Abstract
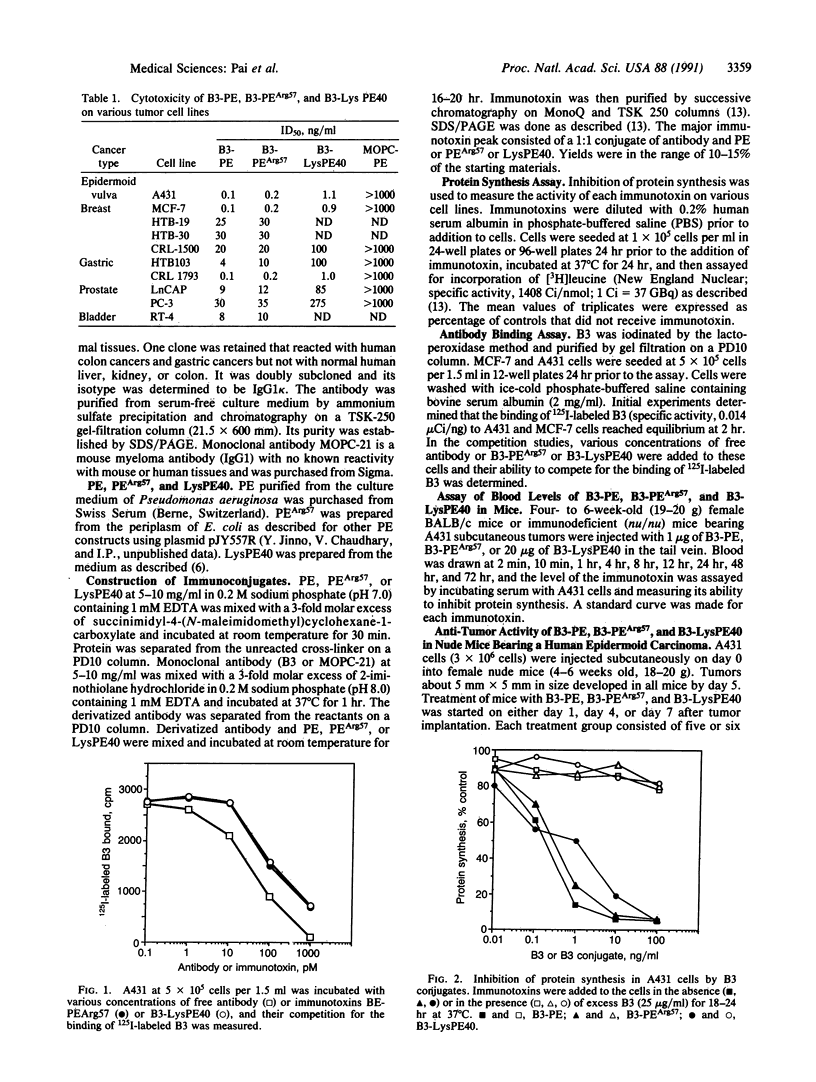
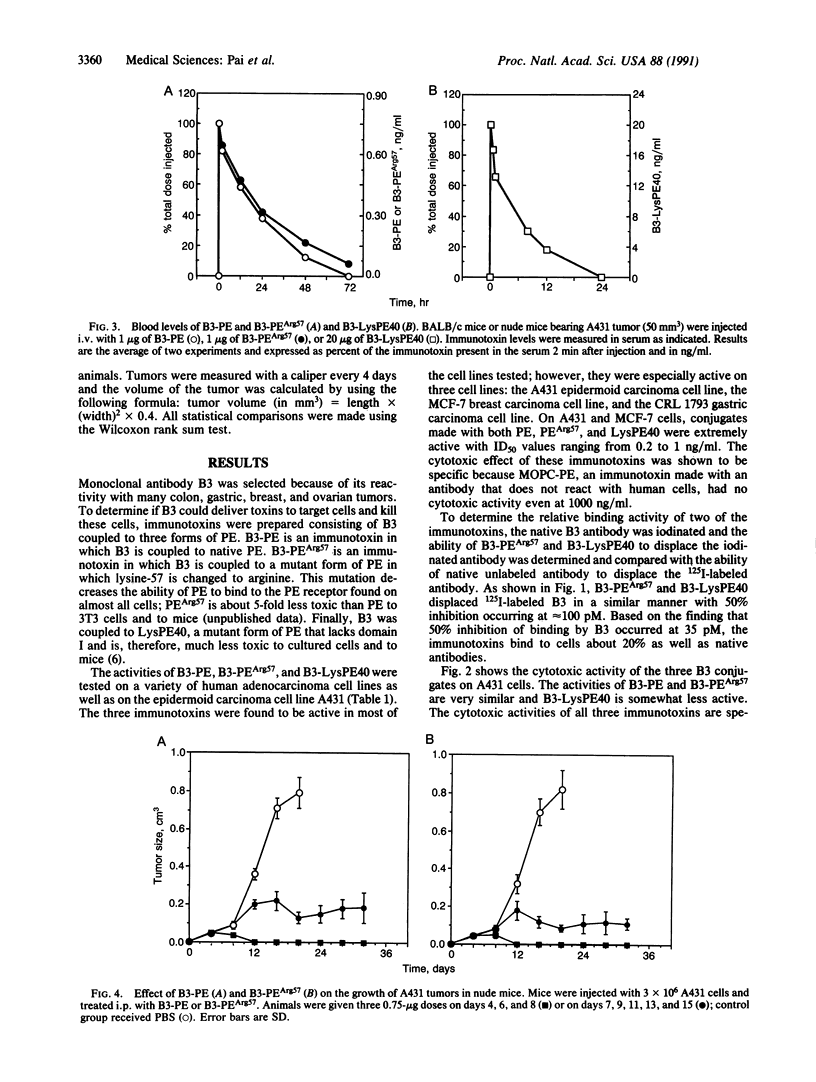
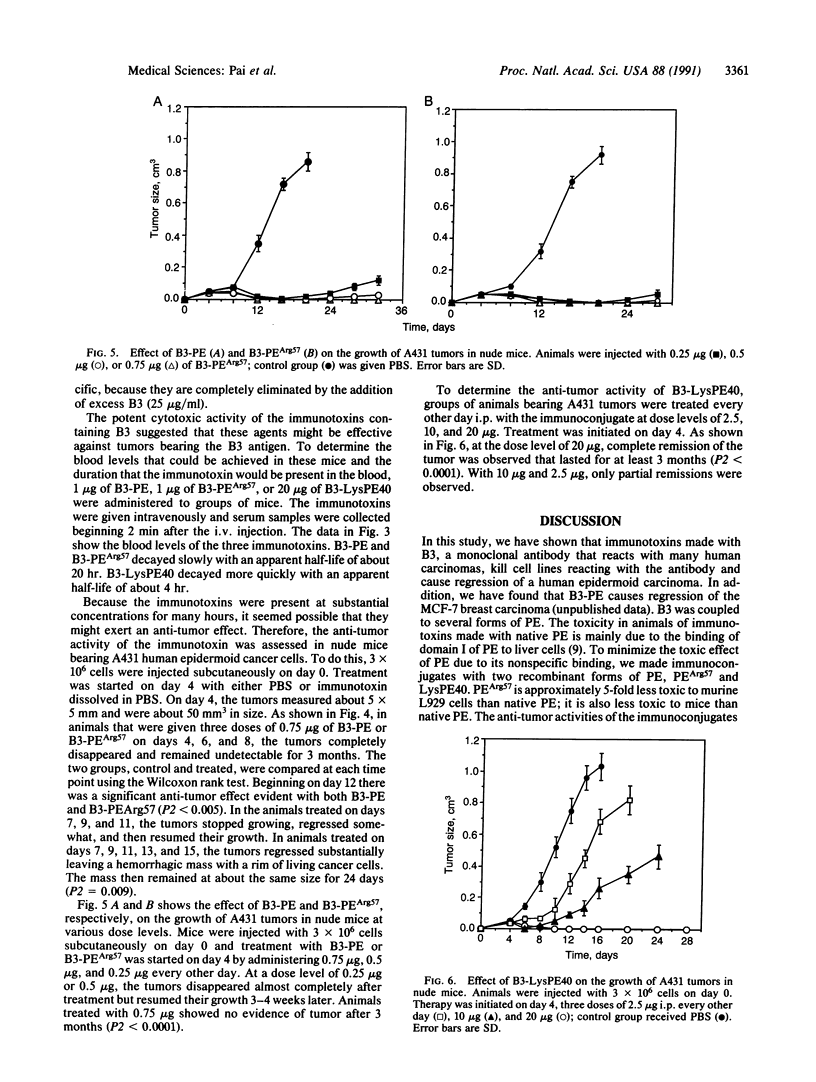
B3 is a monoclonal antibody that reacts with a carbohydrate epitope present on a variety of proteins located on the surface of many cancer cells and a limited number of normal tissues. We evaluated the cytotoxic activity of immunotoxins composed of monoclonal antibody B3 coupled to native Pseudomonas exotoxin (PE) or two recombinant forms of Pseudomonas exotoxin, PEArg57 or LysPE40, a form of PE with a deletion of the cell binding domain. All three conjugates were cytotoxic to human cell lines expressing the B3 antigen on their surface. The survival of each of the three immunotoxins in the circulation of mice was determined after administering the immunotoxin i.v. The half-life in blood of B3-PE and B3-PEArg57 was 20 hr, whereas the half-life of B3-LysPE40 was 4 hr. The short half-life of B3-LysPE40 may be due to the absence of domain I of PE. To determine the therapeutic effects of the three immunotoxins, they were given intraperitoneally to nude mice bearing subcutaneous A431 tumors. All three immunotoxins caused complete regression of 50-mm3 tumors with no toxic effects to the animals at therapeutic doses. Furthermore, substantial regression was also noted with much larger tumors. Our data indicate that the monoclonal antibody B3, when coupled to PE or recombinant forms of PE, may be useful for the treatment of tumors expressing B3 antigen. The therapeutic window was largest with B3-LysPE40, which can be administered in higher doses because it lacks sequences in domain I of PE that enable PE to bind to nontarget cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra J. K., Jinno Y., Chaudhary V. K., Kondo T., Willingham M. C., FitzGerald D. J., Pastan I. Antitumor activity in mice of an immunotoxin made with anti-transferrin receptor and a recombinant form of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8545–8549. doi: 10.1073/pnas.86.21.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers V. S., Henslee P. J., Kernan N. A., Blazar B. R., Gingrich R., Phillips G. L., LeMaistre C. F., Gilliland G., Antin J. H., Martin P. Use of an anti-pan T-lymphocyte ricin a chain immunotoxin in steroid-resistant acute graft-versus-host disease. Blood. 1990 Apr 1;75(7):1426–1432. [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Hellström I., Garrigues H. J., Garrigues U., Hellström K. E. Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Le(y)-related cell surface antigens. Cancer Res. 1990 Apr 1;50(7):2183–2190. [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Chaudhary V. K., Kondo T., Adhya S., FitzGerald D. J., Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988 Sep 15;263(26):13203–13207. [PubMed] [Google Scholar]
- Kondo T., FitzGerald D., Chaudhary V. K., Adhya S., Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., FitzGerald D. J., Pastan I. Pseudomonas exotoxin coupled to a monoclonal antibody against ovarian cancer inhibits the growth of human ovarian cancer cells in a mouse model. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2474–2478. doi: 10.1073/pnas.84.8.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. A reversible multi-well chamber for incubation of cultured cells with small volumes: application to screening of hybridoma fusions using immunofluorescence microscopy. Biotechniques. 1990 Mar;8(3):320–324. [PubMed] [Google Scholar]


