Abstract
Objective
Recently, pancreaticogastrostomy (PG) has attracted renewed interest as a reconstruction technique after pancreaticoduodenectomy (PD), as it may imply a lower risk of clinical pancreatic fistula than reconstruction by pancreaticojejunostomy (PJ). We hypothesise that pancreatic exocrine insufficiency (PEI) is more common during clinical follow-up after PG than it is after PJ.
Research design and methods
This study compares the prevalence of PEI in patients undergoing PD for malignancy with reconstruction by PG versus reconstruction by PJ. PEI during the first year of follow-up was defined as the intake of pancreatic enzyme replacement therapy (PERT) within one year postoperatively and/or an abnormal exocrine function test.
Results
A total of 186 patients, having undergone surgery at two university hospitals, were included in the study. PEI during the first year postoperatively was present in 75.0% of the patients with PG, compared to 45.7% with PJ (p < 0.001). Intake of PERT within one year after surgery was found to be more prevalent in the PG group, i.e. 75.8% versus 38.5% (p < 0.001). There was a trend towards more disturbed exocrine function tests after PG (p = 0.061).
Conclusions
PEI is more common with PG reconstruction than with PJ reconstruction after pancreaticoduodenectomy for malignancy.
Introduction
In recent years, PG has attracted renewed interest as a reconstruction method after PD. This technique might imply a lower risk of clinically relevant pancreatic fistula1 (grades B and C according to the International Study Group for Pancreatic Surgery definition2), although two recent meta-analyses3, 4 were inconclusive with regard to this assumption. However, since patients are increasingly undergoing PD for premalignant disease (intraductal papillary mucinous neoplasm, neuroendocrine tumours, mucinous cysts …), associated with better survival rates, physicians involved in pancreatic surgery should focus more on postoperative function of the pancreatic remnant. Diabetes mellitus, but also steatorrhoea, altered bowel function, and frequent stools have a negative impact on the patient's quality of life.5, 6
Many patients with PG reconstruction after PD seem to present with PEI during clinical follow-up. A recent study also suggests PG as an independent risk factor for PEI.7 We hypothesise that PEI is more common during clinical follow-up after PG than after PJ.
Methods
In this retrospective multicentre observational cohort study, PEI was evaluated in two groups of patients undergoing PD for suspected or histologically proven pancreatic head malignancy at two high-volume pancreatic surgery centres (>30 PD per year8).
The patients having undergone surgery at Antwerp University Hospital had reconstruction with PG, while the patients having undergone surgery at Ghent University Hospital had reconstruction with PJ. As the two standard reconstruction techniques after PD at the respective hospitals, these particular reconstruction techniques were very familiar to the experienced surgeons involved (>200 personal cases). All hospital records of these patients since the introduction of PG at Antwerp University Hospital between 2009 and 2015 have been reviewed. Patients with preoperative clinical PEI (3 fatty stools per day or already requiring PERT) were excluded from the analysis, as were patients lost to follow up or with insufficient data retrieval.
PEI during the first postoperative year was defined as the intake of PERT within one year postoperatively and/or an abnormal pancreatic exocrine function test (with 13C-labelled mixed triglyceride breath test, faecal elastase determination or fat absorption test). PERT was initiated based on clinical grounds, when steatorrhoea was suspected. In most patients, an abnormal function test confirmed the need for PERT. As PERT, Creon® was administered. Creon® or pancreatin 300 mg is an enteric coated capsule, containing 18.000 U amylase, 25.000 U lipase and 1000 U protease.
The most commonly used function test is the 13C-labelled mixed triglyceride breath test. Few patients had a fecal elastase or a fecal fat absorption test. During a 13C-labelled mixed triglyceride breath test, 250 mg of the substrate 1.3-distearyl-(13C-Carboxyl)octanol glycerol is mixed with a test meal and digested in the small bowel through lipase. The breath test analyses the cumulative recovery of 13CO2 (cumulative dose percentage) with isotope ratio mass spectrometry. There is an excellent correlation between bowel lipase activity and 13CO2 concentrations on the breath. This test is considered more useful than the faecal elastase test for evaluating PEI after pancreatic resection. Accuracy rates for clinical symptoms, including clinical steatorrhoea, are 62% for the faecal test and 88% for the breath test.9
Pancreatic consistency was appreciated by the surgeon and noted in the operative report.
Pancreatic head resection was performed as pylorus-preserving PD or as pylorus-resecting PD. With the latter, the stomach is divided just proximal to the pylorus, and nearly the entire stomach can be preserved. Standard regional lymphadenectomy was performed.10
Reconstruction with PG was performed, suturing the pancreatic remnant to the posterior wall of the stomach. The pancreatic remnant was freed over 2 cm and introduced over this length into the stomach. Single-layer separate sutures, PDS 4/0, were applied, with no stent being used. Hepaticojejunostomy and antecolic duodenojejunostomy were performed in pylorus-preserving PD, and hepaticojejunostomy and gastrojejunostomy in pylorus-resecting PD.
Reconstruction with PJ was performed, suturing the pancreatic remnant “duct to mucosa” to the jejunal loop. An outer layer of separate sutures, Prolene 4/0, was applied between the pancreatic remnant capsula and the jejunal seromuscular layer, which was opened over the length of the anastomosis. An inner layer of 3–5 stitches, Prolene 4/0, sutured the pancreatic duct separately to a small jejunal mucosal incision. The patency of the anastomosis was checked with a silicone tube, but no stent was left behind. Hepaticojejunostomy and antecolic duodenojejunostomy were performed in pylorus-preserving PD, and hepaticojejunostomy and gastrojejunostomy in pylorus-resecting PD.
Postoperative pancreatic fistula has been assessed according to the ISPGS definition.2
Since pancreatic duct dilatation7 is considered a marker for obstructive pancreatitis due to a pancreatic head mass leading to atrophy of the pancreatic remnant, the diameter of the main pancreatic duct was assessed on preoperative CT-scan or MRI and/or on intraoperative measurement with dilatation probes. The dilatation of the pancreatic duct in the remnant at the site of implantation in the stomach or small bowel loop was also assessed on postoperative CT-scan or MRI. The pancreatic duct was considered not dilated up to 4 mm, and considered dilated when it was 5 mm or more.
The intake of proton pump inhibitors was also evaluated in the PG group because, theoretically, PEI could be explained by acid disintegration of pancreatic lipase by gastric juice.
Informed consent and approval of the local Ethical Committee was acquired (Belgian registration number: BE300201318590). This study was conducted according to the ethical principles stated in the ‘Declaration of Helsinki’ and in ‘Good Clinical Practice’.
Statistical analysis
Results were analysed using SPSS (version 23.0, Chicago, IL, USA). The data are expressed as means (standard deviation) for normally distributed continuous variables, and as medians (range) for non-normally distributed continuous variables. Categorical data are expressed as numbers (%). The normal distribution of continuous variables was assessed with the Shapiro–Wilk test. To compare medians for outcome parameters, the Mann–Whitney U test for non-normally distributed variables was used. To compare categorical data, the Chi-Square or Fisher's Exact test was used. The Wilcoxon Signed Rank test was used to compare two measurements of a single sample. A p-value <0.05 is considered statistically significant.
Results
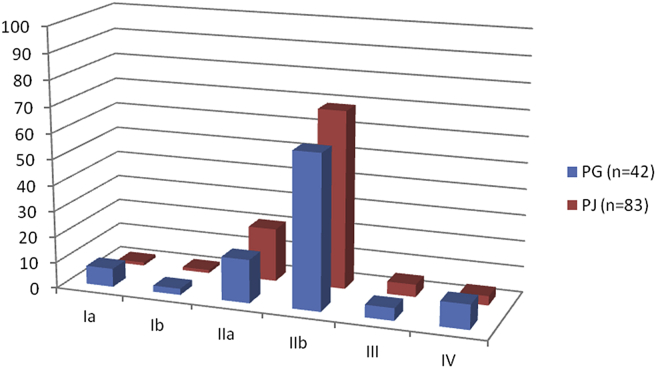
There was no significant statistical difference between groups concerning patients' characteristics (Table 1) such as age, final pathological diagnosis, American Joint Committee on Cancer (AJCC) stage for the patients with a pancreatic head adenocarcinoma (Fig. 1), consistency of the pancreatic parenchyma, diameter of the main pancreatic duct at the pancreatic transsection site on preoperative imaging and/or intraoperative measurement, incidence of clinically relevant pancreatic fistula (grade B or C2), and duration of follow-up.
Table 1.
Patient characteristics
| PG (n = 68) | PJ (n = 118) | p-Value | |
|---|---|---|---|
| Age at surgery, median (range) | 0.929 | ||
| Years | 66 (38–85) | 66 (26–88) | |
| Final pathological diagnosis, n (%) | 0.126 | ||
| Periampullary cancer | 10 (14.7) | 16 (13.6) | |
| Pancreatic adenocarcinoma | 42 (61.8) | 83 (70.3) | |
| Distal bile duct carcinoma | 5 (7.4) | 10 (8.5) | |
| Neuroendocrine carcinoma | 4 (5.9) | 0 (0) | |
| Intraductal papillary mucinous neoplasm | 1 (1.5) | 4 (3.4) | |
| Duodenal carcinoma | 4 (5.9) | 4 (3.4) | |
| Other | 2 (2.9) | 1 (0.8) | |
| Consistency of pancreatic parenchyma, n (%) | 0.573 | ||
| Soft | 26 (41.3) | 35 (43.2) | |
| Hard | 37 (54.4) | 46 (56.8) | |
| Diameter pancreatic duct on preoperative imaging, n (%) | 0.203 | ||
| Not dilated (0–4 mm) | 30 (47.6) | 56 (55.4) | |
| Dilated (5 mm or more) | 33 (52.4) | 45 (44.6) | |
| Clinically relevant postoperative pancreatic fistula, n (%) | 0.412 | ||
| None or grade A fistula | 59 (89.4) | 103 (92.0) | |
| Grade B or C fistula | 7 (10.6) | 9 (8.0) | |
| Duration of follow-up, median (range) days | 342 (45–1760) | 445 (29–2257) | 0.184 |
Figure 1.
AJCC stage of subgroup of patients having undergone surgery for pancreatic head adenocarcinoma (125/186 patients; in percentages)
Postoperative pancreatic exocrine insufficiency, scored as the intake of PERT within one year postoperatively and/or an abnormal function test, was more pronounced in the PG group compared to the PJ group: 75% (51/68) versus 45.7% (54/118) respectively (p < 0.001). The intake of PERT within one year postoperatively alone was also more frequent after PG than after PJ: 75.8% (50/66) versus 38.5% (45/117) (p < 0.001) (Table 2).
Table 2.
Results of pancreatic exocrine insufficiency (PEI) and pancreatic enzyme replacement therapy (PERT)
| PG (n = 68) | PJ (n = 118) | p-Value | |
|---|---|---|---|
| PEI (intake of PERT within 1 year postoperatively and/or abnormal function test) | 75% (51/68) | 45.7% (54/118) | <0.001 |
| PERT within 1 year | 75.8% (50/66) | 38.5% (45/117) | <0.001 |
| PEI in hard parenchymal remnant | 89.1% (33/37) | 47.8% (22/46) | <0.001 |
| PEI in soft parenchymal remnant | 53.8% (14/26) | 40.0% (14/35) | =0.311 |
50.0% (93/186) of the patients had an exocrine function test based on the suspicion of steatorrhoea: 46.8% (87/186) had 13C-labelled mixed triglyceride breath test, and 1.0% (2/186) a postoperative fecal elastase test, 5.4% (10/186) a fecal fat absorption test (some patients had more than one test). In the PG group 72.1% (49/68) patients have been tested, resulting in 55.9 (38/68) disturbed, 16.2% (11/68) normal functional tests. In the PJ group 37.3% (44/118) have been tested, resulting in 34.7% (41/118) disturbed and 2.5% (3/118) normal functional tests. Although not statistically significant because not all patients did undergo a function test, there was a trend towards more disturbed exocrine function tests after PG (p = 0.061).
In a subgroup with a pancreatic remnant with hard consistency, there was also more PEI in the group of PG compared to PJ (89.1% versus 47.8% respectively; p < 0.001). In the subgroup with soft pancreatic remnant consistency, the difference did not reach statistical significance, probably due to the low number of patients included in the analysis (Table 2).
In patients on whom both a preoperative and a postoperative 13C-labelled mixed triglyceride breath test was performed, an important decrease in cumulative dose percentage was observed in the PG group. The median cumulative dose percentage preoperatively was 34.19% (range: 3.24–54.66%) and 11.44% (range: 2.37–60.12%) postoperatively (p < 0.001). A statistically significant difference was not observed in the PJ group.
With postoperative imaging by CT-scan or MRI, the pancreatic duct in the remnant was more frequently dilated in the PG group (p = 0.017). In the PG group, 49.1% (26/53) had a dilated duct, while in the PJ group 27.9% (19/68) had a dilated duct. In the PG group some patients (4/68) also underwent redo surgery at the level of the PG, because the main pancreatic duct was entirely overgrown and could no longer be visualised by endoscopy. In the case of PJ patients, no redo surgery for anastomotic strictures was performed.
In the subgroup of patients with PG, 85.5% (53/62) of patients took proton pump inhibitors after hospital discharge, which is an important finding considering the potential acid disintegration of pancreatic lipase by gastric juice.
Discussion
In recent years, PG has attracted renewed interest as a reconstruction method after PD.1 PG might be associated with a lower risk of leakage than PJ: 8.0% versus 19.8% for clinical pancreatic fistula (grades B and C2). Although this was not under investigation in this study, the incidence of pancreatic fistula was similar. However, this PG technique also has some potential disadvantages, such as PEI.
Conclusive studies on postoperative PEI between different reconstruction techniques are scarce, but some older papers were already suggesting a more prominent functional deterioration after PG. In the case of these papers, however, only a limited number of patients were included.11, 12 In the paper by Rault et al.12 41 PJ patients were compared with only 11 PG patients by clinical evaluation of PEI (steatorrhoea and pasty stools): severe steatorrhoea was considerably more frequent in the PG group (7/10 patients) than in the PJ group (5/23 patients) (p < 0.025). In the paper by Jang et al.11 20 patients with PJ were compared with 14 patients with PG using a faecal elastase test. In the PJ group, 15/20 had severe insufficiency and 4/20 had mild insufficiency, with only one patient having a normal elastase test. In the PG group, all the patients (14 in total) had severe PEI (p = 0.045).
A recent systematic review13 of 3203 articles could identify only nine studies of sufficient quality with regard to PEI in patients with pancreatic or periampullary cancer to be included in their analysis. This review suggests that the type of reconstruction anastomosis may influence the prevalence of PEI. Only two studies with PG reconstruction after PD were retained in the analysis, with only 10 and 11 patients included respectively. Both studies reported a widely varied prevalence of postoperative PEI after PG: between 36% and 100%.6, 14
The present study observed that the prevalence of PEI after PD was higher with PG than with PJ. One could speculate on the origin of this observation.
The diameter of the main pancreatic duct at the transsection site, assessed on preoperative imaging or intraoperative measurement, was not statistically different between the groups. This is important, because pancreatic duct obstruction in the remnant usually results in fibrosis and atrophy of the pancreatic remnant, and consequently possible PEI. Postoperatively, however, there was an increased prevalence of PEI in this subgroup with hard pancreatic remnant when a reconstruction with PG was performed compared to reconstruction with PJ. The higher prevalence of PEI after PG reconstruction might also be explained by intragastric inactivation and disintegration of the pancreatic lipase by gastric acid. Of all the pancreatic enzymes, the most important for relief from symptomatic steatorrhoea, and the most fragile to gastric acid, is lipase. Lipase is less resistant to gastric disintegration than trypsin or chymotrypsin.15 Since the introduction of PERT in the 1970s, the addition of cimetidine (or later proton pump inhibitors) to pancreatic enzymes has been considered useful in patients with severe pancreatic insufficiency.16 The effect produced by acid-protected porcine preparations is equivalent to the effect produced by the conventional porcine pancreatic enzyme, but can be administered at only one-fourth of the dosage.17 Furthermore, the pancreas has a very large functional reserve, with Lankisch et al.18 reporting that only 10% of the normal total lipase activity is required to avoid steatorrhoea. In the present PG group, 85.5% of the patients took proton pump inhibitors after hospital discharge, which should prevent intragastric disintegration of pancreatic lipase activity.
Could the difference in postoperative PEI be explained by the difference in healing capacity between the gastric (PG) and jejunal (PJ) walls? Although this is pure speculation, we believe that the gastric wall heals more quickly and more prominently after performing an anastomosis to a parenchymal organ. On postoperative imaging, a dilated pancreatic duct in the remnant seemed to be more frequent in the PG group than in the PJ group. This finding suggests pancreatic remnant overgrowth by the gastric mucosa.
In the PG group, some patients (4/66) who had PEI evaluated by mixed triglyceride breath test, and with chronic intake need for PERT, even underwent redo surgery. In these patients the PG was converted to PJ in order to prevent further postoperative main pancreatic duct dilatation. The main reason for redo surgery was an entirely overgrown PG anastomosis, which no longer could be visualised by endoscopy. Our PG technique has since been modified since by the introduction of a stent through the anastomosis in order to prevent overgrowth and therefore PEI.
The most commonly used function test in the present study is the 13C-labelled mixed triglyceride breath test. An abnormal faecal elastase test is not frequently used in Belgium, because this test alone is not sufficient to obtain reimbursement and also involves extra costs for the patient. The fat absorption test on faeces is not performed on a regular basis, probably because of its inconvenience for patients and nurses.
Another very recent paper7 identifying the risk factors for PEI after PD, also using the 13C-labelled mixed triglyceride breath test, reported a higher prevalence of PEI after PG than after PJ: 65.6% versus 38.4% (p < 0.001). A hard pancreas (OR = 3.157) and PG reconstruction (OR = 2.3) were identified as independent risk factors for PEI by multivariate analysis. These findings are highly consistent with those of the present study. Another article by the same group19 identified preoperative impaired endocrine function, a hard pancreatic texture induced by pre-existing obstructive pancreatitis, and pancreatic duct dilatation in the remnant due to PG stricture as independent risk factors for PEI.
The RECOPANC trial,20 a German trial in 14 centres published in 2016, concluded very differently. This study focused on clinically relevant postoperative pancreatic fistula after PG and PJ in patients undergoing PD, but as secondary endpoint, also pancreatic exocrine function was evaluated by means of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 and the pancreatic cancer module PAN26. At 6 months after operation the intake of PERT was significantly higher in the PJ group than in the PG group (89% versus 72%, p < 0.001) but this difference did not persist at 12 months follow up. In a study by Schmidt et al.,21 using the same questionnaires, 69.6% of the PG patients required de novo medication to treat PEI compared to 59.5% of the PJ patients. This was also not significantly different probably because only 56 PG and 37 PJ patients have been evaluated. In both studies20, 21 however, no exocrine function test have been performed probably resulting in an underestimation of the incidence of PEI after PD.
The strengths of the present study lie in the fact that only patients of two centres have been evaluated where the main surgeons involved have large experience in their reconstruction technique. Learning effects by performing a new technique and variations between centres are avoided. The study also focuses on a well defined group namely patients with proven malignancies in the pancreatic head. Surgery for premalignant lesions or chronic pancreatitis was not taken into account.
But a retrospective analysis has also many weaknesses. Only patients with suspicion of steatorrhoea had an exocrine function test, which might be an important bias in this study. The drive to look for PEI could differ between centres. To avoid this bias, all patients should have one predefined function test before surgery to eliminate unknown preoperative PEI and after surgery to have a well-documented diagnosis in a prospective trial.
In conclusion
Although there is still no conclusive evidence on which reconstruction technique is to be preferred after PD, PG has attracted renewed interest because of a supposed lower incidence of leakage. However, this technique seems associated with an important functional disadvantage over the longer term, namely a higher incidence of PEI. The present study needs confirmation by a prospective trial comparing the exocrine function before and after PD with both reconstruction techniques. The obvious explanation that pancreatic lipase is disintegrated by gastric juice, and therefore PEI is to be expected in PG, does not – in our opinion – make sense in an era when proton pump inhibitors are largely administered postoperatively.
Conflict of interest
None declared.
References
- 1.Topal B., Fieuws S., Aerts R., Weerts J., Feryn T., Roeyen G. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomized trial. Lancet Oncol. 2013;14:655–662. doi: 10.1016/S1470-2045(13)70126-8. [DOI] [PubMed] [Google Scholar]
- 2.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Guerrini G., Soliani P., D'Amico G., Di Benedetto F., Negri M., Piccoli M. Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis. J Invest Surg. 2016;29:175–184. doi: 10.3109/08941939.2015.1093047. [DOI] [PubMed] [Google Scholar]
- 4.Crippa S., Cirocchi R., Randolph J., Partelli S., Belfiori G., Piccioli A. Pancreaticojejunostomy is comparable to pancreaticogastrostomy after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Langenbecks Arch Surg. 2016;401(4):427–437. doi: 10.1007/s00423-016-1418-z. [DOI] [PubMed] [Google Scholar]
- 5.Hänninen J., Takala J., Keinänen-Kikaaniemi S. Quality of life in NIDDM patients assessed with the SF-20 Questionnaire. Diabetes Res Clin Pract. 1998;42:17–27. doi: 10.1016/s0168-8227(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong T., Walters E., Varsney S., Johnson C. Deficiencies of micronutrients, altered bowel function, and quality of life during late follow-up after pancreaticoduodenectomy for malignancy. Pancreatology. 2002;2:528–534. doi: 10.1159/000066095. [DOI] [PubMed] [Google Scholar]
- 7.Hirono S., Murakami Y., Tani M., Kawai M., Okada K., Uemura K. Identification of risk factors for pancreatic exocrine insufficiency after pancreaticoduodenectomy using a 13C-labeled mixed triglyceride breath test. World J Surg. 2015;39:516–525. doi: 10.1007/s00268-014-2832-4. [DOI] [PubMed] [Google Scholar]
- 8.Coupland V., Konfortion J., Jack R., Allum W., Kocher H., Riaz S. Resection rate, hospital procedure volume and survival in pancreatic cancer patients in England: population-based study, 2005–2009. Eur J Surg Oncol. 2016;42:190–196. doi: 10.1016/j.ejso.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H., Morifuji M., Murakami Y., Uemura K., Ohge H., Hayashidani Y. Usefulness of 13C-labeled mixed triglyceride breath test for assessing pancreatic exocrine function after pancreatic surgery. Surgery. 2009;145:168–175. doi: 10.1016/j.surg.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Tol J., Gouma D., Bassi C., Dervenis C., Montorsi M., Adhm M. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang J., Kim S., Park S., Park Y. Comparison of the functional outcome after pylorus-preserving pancreaticoduodenectomy: pancreatogastrostomy and pancreatojejunostomy. World J Surg. 2002;26:366–371. doi: 10.1007/s00268-001-0234-x. [DOI] [PubMed] [Google Scholar]
- 12.Rault A., SaCunha A., Klopfenstein D., Larroudé D., Epoy F., Collet D. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg. 2005;201:239–244. doi: 10.1016/j.jamcollsurg.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Tseng D., Molenaar I., Besselink M., van Eijck C., Borel Rinkes I., van Santvoort H. Pancreatic exocrine insufficiency in patients with pancreatic or periampullary cancer. A systematic review. Pancreas. 2016;45:325–330. doi: 10.1097/MPA.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 14.Ong H., Ng E., Heng G., Soo K. Pancreaticoduodenectomy with pancreaticogastrostomy: assessment of patients' nutritional status, quality of life and pancreatic exocrine function. Aust N Z J Surg. 2000;70:199–203. doi: 10.1046/j.1440-1622.2000.01786.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiruvengadam R., DiMagno E. Inactivation of human lipase by proteases. Am J Physiol. 1988;255:G476–G481. doi: 10.1152/ajpgi.1988.255.4.G476. [DOI] [PubMed] [Google Scholar]
- 16.Regan P., Malagelada J., DiMagno E., Glanzman S., Go V. Comparative effects of antiacids, cimetidine and enteric coating on the therapeutic response to oral enzymes in severe pancreatic insufficiency. N Eng J Med. 1977;297:854–858. doi: 10.1056/NEJM197710202971603. [DOI] [PubMed] [Google Scholar]
- 17.Schneider M., Knoll-Ruzicka M., Domschke S., Heptner G., Domschke W. Pancreatic enzyme replacement therapy: comparative effects of conventional and enteric-coated microspheric pancreatin and acid-stable fungal enzyme preparations on streatorrhea in chronic pancreatitis. Hepatogastroenterology. 1985;32:97–102. [PubMed] [Google Scholar]
- 18.Lankisch P., Lembcke B., Wenken G., Creutzfeldt W. Functional reserve capacity of the exocrine pancreas. Digestion. 1986;35:175–181. doi: 10.1159/000199364. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura H., Murakami Y., Uemura K., Hayashidani Y., Sudo T., Ohge H. Predictive factors for exocrine pancreatic insufficiency after pancreaticoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 20.Keck T., Wellner U., Bahra M., Klein F., Sick O., Niedergethmann M. Pancreatogastrostomy versus pancreatojejunostomy for RECOnstruction after PANCreatoduodenectomy (RECOPANC, DRKS 00000767) Ann Surg. 2016;263:440–449. doi: 10.1097/SLA.0000000000001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt U., Simunec D., Piso P., Klempnauer J., Schlitt H.J. Quality of life and functional long-term outcome after partial pancreaticoduodenectomy: pancreatogastrostomy versus pancreatojejunostomy. Ann Surg Oncol. 2005;12:1–6. doi: 10.1245/ASO.2005.04.005. [DOI] [PubMed] [Google Scholar]