Abstract
Scope
Obese and overweight women are at high risk of developing endometrial cancer; indeed, many of endometrial cancer patients are obese. The increased number and size of adipocytes due to obesity elevate levels of circulating estrogens that stimulate cell proliferation in the endometrium. However, black raspberries (BRBs) are a promising approach to preventing endometrial cancer.
Methods and results
We examined 17 BRB constituents and metabolites (10μM or 10μg/ml, 48h) for their ability to prevent endometrial cancer cells from proliferating. Urolithin A (UA) was most able to suppress proliferation in a time- and dose-dependent manner (P<0.05). It arrested the G2/M phase of the cell cycle by upregulating cyclin-B1, cyclin-E2, p21, phospho-cdc2, and CDC25B. UA also acted as an estrogen agonist by modulating estrogen receptor-α (ERα)-dependent gene expression in estrogen receptor-positive endometrial cancer cells. UA enhanced the expression of ERβ, PGR, pS2, GREB1 while inhibiting the expression of ERα and GRIP1. Co-incubating UA-treated cells with the estrogen antagonist ICI182,780 abolished UA's estrogenic effects. Knocking down ERα suppressed PGR, pS2, and GREB gene expression but increased GRIP1 expression. Thus, UA's actions appear to be mediated through ERα.
Conclusion
This study suggests that UA modulates ERα-dependent gene expression, thereby inhibiting endometrial cancer proliferation.
Keywords: black raspberry, urolithin A, estrogen receptor, cell proliferation, endometrial cancer
1 Introduction
Uterine cancer is the most common gynecologic cancer in the Unites States, with an estimated 60,050 women newly diagnosed in 2016 and 10,470 deaths [1]. The most common histological type of endometrial cancer (which represents the majority of uterine cancers) is endometrioid endometrial cancer (EEC), typically treated by surgical removal of the uterus, fallopian tubes, and ovaries.
Epidemiological studies point to obesity as a significant risk factor for the development of endometrial cancer [2]. Obese and overweight women have 2-4 times the risk than women of normal weight, regardless of menopausal status, with ∼70-90% of EEC patients being obese [3]. Obesity typically associates with an increase in the number and size of adipocytes, which convert androgens to estrogens. The elevated levels of circulating estrogens, which act as agonists by binding to estrogen receptors, stimulate cell proliferation in the endometrium [4,5]. In contrast, tamoxifen, a selective estrogen receptor modulator (SERM) and a preventive and therapeutic regimen for breast cancer, counteracts estrogen's effects.
One promising means of restoring adipose tissue homeostasis is with dietary supplements, as whole foods contain a number of putative chemoprotective agents that might be expected to act at multiple stages in carcinogenesis [6]. This approach is problematic, however, given the critical gap in knowledge about which components in these supplements are beneficial.
Black raspberry (BRB) powders have been reported to be chemopreventive in both animal model systems and humans [7-10]. However, the effects of BRBs on endometrium have never been evaluated. Bio-fractionation studies indicate that some of BRB's preventive effects can be ascribed to their component anthocyanins and one anthocyanin metabolite, protocatechuic acid (PCA) [8,11,12]. In addition, ellagic acid (EA), a hydrolyzed form of the ellagitannins in BRB pulp and seeds (though not juice), was shown to prevent colon cancer [13,14]. Ellagitannins and EA are further metabolized by gut microbiota to urolithin A (UA) and urolithin B (UB), both of which demonstrate chemopreventive actions [13-16]. In humans, both ellagitannins and EA are poorly absorbed, but UA and UB are biodistributed throughout plasma, urine, feces, and colon and prostate tissues following consumption of ellagitannin-enriched foods or juices [17,18]. Blood concentrations of UA and UB can reach micromolar levels, suggesting far greater biological and physiological potential for UA and UB than for their precursor compounds.
More recently, seven berry types (black or red raspberries, strawberries, blueberries, noni, açaí, and wolfberry) with varying anthocyanin and ellagitannin content were shown to be almost equally capable of inhibiting tumor progression in a N-nitrosomethylbenzylamine (NMBA)-induced model of rat esophageal cancer [19]. Residue fractions of three berry types (BRBs, strawberries, and blueberries) that differ in ellagitannin content [20] were equally effective in reducing NMBA tumorigenesis in that study, suggesting that the chemopreventive effects of BRBs might derive from components other than ellagitannins and anthocyanins.
Other constituents of BRBs such as the fiber fraction (alcohol/water-insoluble)-though not the sugar fraction-also appear to contribute to cancer prevention [12]. Dietary fiber has been suggested to suppress tumor growth by altering gut microbiota and their metabolites. Short-chain fatty acids, such as acetate, butyrate, and propionate, are metabolites of fiber that appear to have protective effects. For example, dietary fiber was shown to protect against colorectal carcinoma by increasing colon levels of butyrate, which inhibits histone deacetylase (HDAC), thus impairing the proliferation of colon cancer cells and promoting their apoptosis [21]. The potential chemoprotective role of fiber and its metabolites is unclear, however, as epidemiological studies have associated the intake of lignans (phenolic components coexisting within dietary fiber) with reduced endometrial cancer in some studies [22,23] but not others [24-26]. Some studies suggest that indigestible oligosaccharides (fructooligosaccharides, galactooligosaccharides, and xylooligosaccharides) exert chemopreventive effects by fermenting sugars in the gastrointestinal tract into short-chain carboxylic acids [27] or promoting the proliferation of desirable bacterial species [28,29].
In this study, we investigated a number of reportedly bioactive BRB constituents to identify the functional modulator(s) that inhibit endometrial cancer proliferation. We evaluated the following molecules for their ability to inhibit the proliferation of human endometrial cancer cells: EA, UA, UB; two prominent BRB anthocyanins (cyandin-3-glucoside and cyanidin-3-rutinoside) and PCA; four fiber metabolites (acetic acid, butyric acid, propionic acid, and valeric acid); four lignans (lariciresinol, matairesinol, pinoresinol, and secoisolariciresinol); and three oligosaccharides (fructooligosaccharides, galactooligo-saccharides, and xylooligosaccharides). Of these 17 constituent molecules, only UA and UB suppressed endometrial cancer cell proliferation. UA, the more potent, arrested the cell cycle at the G2/M phase, while also exhibiting some estrogenic actions that might contribute to the mechanism for preventing endometrial cancer.
2 Materials and methods
2.1 Reagents and chemicals
Cyanidin-3-glucoside, cyanidin-3-rutinoside, acetic acid, butyric acid, ellagic acid, propinoic acid, protocatechuic acid, valeric acid, matairesinol, lariciresinol, pinoresinol, secoisolariciresinol, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Fructooligosaccharides, xylooligosaccharides, and galactooligosaccharides were obtained from Creative Dynamics (Shirley, NY). Charcoal/dextran-treated fetal bovine serum (FBS) was procured from HyClone (Logan, Utah). Antibodies against cyclin-B1, cyclin-E2, p21, phospho-cdc2, Myt1, CDC25B, and β-actin were obtained from Cell Signaling Technology (Danvers, MA). Primers for RT-qPCR and siRNA for estrogen receptor-α (ERα) were purchased from Thermo Fisher Scientific (Grand Island, NY).
2.2 Urolithins A and B synthesis
Urolithin A and B were synthesized in the laboratory of Dr. Chung-Wai Shiau. Briefly, resorcinol and 2-bromo-5-methoxybenzoic acids were condensed in the presence of copper sulphate and NaOH to generate dibenzopyranone, from which the methyl group was removed (via catalysis, using boron tribromide) to yield urolithin A. Resorcinol was reacted with 2-bromobenzoic acid in the presence of NaOH and copper sulphate, to produce urolithin B. Chemical structures and reactions are described in Supporting Information Fig. 1. The purities of both urolithin A and B were 94.34% (measured by NMR).
2.3 Cell culture
Endometrial cancer cells (HEC1A and Ishikawa) were kindly provided by Dr. Paul Goodfellow (Ohio State University). HEC1A cells were grown in DMEM and RRMPI-1640 (1:1) medium with 10% FBS. Ishikawa cells were cultured in DMEM medium with 10% FBS, 1% NEAA. ECC-1 cells were obtained from ATCC (Manassas, VA) and grown in RPMI-1640 medium with 5% FBS, 1% HEPES, 1% glucose, and 1% sodium pyruvate. T HESCs cells from ATCC were maintained in DMEM/F12 medium with 1% ITS+ Premix (Becton Dickinson, Franklin Lakes, NJ), 500ng/mL puromycin, and 10% charcoal/dextran-treated FBS. All the cell lines were maintained in a 37°C incubator with 5% CO2. Experiments were performed within 6 months of the cell lines being thawed or obtained from the original sources. Cell line authentication was performed by IDEXX (Westbrook, ME), which utilizes short tandem repeat (STR) profiling for characterization and authentication. To evaluate cell proliferation, endometrial cancer cells were cultured in full medium and treated with BRB components and metabolites as indicated. In experiments aimed at examining estrogenic effects, ECC-1 and Ishikawa cells were grown in phenol red-free medium with 5% charcoal/dextran-treated FBS.
2.4 Cell proliferation
Cell proliferation was monitored using the CellTiter 96 AQueous One Solution Assay (Promega, Madison, WI), according to the manufacturer's protocol and as described previously [30]. Endometrial cancer cells (2,000 per well) were seeded into 96-well plates. At each time point, each group was represented by 6-8 replicates. Cell proliferation was documented at indicated times, using a microplate reader at 490nm, with medium as the blank.
2.5 Knocking down estrogen receptor-α (ERα)
ECC-1 and Ishikawa cells (2×105) were seeded into 6-well plates and transfected with siRNA of human ERα or stealth RNAi-negative control (75pmol), using Lipofectamine RNAiMAX (Thermo Fisher Scientific) according to the manufacturer's instructions.
2.6 Reverse transcription and quantitative PCR (RT-qPCR)
Total RNA (1μg) isolated by TRIzol reagent was reverse-transcribed, using Superscript III reverse transcriptase (Thermo Fisher Scientific) at 37°C for 50m. The reaction was terminated by heating to 95°C for 3m. The synthesized cDNAs were used as templates for qPCR, which was performed as described previously [30], using the primer sequences described in Supporting Information Table 1. The reactions were amplified with 40 cycles consisting of 95° for 10s, 60°C for 30s, and 72°C for 35s. The relative expression of a gene in cells was determined by comparing the threshold cycle (Ct) of the gene against the Ct of the housekeeping gene 36B4, which is not regulated by estrogens [31], unlike GAPDH, β-actin, and 18S RNA [32,33].
2.7 Western blotting
Western blot analysis was performed as described previously [30]. Proteins were lysed in RIPA buffer with proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich). Briefly, 50μg of protein was loaded on to 10% Mini-PROTEAN TGX Gel (Bio-Rad, Hercules, CA) and then transferred to polyvinylidene difluoride (PVDF) membranes. After the membranes were blocked with BSA and incubated with primary and second antibodies, they were exposed to ECL-plus (GE Healthcare, Pittsburgh, PA). Protein bands were visualized using the ChemiDoc image system (Bio-Rad). Densitometry was analyzed by ImageJ software.
2.8 Statistical analyses
Individual results from the cell proliferation and mRNA expression were compared using Student's t-test two-sided analyses by GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). The differences in cell cycle analyses were determined by two-way ANOVA with Post-hoc tests using SigmaPlot (Systat Software, San Jose, CA).
3 Results
3.1 Urolithin A and B inhibit endometrial cancer cell proliferation
To search for and identify bioactive berry components, we evaluated the anti-proliferative effects of 17 compounds and metabolites of BRBs, using two human endometrial cancer cell lines: ECC-1 and Ishikawa (Fig. 1A). The compounds included: polyphenols (EA, UA, and UB); color compounds (cyandin-3-glucoside, cyanidin-3-rutinoside, and PCA); fiber metabolites (butyric acid, acetic acid, propionic acid, and valeric acid), lignans (secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol); and oligosaccharides (fructooligosaccharides, xylooligosaccharides, galactooligosaccharides). Most of the molecules were incubated at 10μM for 48h except for the oligosaccharides, which were used at 10μg/ml. In cell proliferation assays, UA and UB were the most effective inhibitors of cell proliferation in both cell lines (Figs. 1B-C). In contrast, the other molecules either had no effect or were stimulatory.
Figure 1.
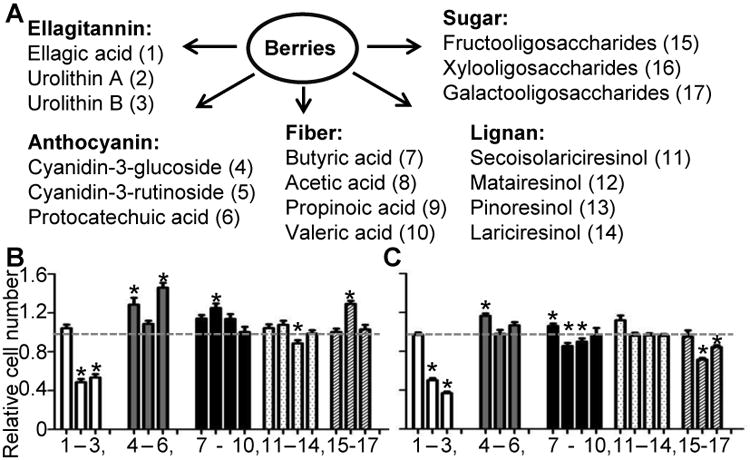
Urolithin A and B inhibit endometrial cancer cell proliferation. (A) List of 17 constituents and metabolites in BRBs. (B) ECC-1 and (C) Ishikawa cells were treated with the indicated compounds for 48h, and cell proliferation was measured using the CellTiter solution. Relative cell number was compared against vehicle-only (gray dotted line). *: P<0.05.
3.2 Urolithin A inhibits cell proliferation more effectively than urolithin B
To further examine the anti-proliferative effects of UA and UB, we investigated the time- and dose-response effects of EA, UA, and UB in three endometrial cancer cell lines (ECC-1, Ishikawa, and HEC1A) and a normal cell line (T HESCs) by treating the cells with doses up to 50μM for 48h. At the lower doses (0.1 and 1μM), UA inhibited cell proliferation more effectively than EA or UB (Fig. 2, upper panels). When these cells were treated at 10μM, UA inhibited cell proliferation to a greater extent over 7 days (Fig. 2, lower panels). Fig. 2 thus demonstrates that UA inhibits endometrial cell proliferation in a time- and dose-dependent manner, including in the normal endometrial cell line.
Figure 2.

Urolithin A suppresses endometrial cancer cell proliferation. Human endometrial cancer (ECC-1, Ishikawa, and HEC1A) and normal (T HESCs) cells were treated with UA at 0-50μM for 48h (top panels) or at 10μM for 1-7 days (lower panels). Cell proliferation was measured by the CellTiter solution. #: P<0.05 UA compared with untreated. *: P<0.05 UA compared with vehicle treatment.
3.3 UA arrests the cell cycle at the G2/M phase
To determine the anti-proliferative effects of UA, we treated endometrial cancer cells (ECC-1, Ishikawa, and HEC1A) for 48h with UA at 10μM and 50μM or with vehicle as a control. Cell cycle analysis using flow cytometry revealed that UA induced cell cycle arrest at the G2/M phase (Figs. 3A-B). Interestingly, little or no apoptosis (sub-G1 phase) was noted, suggesting that it is cell cycle arrest per se that associates with UA's anti-proliferative effect. We next used western blotting to examine UA's effects on major cell cycle regulators of the G2/M phase. UA upregulated the expression of cyclin-B1, cyclin-E2, p21, phosphor (p)-CDC2 (on Tyr15), Myt1, and CDC25B proteins (Fig. 3C and Supporting Information Fig. 2) without influencing levels of cyclin-A, p-histone H3 (on Ser10), p-WEE1 (on Ser642), or CDC25C (data not shown). These results, shown in Fig. 3, suggest that UA inhibits endometrial cancer cell proliferation by modulating genes that specifically regulate the cell cycle at the G2/M phase.
Figure 3.

Urolithin A arrests the cell cycle at the G2/M phase. (A-B) ECC-1, Ishikawa, and HEC1A cells were treated with 10μM or 50μM UA or with vehicle for 48h. Cell cycles were determined by flow cytometry. (C) Expression of cell cycle regulators was analyzed by western blotting. β-actin was the loading control. *: P<0.05; **: P<0.01; ***: P<0.001 compared with vehicle only in the same cell line. ###: P<0.001 compared between two treatment groups in the same cell line.
3.4 Urolithin A affects ERα-modulated gene expression
Urolithin A has been reported to have both estrogenic and anti-estrogenic effects in the human breast cancer cell line MCF-7 [34], but whether it specifically regulates ERα-modulated gene expression is unknown. To examine whether UA changes the expression of genes regulated by the estrogen receptors (PGR, pS2, GREB1, and GRIP1), we pretreated two estrogen receptor-positive human endometrial cancer cell lines, ECC-1 and Ishikawa, with 17β-estradiol (E2, 10nM) or the pure estrogen antagonist ICI182,780 (10μM) for 1h and then exposed them to UA (10μM) for 48h. HEC1A was not used in further studies because the expression of ERα is not clear [35,36]. Measuring mRNA levels by RT-qPCR revealed that UA suppresses ERα (Supporting Information Fig. 3) but enhances ERβ expression, whereas E2 treatments inhibit both ERα and ERβ (Fig. 4). Both UA and E2 increased the amounts of PGR, pS2, and GREB1 but decreased the level of GRIP1. In contrast, ICI182,780 either failed to modulate the expression of those genes or did so to a much lesser extent. When we treated the cells with both UA and E2, we observed changes in mRNA levels similar to those seen with UA alone. When UA-treated cells were co-treated with ICI182,780, the antagonist blocked the effects of UA (Fig. 4). ERE reporter assays demonstrated that both UA and E2 bound to estrogen receptors (Supporting Information Fig. 4). These results suggest that UA modulates gene expression that is mediated by the estrogen receptors in a manner similar to E2, thus acting as an estrogen agonist.
Figure 4.
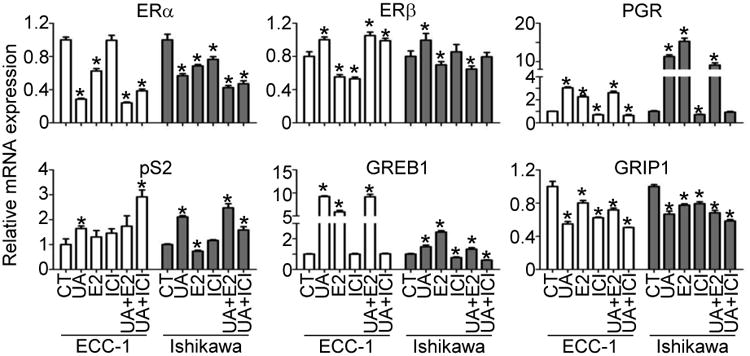
Urolithin A modulates estrogen receptor-regulated gene expression. ECC-1 and Ishikawa cells were pretreated with E2 (10nM) or ICI182,780 (ICI, 10μM) for 1h and then exposed to UA (10μM) for 48h. Total RNA was isolated, and real-time PCR was conducted using 36B4 as an internal control. *: P<0.05 compared with control (CT = vehicle-treated) cells.
3.5 Urolithin A depends on ERα to regulate estrogen receptor-mediated gene expression
To investigate whether UA regulates cell proliferation and estrogen receptor-regulated genes through ERα, we transfected ECC-1 and Ishikawa cells with siRNA of ERα. RT-qPCR confirmed that ERα mRNA was reduced by 25% in ECC-1 cells and by 68% in Ishikawa cells (Fig. 5A). Western blots agreed with RT-qPCR, revealing that levels of ERα proteins were further reduced (Fig. 5B). This inhibition of ERα induced a feedback loop to increase ERβ expression (Fig. 5A). Knocking down ERα resulted in suppression of the estrogen-upregulated genes PGR, pS2, and GREB1 and enhancement of the estrogen-downregulated gene GRIP1 in both cell lines, which is consistent with previous reports [37]. In the ECC-1 and Ishikawa cells in which ERα had been knocked-down, UA was less effective at regulating both mRNA levels of the estrogen-regulated genes (Fig. 5A) and cell proliferation (Fig. 5C). In the Ishikawa cells with reduced ERα, UA showed a synergistic effect by suppressing cell proliferation, an effect not seen in the ECC-1 cells (Fig. 5C). These results indicate that UA functions as an estrogen agonist in human endometrial cancer cells, acting through ERα.
Figure 5.

Urolithin A's actions on estrogen-regulated gene expression and cell proliferation are ERα dependent. ECC-1 and Ishikawa cells were transfected with ERα siRNA (siRNA) and/or treated with UA (10μM) for 48h. mRNA (A) and protein (B) levels. Relative cell numbers (C) are shown as a function of these variables. Total RNA was isolated and measured following real-time PCR, using 36B4 as an internal control. Cell proliferation was detected using the CellTiter solution. *: P<0.05 compared with control (CT = vehicle-treated) cells.
4 Discussion
The current study found that UA is the most effective BRB metabolite for inhibiting endometrial cancer cell proliferation. Its mechanisms include inducing cell cycle arrest at the G2/M phase and regulating protein expression associated with that phase. UA also acts as an estrogen agonist by binding to estrogen receptors at the estrogen response element (ERE) and modulating estrogen receptor-mediated gene expression. These effects are at least partly mediated through estrogen receptor-α (ERα), because knocking down ERα eliminates UA's effects on estrogen receptor-regulated gene expression.
When identifying anti-proliferative BRB metabolites, we first found that UA and UB inhibit cell proliferation in endometrial cancer cells more effectively than either ellagic acid or any of the other berry constituents or metabolites we tested. Although we and others have reported that anthocyanin fractions and their metabolite PCA are chemopreventive in rodents [8,38,39], we found no significant inhibitive effects of two commonly found anthocyanins or PCA, though this could be due to our treatment doses or short time frames. Four short-chain fatty acids, four lignans, and three oligosaccharides all failed to prevent cell proliferation in our cell culture systems. Those molecules help prevent certain other forms of cancer but, from our study, t appears that they have to be metabolized in vivo to exert chemopreventive effects.
Further characterization demonstrated that UA was much more effective than EA and UB at suppressing cell proliferation in the cell lines studied. UA suppressed proliferation partly by inducing cell cycle arrest at the G2/M phase while also modulating the expression of certain cell cycle-related proteins. Our results agree with previous findings that UA arrests the cell cycle at the G2/M phase and has the highest antiproliferative ability of the four urolithins [13-15]. In this study, UA at 10μM and 50μM induced the G2/M phase by decreasing the G1 and S phases; it did not increase the sub-G0 phase. This could be due to the low doses and short periods of UA treatment used. When we analyzed gene expression with microarrays (data not shown), we found that UA at 10μM regulated many gene pathways, including those related to apoptosis and survival. These results indicate that UA might also induce cell death, but this would need to be confirmed and the mechanisms investigated in future studies.
In breast and endometrial cells, estrogens as agonists stimulate cell proliferation. In contrast, tamoxifen, a selective estrogen receptor modulator (SERM), is used to prevent breast cancer for high-risk women and to treat breast cancer patients. Clinical trials have indicated that tamoxifen treatment increases the risk of endometrial cancer in both premenopausal and postmenopausal women [40], suggesting that it acts as an agonist in endometrial cells. Therefore, it is important to understand the effects of each estrogen agonist and antagonist in individual organ systems. In the human breast cancer cell line MCF-7, UA was reported to exhibit weaker estrogenic and slightly greater antiestrogenic activities than the phytoestrogens genistein and resveratrol [34]. UA was bound to ERα and ERβ at 0.4μM and 0.75μM IC50, respectively. Our study indicated that UA functions as an estrogen agonist by binding to ERE and regulating the expression of estrogen-regulated genes. For example, UA mimics E2 in upregulating PGR, pS2, and GREB1 while downregulating GRIP1 in estrogen receptor-positive endometrial cancer cells. Interestingly, UA suppresses ERα but enhances ERβ mRNA expression. Co-treating estrogen-regulated genes with UA and ICI182,780 greatly diminished mRNA expression, additionally supporting the hypothesis that UA is an estrogen agonist. In endometrial cancer cells with knocked-down ERα, estrogen-regulated gene expression was shown to be dependent upon ERα. In agreement with this dependence, cell proliferation was further inhibited by UA in those cells.
In vivo, UA is one of the major metabolites detected in colon tissues from colorectal cancer patients [18] and in mouse prostate, colon, and intestinal tissues [41] following the consumption of ellagitannin-enriched foods. In contrast, the ellagitannins and EA are barely detectable in these tissues, suggesting that UA is a bioactive metabolite and a potentially useful antitumor agent. In humans, both UA and its conjugated glucuronide can be detected in plasma, but the conjugated molecule is the major metabolite [16,18]. The in vitro effects of this conjugate are similar to those of UA alone: inhibition of TNFα-stimulated cell migration and regulation of inflammation-related cytokines in human endothelial cells [42]. In most of our study, we exposed endometrial cancer cells to 10μM UA. In healthy volunteers consuming 1 liter of pomegranate juice (which contains 5.13g/L ellagic acid equivalents), total ellagitannin metabolites reached concentrations of 3.3-5.4μM in plasma. Fresh and freeze-dried BRBs can contain up to 90 mg/100g and 160-225 mg/100g, respectively, of ellagic acid [6,43]. In clinical trials, urolithin levels in heathy individuals or cancer patients have not been measured after BRB consumption [7,44]. Based on the amounts of ellagic acid in freeze-dried BRBs, a very significant ingestion would be needed to reach 10μM UA in plasma. However, dietary administration of 0.4-4g/kg ellagic acid has prevented esophageal and mammary tumors in rats [45,46]. Oral or intraperitoneal injection of 40-100mg/kg ellagic acid inhibited tumor growth in nude mice [47,48], suggesting that UA can exert chemopreventive effects in vivo. In addition, the preventive effects of BRBs stem from not only ellagic acid but also from metabolites of the anthocyanins, fibers, and other trace chemicals found in these berries [12,42].
This study utilized in vitro cell proliferation assays to identify UA as the most potent inhibitor of cell proliferation of the 17 BRB constituents and metabolites examined. This inhibition by UA is mediated partly by arrest of the cell cycle at the G2/M phase, upregulation of cyclin-B1, cyclin-E2, p21, CDC25B, and p-cdc2 expession, and downregulation of Myt1. UA also acts as an estrogen by modulating the expression of estrogen-regulated genes via an ERα-dependent mechanism. Through this mechanism, UA could be chemopreventive in endometrial cancer cells. However, further mechanistic studies are needed to determine how estrogen signaling could mediate these effects.
Supplementary Material
Acknowledgments
We thank Chieh-Ti Kuo and Robert Keyes for their technical assistance and Dr. Glenn Krakower at the Clinical and Translational Scientific Institute (CTSI) of Southeast Wisconsin (NIH grant 8UL 1TR000055) for providing assistance in the preparation of this manuscript. We thank the Flow Cytometry Facility at Medical College of Wisconsin for assistance. This study was supported by the Women's Health Research Program and Faculty Affairs Committee at the Medical College of Wisconsin and the Foundation of Women's Cancer (Y.-W. H) and by NIH R01 CA148818 and American Cancer Society RSG-13-138-01-CNE (L.-S. W.).
Abbreviations
- BRB
black raspberry
- EA
ellagic acid
- UA
urolithin A
- UB
urolithin B
- PCA
protocatechuic acid
- ERα
estrogen receptor α
Footnotes
Author contributions: I.A-.B., X.S., L-S.W., G.D.S., and Y.-W.H. conceived and designed the experiments. W.Z and J.-H.C. performed the experiments. C.-W.S. synthesized UA and UB. J-.H.C. and Y.-W.H. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement: The authors have declared no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 3.von Gruenigen VE, Gil KM, Frasure HE, Jenison EL, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. Am J Obstet Gynecol. 2005;193:1369–1375. doi: 10.1016/j.ajog.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Shen Q, Celestino J, Milam MR, et al. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200:186.e1–8. doi: 10.1016/j.ajog.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi A, Wang H, Jiang G, Douglas W, et al. Endometrial tumorigenesis in Pten(+/−) mice is independent of coexistence of estrogen and estrogen receptor α. Am J Pathol. 2012;180:2536–2547. doi: 10.1016/j.ajpath.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res. 2009;2:187–194. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LS, Burke CA, Hasson H, Kuo CT, et al. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev Res. 2014;7:666–674. doi: 10.1158/1940-6207.CAPR-14-0052. [DOI] [PubMed] [Google Scholar]
- 8.Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, et al. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res. 2014;7:574–584. doi: 10.1158/1940-6207.CAPR-14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mallery SR, Tong M, Shumway BS, Curran AE, et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo-controlled clinical trial. Clin Cancer Res. 2014;20:1910–1924. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shumway BS, Kresty LA, Larsen PE, Zwick JC, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–2430. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Zhang D, Stoner GD, Huang C. Differential effects of black raspberry and strawberry extracts on BaPDE-induced activation of transcription factors and their target genes. Mol Carcinog. 2008;47:286–294. doi: 10.1002/mc.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LS, Hecht SS, Carmella SG, Yu N, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho H, Jung H, Lee H, Yi HC, et al. Chemopreventive activity of ellagitannins and their derivatives from black raspberry seeds on HT-29 colon cancer cells. Food Funct. 2015;6:1675–1683. doi: 10.1039/c5fo00274e. [DOI] [PubMed] [Google Scholar]
- 14.Kasimsetty SG, Bialonska D, Reddy MK, Ma G, et al. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem. 2010;58:2180–2187. doi: 10.1021/jf903762h. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Sarrias A, Gimenez-Bastida J, Nunez-Sanchez M, Larrosa M, et al. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr. 2014;53:853–864. doi: 10.1007/s00394-013-0589-4. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Sarrias A, Gimenez-Bastida JA, Garcia-Conesa MT, Gomez-Sanchez MB, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. doi: 10.1002/mnfr.200900152. [DOI] [PubMed] [Google Scholar]
- 17.Seeram NP, Henning SM, Zhang Y, Suchard M, et al. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006;136:2481–2485. doi: 10.1093/jn/136.10.2481. [DOI] [PubMed] [Google Scholar]
- 18.Nunez-Sanchez MA, Garcia-Villalba R, Monedero-Saiz T, Gomez-Sanchez MB, et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res. 2014;58:1199–1211. doi: 10.1002/mnfr.201300931. [DOI] [PubMed] [Google Scholar]
- 19.Stoner GD, Wang LS, Seguin C, Rocha C, et al. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res. 2010;27:1138–1145. doi: 10.1007/s11095-010-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LS, Hecht S, Carmella S, Seguin C, et al. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J Agric Food Chem. 2010;58:3992–3995. doi: 10.1021/jf9030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donohoe DR, Holley D, Collins LB, Montgomery SA, et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4:1387–1397. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aarestrup J, Kyro C, Knudsen KE, Weiderpass E, et al. Plasma enterolactone and incidence of endometrial cancer in a case-cohort study of Danish women. Br J Nutr. 2013;109:2269–2275. doi: 10.1017/S0007114512004424. [DOI] [PubMed] [Google Scholar]
- 23.Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95:1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 24.Neill AS, Ibiebelem TI, Lahmann PH, Hughes MC, et al. Dietary phyto-oestrogens and the risk of ovarian and endometrial cancers: findings from two Australian case-control studies. Br J Nutr. 2014;111:1430–1440. doi: 10.1017/S0007114513003899. [DOI] [PubMed] [Google Scholar]
- 25.Bandera E, Williams M, Sima C, Bayuga S, et al. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009;20:1117–1127. doi: 10.1007/s10552-009-9336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeleniuch-Jacquotte A, Lundin E, Micheli A, Koenig KL, et al. Circulating enterolactone and risk of endometrial cancer. Int J Cancer. 2006;119:2376–2381. doi: 10.1002/ijc.22140. [DOI] [PubMed] [Google Scholar]
- 27.Sabater-Molina M, Larque E, Torrella F, Zamora S. Dietary fructooligosaccharides and potential benefits on health. J Physiol Biochem. 2009;65:315–328. doi: 10.1007/BF03180584. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr. 2004;134:1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 29.Bruno-Barcena JM, Azcarate-Peril MA. Galacto-oligosaccharides and colorectal cancer: feeding our intestinal probiome. J funct foods. 2015;12:92–108. doi: 10.1016/j.jff.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang YW, Liu JC, Deatherage DE, Luo J, et al. Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038–9046. doi: 10.1158/0008-5472.CAN-09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 32.Schroder AL, Pelch KE, Nagel SC. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine. 2009;35:211–219. doi: 10.1007/s12020-009-9154-6. [DOI] [PubMed] [Google Scholar]
- 33.Filby AL, Tyler CR. Appropriate ‘housekeeping’ genes for use in expression profiling the effects of environmental estrogens in fish. BMC Mol Biol. 2007;8:1–13. doi: 10.1186/1471-2199-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem. 2006;54:1611–1620. doi: 10.1021/jf0527403. [DOI] [PubMed] [Google Scholar]
- 35.Navari JR, Roland PY, Keh P, Salvesen HB, et al. Loss of estrogen receptor (ER) expression in endometrial tumors is not associated with de novo methylation of the 5′ end of the ER gene. Clin Cancer Res. 2000;6:4026–4032. [PubMed] [Google Scholar]
- 36.Castro-Rivera E, Safe S. Estrogen- and antiestrogen-responsiveness of HEC1A endometrial adenocarcinoma cells in culture. J Steroid Biochem Mol Biol. 1998;64:287–295. doi: 10.1016/s0960-0760(97)00202-1. [DOI] [PubMed] [Google Scholar]
- 37.Deschenes J, Bourdeau V, White JH, Mader S. Regulation of GREB1 transcription by estrogen receptor α through a multipartite enhancer spread over 20 kb of upstream flanking sequences. J Biol Chem. 2007;282:17335–17339. doi: 10.1074/jbc.C700030200. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Torikai K, Ohto Y, Murakami A, et al. A simple phenolic antioxidant protocatechuic acid enhances tumor promotion and oxidative stress in female ICR mouse skin: dose- and timing-dependent enhancement and involvement of bioactivation by tyrosinase. Carcinogenesis. 2000;21:1899–1907. doi: 10.1093/carcin/21.10.1899. [DOI] [PubMed] [Google Scholar]
- 39.Tseng TH, Hsu JD, Lo MH, Chu CY, et al. Inhibitory effect of Hibiscus protocatechuic acid on tumor promotion in mouse skin. Cancer Lett. 1998;126:199–207. doi: 10.1016/s0304-3835(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 40.Swerdlow AJ, Jones ME. British Tamoxifen Second Cancer Study Group., Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–384. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 41.Seeram NP, Aronson WJ, Zhang Y, Henning SM, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 42.Gimenez-Bastida JA, Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan F, et al. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol Nutr Food Res. 2012;56:784–796. doi: 10.1002/mnfr.201100677. [DOI] [PubMed] [Google Scholar]
- 43.Wada L, Ou B. Antioxidant activity and phenolic content of oregon caneberries. J Agric Food Chem. 2002;50:3495–3500. doi: 10.1021/jf011405l. [DOI] [PubMed] [Google Scholar]
- 44.Stoner GD, Sardo C, Apseloff G, Mullet D, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 45.Aiyer HS, Gupta RC. Berries and ellagic acid prevent estrogen-induced mammary tumorigenesis by modulating enzymes of estrogen metabolism. Cancer Prev Res. 2010;3:727–737. doi: 10.1158/1940-6207.CAPR-09-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandal S, Stoner GD. Inhibition of N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 47.Wang N, Wang ZY, Mo SL, Loo TY, et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat. 2012;134:943–955. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M, Tang SN, Marsh JL, Shankar S, Srivastava RK. Ellagic acid inhibits human pancreatic cancer growth in Balb c nude mice. Cancer Lett. 2013;337:210–217. doi: 10.1016/j.canlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


