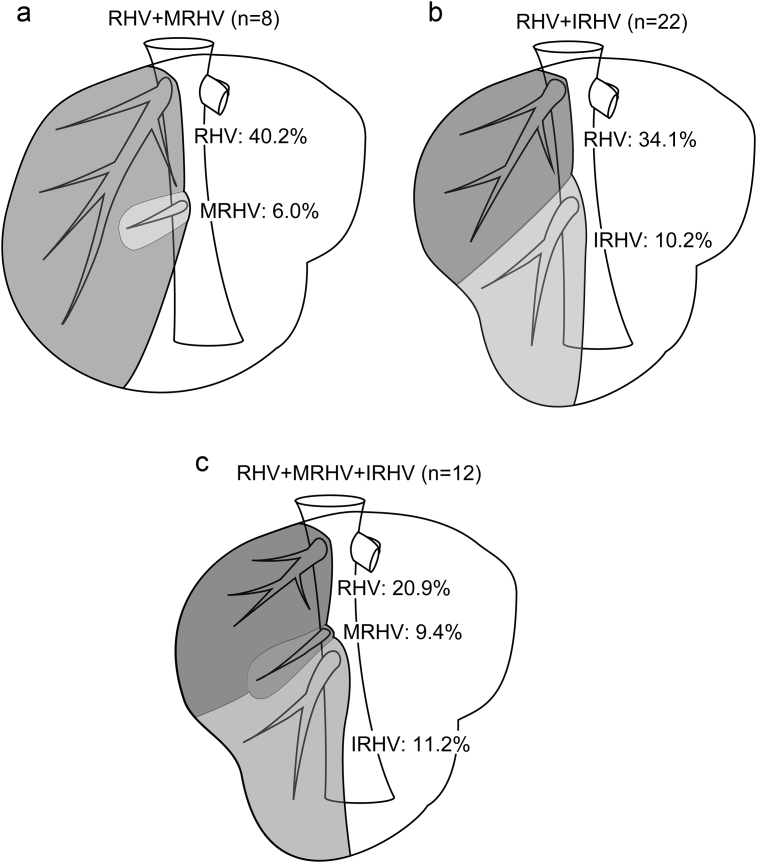
The RHV drainage territory is unique in having accessory veins, such as the middle MRHV, and the IRHV. Of note, IRHV is defined as an isolated vein which drains segment VI and joins IVC independently. Any accessory hepatic vein found to drain segment VII, but with no contribution to drainage of segment VI, is defined as MRHV rather than IRHV. In the present study population, 20% and 34% of subjects were found to have MRHV and IRHV with significant diameters (>3 mm) on CT imaging, respectively. MRHV and IRHV were found to account for a mean proportion of 8.0% and 10.6% of the entire hepatic venous drainage, respectively. Multiple MRHV with diameters of less than 3 mm may be observed as short hepatic veins intraoperatively; however, such veins are not clearly identifiable on CT imaging and, as such, were not taken into account in the 3D analysis of the present study. Similarly, IRHV with diameters of less than 3 mm may present but not observed on CT imaging. By contrast, patients in whom IRHV was detected had only one IRHV, and no donor had two or more IRHV. Of note, in patients with the presence of MRHV alone (n = 8), IRHV alone (n = 22), and coexistence of MRHV and IRHV (n = 12), RHV accounted for a mean proportion of 40.2%, 34.1%, and 20.9% of the entire hepatic venous drainage, respectively (Fig. 4a–c).
Figure 4.
(a) Drainage area of the right hepatic vein (RHV) region in cases where the middle right hepatic vein. (MRHV) was present (n = 8). (b) Drainage area of the right hepatic vein (RHV) region in cases where the inferior right hepatic vein. (IRHV) was present (n = 22). (c) Drainage area of the right hepatic vein (RHV) region in cases where the middle right hepatic vein. (MRHV) and inferior right hepatic vein (IRHV) were present (n = 12)
Based on volumetric analysis, segment VI was found to be drained by RHV, IRHV, and rarely V6, which drains into MHV as reported previously.13 In patients with an IRHV present (n = 34), a mean proportion of 70.8% of segment VI was found to be drained by IRHV, with the entire part of segment VI drained exclusively by a large IRHV in 11 cases (11/34, 32%). In the 11 cases found to have a large IRHV in the present study, IRHV was found to drain the entire part of segment VI, in addition to a proportion of segment VII, accounting for a mean proportion of 28.8% of the drainage area of segment VII and 14.5% of the drainage area of the entire liver, respectively.
Venous drainage of the caudate lobe
The caudate lobe is typically drained by short hepatic veins directly connecting with the inferior vena cava. In the present study, the short hepatic veins contributed to a mean proportion of 1.8% [interquartile range (IQR), 1.1%–2.6%] of the total hepatic venous drainage, which corresponds to 24.5% (IQR, 14.4%–41.7%) of segment I. The contribution of the short hepatic veins was calculated by subtracting the drainage area of all tributaries from the total liver volume. Of the 100 donors, 80 (80%) donors had relatively thick caudate veins detectable on CT imaging. The caudate veins were named as the proper hepatic veins of the caudate lobe by Kogure et al.14 and are reportedly detected in all adult cadaveric livers. However, no caudate veins with a significant diameter (>3 mm) were observed on CT imaging in the present study. Accordingly, detailed volumetric analysis was difficult for segment I.
Discussion
To date, several reports15, 16, 17 described the anatomic variation of hepatic veins. However, no nomenclature for the hepatic venous tributaries has yet been established, and there is a lack of data regarding the hepatic regions drained by each tributary. In the present study, we describe major tributaries of hepatic veins and created a “venous drainage map” to clarify the typical ramifications and distribution of the venous tributaries. The nomenclature of venous tributaries used in the present study was based on the segment drainage areas, with characteristic veins named individually. Additionally, the appellation commonly used to describe the hepatic territories during living donor liver transplantation was adopted as a priority.
LHV drains approximately 20% of the entire liver. Congestion of LHV territory seldom becomes a clinical issue as the liver volume drained by LHV is typically small enough relative to remnant liver volume. The main trunk of LHV, supplied by the veins draining segment II (V2) and segment III (V3) and running between segment II and III, forms a common trunk with MHV and drains into IVC. In rare cases, LHV and MHV do not form a common trunk and independently join IVC, as reported by Nakamura;17 however, LHV and MHV were observed creating a common trunk in the present study population. LSV running beneath the diaphragmatic surface of segment II and draining the cranial part of segment II is rarely observed in the transection plane during hepatectomy. However, LSV occasionally communicates with the left inferior phrenic vein and directly drains into IVC6 despite this communication being rarely identified on CT imaging. Surgeons should take care to avoid injury to LSV during mobilization of the left liver. The UFV runs between segments III and IV and drains into LHV, and occasionally into MHV. Therefore, the UFV is used as a landmark vein between segments III and IV during liver transection, for instance during anatomic resection of segment IV.
From a clinical standpoint, congestion of the MHV region, which was found to drain approximately 30% of the entire liver in the present study, is often more important than the other major hepatic veins as MHV runs along the mid-plane of the liver, known as the Rex-Cantlie line, and drains both sides of the liver. As discussed above, MHV drains 76.1% of segment IV. Accordingly, deprivation of MHV after extended right hepatectomy or left liver grafting without MHV may result in significant venous congestion in the majority of segment IV. The findings of the present study indicate approximately 26% of the left liver will be congested after such surgery unless the majority of segment IV is drained by LHV. The line of resection in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is usually determined along the falciform ligament but sometimes occurs along the mid-plane of the liver. In the cases where the transection line is set along the mid-plane of the liver, significant reliance on MHV for segment IV drainage may contribute to the high morbidity associated with this procedure.18, 19 In the present study, the combined proportion of the entire liver drained by V8i, V8v, and V5 was 19.5%, representing 31.0% of drainage of the right liver. This finding highlights the importance of V8i and V5 reconstruction during right liver grafting without MHV.20
RHV drains the largest hepatic territory of all hepatic veins, accounting for 39.6% of the venous drainage of entire liver. Although RSV is the counterpart of LSV, RSV was found to have a significant diameter in almost all patients included in the present study, whereas LSV had a significant diameter in approximately half of all patients. However, LSV was observed to have a significant diameter in almost all cases on intraoperative ultrasonography, differing from the results of CT imaging. This discrepancy may be attributable to the fact that LSV runs immediately inferior to the left diaphragm and the effect of the heart beating may interfere with visualization of LSV on CT imaging. RSV seldom communicates with the right inferior phrenic vein, which directly communicates with IVC,21 whereas LSV is occasionally observed communicating with the left inferior phrenic vein.6 A clinically important finding of the present study is the demonstration that RSV frequently runs immediately inferior to the caval ligament. This finding indicates careful handling of the caval ligament by ligation or sealing with adequate energy devices is particularly important during mobilization of the right hemiliver. The main trunk of RHV is formed by veins of segments VI (V6) and VII (V7), whose ramifications are multifarious and difficult to simply classify, as reported elsewhere.22 The presence of IRHV, which drains the entirety of segment VI, is associated with the absence of V6, with the main trunk of RHV instead formed exclusively by V7. In the present study, V8d was observed in all cases and always drained into RHV. Generally, division of V8d is not a clinical concern during extended right lateral sectorectomy, sacrificing RHV, as the proportion of the remnant liver volume drained by V8d is relatively small.
Precise understanding of the venous drainage pattern of segment VI is clinically important as preservation of IRHV reportedly expands the indications for surgery in cases requiring concomitant resection of RHV because of tumor invasion.23 The presence of a sizable MRHV or IRHV is also clinically important as they are frequently reconstructed during living donor transplantation using right liver grafts at our institution.24, 25
Although detailed descriptions of the hepatic venous drainage patterns have not previously been reported, the gross venous drainage territories have been reported in several studies. Newmann et al.26 calculated the drainage volume of four major branches of MHV by 3D CT imaging and classified the branching pattern of MHV into three types, with a particular focus on V4inf and V5. In their report, V5 (which extends to segment VI) was found to be present in 10% of cases, a result corroborated by the findings of the present study. Radtke et al.11 investigated the drainage territories of major hepatic veins, including the accessory hepatic veins, and provided classifications according to venous dominance type. Two categories, the large MHV territory type and small RHV with large accessory vein (MRHV or IRHV) territory type, were defined as high risk for venous congestion after liver transplantation. In the present study, V8i and V5 accounted for a mean proportion of 5.6% and 10.8% of total hepatic venous drainage, a relatively large proportion of the venous drainage of the remnant liver volume even in average-sized donor grafts. Hence, donor grafts with a larger MHV than RHV are considered high risk for congestion after living donor transplantation if the corresponding veins are not reconstructed. As described previously, RHV region tended to be smaller (20.9%) in individuals in whom MRHV and IRHV were present than in those in whom either of these veins where absent. In such cases, congestion in the territories of the accessory hepatic veins may be large enough to be clinically significant.
Although a proportion of patients do not require venous reconstruction despite deprivation of major venous drainage routes because of the presence of peripheral venous connections offering a bypass route for venous drainage,27 such venous connections are typically thin and difficult to detect by preoperative imaging studies. Therefore, detailed surgical planning and knowledge of vascular anatomy is crucial for reducing surgical complications and poor outcomes.
Calculation of venous drainage area is not always required in surgical planning for typical hepatectomies. However, for complex liver resections or living donor liver transplantation, it is strongly recommended to calculate drainage areas for major venous tributaries to determine whether venous reconstruction is necessary or nor4 to avoid excessive venous congestion or preservation of hepatic functional reserve especially for the cases with marginal future liver remnant volumes.
There is currently a lack of consensus regarding the definition of the hepatic venous tributaries, even among liver surgeons. In addition, the names of the hepatic venous tributaries have not been summarized in detail by previous studies. In a proportion of previous reports, LSV and UFV are termed the left superior vein and left medial vein, respectively,17, 28 with the UFV occasionally referred to as the fissural vein. Regarding MHV tributaries, the names of V4, V5 and V8 are widely accepted, particularly in the setting of liver transplantation; however, they are not typically classified in detail into V4sup, V4inf, V8i, and V8v. In a small proportion of previous reports, V4sup, V4inf, V8i, and V5 were termed the left superior branch, left inferior branch, right superior branch, and right inferior branch, respectively.26 Further, RSV is often referred to as the right superior vein, similar to LSV. Few reports have provided definitions of V8d.12 The terms MRHV and IRHV are also widely used among hepatobiliary surgeons.
In the present study, we provide simple definitions of major hepatic venous tributaries based on the findings of 3D venography using CT imaging. We believe these unified definitions will have utility in increasing knowledge of hepatic venous anatomy. In the present report, the drainage area of every major tributary was defined and demonstrated to contribute to the drainage of significant corresponding liver volumes. This “venous drainage map” derived from the results of the present study demonstrates the typical drainage pattern of hepatic veins and may have utility in increasing understanding of hepatic venous anatomy.
In conclusion, we defined major hepatic venous tributaries and investigated the drainage volumes for each territory using 3D liver analysis software. The demonstration of the hepatic venous anatomy and corresponding drainage patterns may provide practically useful guides for decision making related to vascular reconstruction during complex hepatobiliary surgery.
Funding sources
None.
Conflicts of interest
None to declare.
Contributor Information
Junichi Shindoh, Email: shindou-tky@umin.ac.jp.
Norihiro Kokudo, Email: KOKUDO-2SU@h.u-tokyo.ac.jp.
References
- 1.Maema A., Imamura H., Takayama T., Sano K., Hui A.M., Sugawara Y. Impaired volume regeneration of split livers with partial venous disruption: a latent problem in partial liver transplantation. Transplantation. 2002;73:765–769. doi: 10.1097/00007890-200203150-00019. [DOI] [PubMed] [Google Scholar]
- 2.Lee S., Park K., Hwang S., Lee Y., Choi D., Kim K. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. doi: 10.1097/00007890-200103270-00021. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi Y., Ishizawa T., Miyata Y., Yamashita S., Masuda K., Satou S. Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol. 2013;58:247–253. doi: 10.1016/j.jhep.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Mise Y., Hasegawa K., Satou S., Aoki T., Beck Y., Sugawara Y. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. 2011;98:1742–1751. doi: 10.1002/bjs.7670. [DOI] [PubMed] [Google Scholar]
- 5.Shindoh J., Mise Y., Satou S., Sugawara Y., Kokudo N. The intersegmental plane of the liver is not always flat–tricks for anatomical liver resection. Ann Surg. 2010;251:917–922. doi: 10.1097/SLA.0b013e3181d773ae. [DOI] [PubMed] [Google Scholar]
- 6.Shindoh J., Kokudo N., Satou S., Sugawara Y., Makuuchi M. Volumetric analyses of venous variations in the left liver using 3D-CT venography. Hepato-gastroenterology. 2006;53:831–835. [PubMed] [Google Scholar]
- 7.Belgihiti J., Clavien P., Gadzijev The Brisbane 2000 terminology of liver anatomy and resections. HPB Off J Int Hepato Pancreato Biliary Assoc. 2000;2:333–339. [Google Scholar]
- 8.Shindoh J., Satou S., Aoki T., Beck Y., Hasegawa K., Sugawara Y. Hidden symmetry in asymmetric morphology: significance of Hjortsjo's anatomical model in liver surgery. Hepato-gastroenterology. 2012;59:519–525. doi: 10.5754/hge11529. [DOI] [PubMed] [Google Scholar]
- 9.Cheng Y.F., Huang T.L., Chen C.L., Chen T.Y., Huang C.C., Ko S.F. Variations of the middle and inferior right hepatic vein: application in hepatectomy. J Clin Ultrasound JCU. 1997;25:175–182. doi: 10.1002/(sici)1097-0096(199705)25:4<175::aid-jcu4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Makuuchi M., Hasegawa H., Yamazaki S., Bandai Y., Watanabe G., Ito T. The inferior right hepatic vein: ultrasonic demonstration. Radiology. 1983;148:213–217. doi: 10.1148/radiology.148.1.6304809. [DOI] [PubMed] [Google Scholar]
- 11.Radtke A., Sotiropoulos G.C., Sgourakis G., Molmenti E.P., Schroeder T., Saner F.H. Hepatic venous drainage: how much can we learn from imaging studies? Anatomic-functional classification derived from three-dimensional computed tomography reconstructions. Transplantation. 2010;89:1518–1525. doi: 10.1097/TP.0b013e3181dd6bac. [DOI] [PubMed] [Google Scholar]
- 12.Cho A., Okazumi S., Makino H., Miura F., Ohira G., Yoshinaga Y. Relation between hepatic and portal veins in the right paramedian sector: proposal for anatomical reclassification of the liver. World J Surg. 2004;28:8–12. doi: 10.1007/s00268-003-7038-0. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T., Sugawara Y., Kishi Y., Akamatsu N., Matsui Y., Kokudo N. Reconstruction of the middle hepatic vein tributary in a right lateral sector graft. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2005;11:309–313. doi: 10.1002/lt.20349. [DOI] [PubMed] [Google Scholar]
- 14.Kogure K., Kuwano H., Yorifuji H., Ishikawa H., Takata K., Makuuchi M. The caudate processus hepatic vein: a boundary hepatic vein between the caudate lobe and the right liver. Ann Surg. 2008;247:288–293. doi: 10.1097/SLA.0b013e31815efd8d. [DOI] [PubMed] [Google Scholar]
- 15.Couinaud C. 1989. Surgical anatomy of the liver revised. Paris, France. [Google Scholar]
- 16.Gupta S.C., Gupta C.D., Gupta S.B. Hepatovenous segments in the human liver. J Anat. 1981;133(Pt 1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura S., Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 1981;152:43–50. [PubMed] [Google Scholar]
- 18.Aloia T.A., Vauthey J.N. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): what is gained and what is lost? Ann Surg. 2012;256:e9. doi: 10.1097/SLA.0b013e318265fd3e. author reply e16–9. [DOI] [PubMed] [Google Scholar]
- 19.Schadde E., Raptis D.A., Schnitzbauer A.A., Ardiles V., Tschuor C., Lesurtel M. Prediction of mortality after ALPPS Stage-1: an analysis of 320 patients from the international ALPPS registry. Ann Surg. 2015;262:780–785. doi: 10.1097/SLA.0000000000001450. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 20.Akamatsu N., Sugawara Y., Nagata R., Kaneko J., Aoki T., Sakamoto Y. Adult right living-donor liver transplantation with special reference to reconstruction of the middle hepatic vein. Am J Transpl. 2014;14:2777–2787. doi: 10.1111/ajt.12917. [DOI] [PubMed] [Google Scholar]
- 21.Torzilli G., Montorsi M., Palmisano A., Del Fabbro D., Gambetti A., Donadon M. Right inferior phrenic vein indicating the right hepatic vein confluence into the inferior vena cava. Am J Surg. 2006;192:690–694. doi: 10.1016/j.amjsurg.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Hata F., Murakami G., Hirata K., Kitagawa S., Mukaiya M. Configuration of hepatic veins in the right surgical lobe of the human liver with special reference to their complementary territorial relationships: morphometric analysis of controlled specimens with clearly defined portal segmentation. Okajimas Folia Anat Jpn. 1999;76:1–16. doi: 10.2535/ofaj1936.76.1_1. [DOI] [PubMed] [Google Scholar]
- 23.Makuuchi M., Hasegawa H., Yamazaki S., Takayasu K. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet. 1987;164:68–72. [PubMed] [Google Scholar]
- 24.Sugawara Y., Makuuchi M., Akamatsu N., Kishi Y., Niiya T., Kaneko J. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2004;10:541–547. doi: 10.1002/lt.20129. [DOI] [PubMed] [Google Scholar]
- 25.Ito K., Akamatsu N., Tani K., Ito D., Kaneko J., Arita J. The reconstruction of hepatic venous tributary in right liver living-donor liver transplantation: the importance of the inferior right hepatic vein. Liver Transplant. 2015;22:410–419. doi: 10.1002/lt.24386. [DOI] [PubMed] [Google Scholar]
- 26.Neumann J.O., Thorn M., Fischer L., Schobinger M., Heimann T., Radeleff B. Branching patterns and drainage territories of the middle hepatic vein in computer-simulated right living-donor hepatectomies. Am J Transpl. 2006;6:1407–1415. doi: 10.1111/j.1600-6143.2006.01315.x. [DOI] [PubMed] [Google Scholar]
- 27.Sano K., Makuuchi M., Miki K., Maema A., Sugawara Y., Imamura H. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241–247. doi: 10.1097/00000658-200208000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawasaki S., Makuuchi M., Miyagawa S., Matsunami H., Hashikura Y., Ikegami T. Extended lateral segmentectomy using intraoperative ultrasound to obtain a partial liver graft. Am J Surg. 1996;171:286–288. doi: 10.1016/S0002-9610(97)89570-0. [DOI] [PubMed] [Google Scholar]