Sir
Retinoic acid orphan receptor-α (RORα aka NR1F1) is a member of the ROR subfamily of nuclear receptors (RORα-γ), which contain an N-terminal domain, a highly conserved DNA-binding domain (DBD), a ligand-binding domain (LBD), and a hinge domain between the DBD and LBD [1]. RORs are ligand-dependent transcription factors that regulate transcription by binding as a monomer to ROR response elements (RORE) in the promoter region of target genes [1]. RORα plays a critical role in the regulation of many physiological processes, including embryonic development, cell differentiation, and several immune, metabolic and circadian activities [1]. RORα has been implicated in several pathologies, including neoplasia, and autoimmune and neurological disorders.
Melatonin is produced and metabolized in almost all living organisms. Melatonin and its various metabolites exhibit many diverse pleiotropic activities triggered by receptor dependent and independent signaling pathways [2]. These include immunomodulatory and beneficial effects in a devastating disease, multiple sclerosis (MS), as was nicely discussed by Farez et al. [3]. As much we agree with the majority of opinions in that paper, we are obliged to correct the cited theory on melatonin acting as a ligand for RORα or RORγ [3].
Specifically, and most importantly, crystallography studies on the LBD of RORα have clearly indicated that melatonin is not a ligand for this nuclear receptor, but that sterols and oxysterols function as natural ligands [4]. The list of endogenous ligands for RORα includes cholesterol, cholesterol sulfate, certain hydroxycholesterols, intermediates of cholesterol synthesis pathway, such as 7-dehydrocholesterol (pro-vitamin D3), and secosteroids that may act as agonists or inverse agonists, depending on the context and the structure of the molecule [1,4,5]. Melatonin, and products of its metabolism via indolic, kynuric and other pathways [2], have very different chemical structures and little in common with those of sterols. Finally, functional studies have provided evidence that melatonin or its metabolites do not directly activate RORα or RORγ [5]. This is consistent with relatively low docking scores in molecular modelling using crystal structure of RORα LBD in comparison to native ligands, hence disqualifying RORα as a high affinity receptor for melatonin and its metabolites [5].
The challenge is how to explain the amplifying effects of melatonin on transcriptional activities that are downstream of RORα, as discussed in [3,5]. We believe that these events may be induced through indirect mechanisms of action (Fig. 1). Thus, it can be proposed that melatonin and/or its metabolites acting through membrane bound melatonin receptors (MT1 or MT2) or other receptors/regulatory proteins (yet to be defined) would stimulate RORα gene transcription or modulate RORα and/or RORγ translation/processing or ROR-protein interactions that result in the modification of ROR transcriptional activity and subsequently altered expression of ROR target genes (Fig. 1). Another possible route is melatonin’s or its metabolites’ action on mitochondria, with induced changes in mitochondrial activity affecting RORα activity (Fig. 1). Finally, melatonin signalling may bypass RORα by indirectly affecting the activity of the nuclear receptor, REV-ERBα, which also interacts with ROREs, thereby influencing the expression of the same target genes as RORs. Inclusion of these multiple pathways downstream of melatonin, and potentially affecting RORα signalling, appears to be justified by the observed pleiotropic phenotypic effects mediated by either receptor-dependent or –independent activities of melatonin and its biologically active intermediates, and may include their anti-oxidative properties. Although speculative, melatonin and its intermediates might affect the cell redox status, leading to increased NADH/NAD and NADPH/NADP ratios, possibly via indirect effects on the oxidative phosphorylation and pentose phosphate pathway, subsequently resulting in changes in circadian rhythm programs, including the expression and activities of RORα. These effects would provide a functional link between melatonin and ROR signalling pathways. Also, NADPH is necessary for corresponding cytochrome P450 hydroxylation of sterols to oxysterols, ligands for RORα and γ. In addition, receptors or regulatory proteins interacting with melatonin metabolites remain to be identified [2]. Experimental efforts in this direction would be of value, because RORα and other RORs are implicated in the pathophysiology of different inflammatory, metabolic and neuropsychiatric disorders and cancer. Moreover, it may shed light on the pleiotropic and sometimes contradictory effects of melatonin, revealing them to be dependent on the context and availability of other signaling pathways.
Figure 1. Putative indirect effects of melatonin or its metabolites on RORα activity.
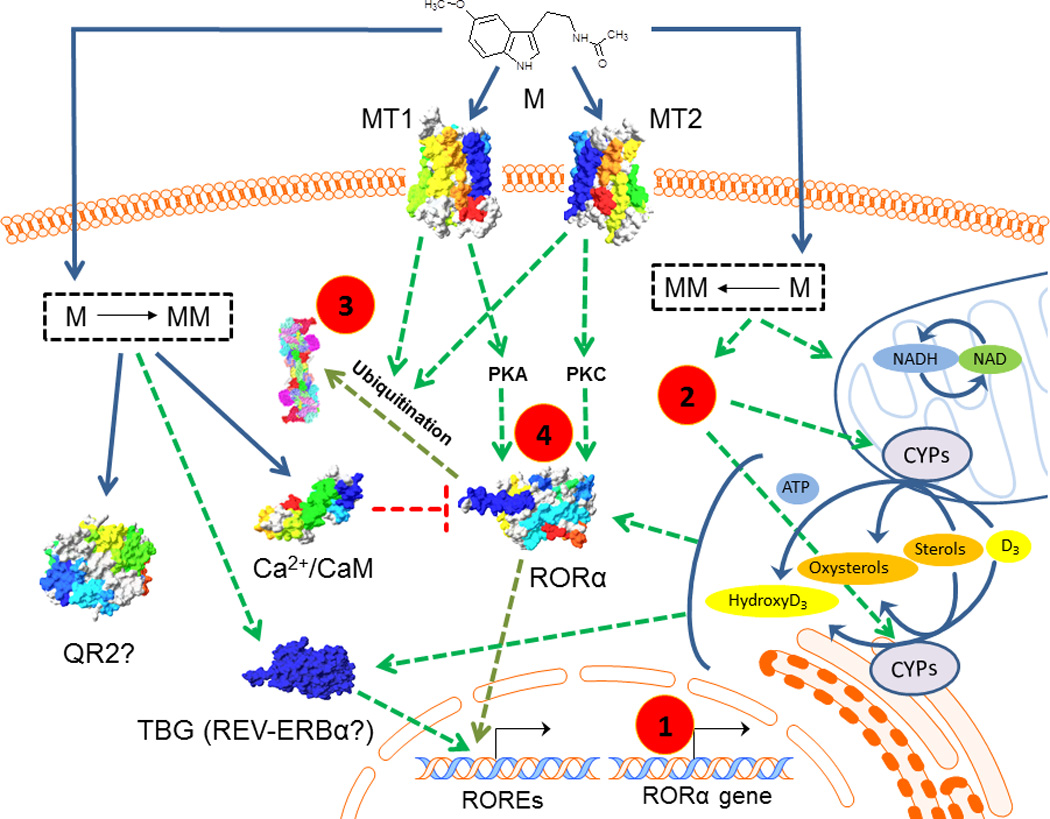
1: MT1/MT2-dependent or independent regulation of RORα expression, 2: pentose phosphate pathway generated NADPH, 3: proteasomal degradation, 4. Phosphorylation, M: melatonin, MM: melatonin metabolites, MT1/MT2: G-protein coupled membrane bound melatonin receptors type 1 and 2, QR2: quinone reductase 2, CAL: caldmodulin, TBR: to be defined receptor for M or MM.
In summary, RORα is not a receptor for melatonin or its metabolites; however, melatonin or its metabolites may indirectly modulate RORα and other ROR activities.
Acknowledgments
Per journal’s regulations we are restricted to 5 references. However, the information listed above is supported by papers cited in these 5 references. We acknowledge a partial support by grants R21AR066505, 1R01AR056666 and 2R01AR052190 from NIH to ATS, and N402 662840 from the Polish Ministry of Science and Higher Education to MAZ.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cook DN, Kang HS, Jetten AM. Retinoic Acid-Related Orphan Receptors (RORs): Regulatory Functions in Immunity, Development, Circadian Rhythm, and Metabolism. Nucl Receptor Res. 2015;2 doi: 10.11131/2015/101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski AT, Semak I, Fischer TW, Kim T-K, et al. Metabolism of melatonin in the skin: why is it important? Experimental Dermatology. 2016 doi: 10.1111/exd.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farez MF, Calandri IL, Correale J, Quintana FJ. Anti-inflammatory effects of melatonin in multiple sclerosis. BioEssays. 2016 doi: 10.1002/bies.201600018. [DOI] [PubMed] [Google Scholar]
- 4.Kallen J, Schlaeppi JM, Bitsch F, Delhon I, et al. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279:14033–14038. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- 5.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]


