Abstract
Background.
Although sepsis is a major health problem, data on sepsis epidemiology are scarce. The aim of this study was to assess the incidence of sepsis, based on clinical findings in all adult patients treated with intravenous antibiotic in all parts of all hospitals in an entire population.
Methods.
This is a retrospective chart review of patients ≥18 years, living in 2 regions in Sweden, who were started on an intravenous antibiotic therapy on 4 dates, evenly distributed over the year of 2015. The main outcome was the incidence of sepsis with organ dysfunction. The mean population ≥18 years at 2015 in the regions was 1275753. Five hundred sixty-three patients living in the regions were started on intravenous antibiotic treatment on the dates of the survey. Patients who had ongoing intravenous antibiotic therapy preceding the inclusion dates were excluded, if sepsis was already present.
Results.
Four hundred eighty-two patients were included in the study; 339 had a diagnosed infection, of those, 96 had severe sepsis according to the 1991/2001 sepsis definitions, and 109 had sepsis according to the sepsis-3. This is equivalent to an annual incidence of traditional severe sepsis of 687/100000 persons (95% confidence interval [CI], 549–824) or according to the sepsis-3 definition of 780/100000 persons (95% CI, 633–926). Seventy-four patients had sepsis according to both definitions.
Conclusions.
The incidence of sepsis with organ dysfunction is higher than most previous estimates independent of definition. The inclusion of all inpatients started on intravenous antibiotic treatment of sepsis in a population makes an accurate assessment of sepsis incidence possible.
Keywords. : incidence, qSOFA, sepsis, SIRS.
The complexity of sepsis and its interactions with other diseases contribute to the difficulty to diagnose sepsis and to estimate the incidence.
There is no gold standard for diagnosing sepsis. The diagnostic criteria for sepsis, which have been applied since 1991, with additions in 2001, are insufficient to discriminate between uncomplicated infection and severe disease and have proven a lack of sensitivity [1–4].
The Sepsis Definitions Task Force, endorsed by the European Society of Intensive Care and the Society of Critical Care Medicine, recommends sepsis to be defined as organ dysfunction, characterized by a rise in Sequential (Sepsis-Related) Organ Failure Assessment (SOFA) score ≥2, due to a dysregulated host response to infection. The discrimination between sepsis and severe sepsis is not advocated.
The new sepsis definition is designated sepsis-3, which it will be called throughout this paper [5]. We use the term traditional severe sepsis in accordance with the 1991 and 2001 conferences for sepsis definitions [1, 2]. The Sepsis Definitions Task Force also launches a tool to recognize patients who should be further examined to discover sepsis, known as quick SOFA (qSOFA), based on ≥2 of altered mental status, hypotension (systolic blood pressure ≤100 mmHg), and tachypnea (respiratory rate ≥22 breaths/minute) [5].
The annual incidence of sepsis varies in studies. The most cited studies on incidence of traditional severe sepsis are based on International Classification of Diseases, Ninth and Tenth Revision codes (ICD-9 and ICD-10) in hospital discharge databases and vary between 132 and 300/100000 [6–8]. Depending on which code abstraction method is used, the incidence varies by 3- to 3.5-fold within a cohort and with poor accuracy. The incidence estimates have risen over the years to 300–1031/100000 (2004–2009), but these estimates are remarkably lower from Swedish databases [9, 10]. Comparisons between ICD code abstractions and medical record review for identification of traditional severe sepsis have shown good specificity but poor sensitivity [11]. Prospective collection of sepsis incidence data is labor intensive; however, Henriksen et al [12] collected data and recognized an incidence of traditional severe sepsis of 457/100000 (95% confidence interval [CI], 430–485). The study was restricted to patients presenting at a medical emergency department (ED) with community-acquired sepsis and excluded large patient groups such as surgery and dialysis patients and nosocomial sepsis [12].
Although sepsis is a major challenge for clinicians, healthcare systems, and researchers, comprehensive data on the epidemiology of sepsis are scarce. Because the criteria for sepsis were recently changed, a comparison of incidence based on both earlier and forthcoming criteria is valuable in understanding how this change of definitions will affect epidemiological estimates.
The ability of qSOFA to predict poor outcome in patients with suspected infection has thus far only been evaluated with inhospital mortality and/or prolonged intensive care unit (ICU) stay as outcome [13]. The aim of this study was to estimate the incidence of sepsis based on clinical findings in all patients treated with intravenous (IV) antibiotics in all parts of all hospitals in 2 entire regions. We estimated the incidence based on both earlier and forthcoming criteria, and we assessed systemic inflammatory response syndrome (SIRS)’s and qSOFA’s ability to identify traditional severe sepsis and sepsis-3.
METHODS
Study Design, Population, and Setting
We conducted a retrospective chart review of patients ≥18 years living in the regions of Skåne and Halland in southern Sweden. We reviewed medical records for all patients who were started on an IV antibiotic therapy at hospitals on 4 dates evenly distributed over the year of 2015: January 12, April 13, July 13, and October 12.
The Regions of Skåne and Halland are geographic regions with self-governing local authorities with responsibility for providing healthcare. The mean population in the regions was 1275753 ≥18 years at 2015: 146401 were 18–24 years, 208282 were 25–34 years, 208483 were 35–44 years, 212104 were 45–54 years, 183976 were 55–64 years, 178073 were 65–74 years, 96 749 were 75–84 years, 38 478 were 85–94 years, and 3207 were ≥95 years [14]. The regions are served by 11 hospitals, 2 of which are tertiary, academic hospitals.
Patients were identified by a national database, the anti-infection tool, which registers all out- and inpatients who are started on antibiotic therapy at the hospitals [15]. It is impossible to prescribe antibiotics and circumvent the registration in the anti-infection tool, which is aimed for surveillance of antibiotic use and healthcare-associated infections. For a few departments (8 of 56) at hospitals in the regions that are not affiliated to this database, medical records were reviewed manually to identify patients who received antibiotics.
Data Collection
Patients were included in the study if they fulfilled at least 1 of the 4 SIRS criteria or had experienced fever or chills, blood pressure ≤110, or had an altered mental status within ±12 hours from the initiation of antibiotic therapy. Patients under palliation and hospice care at home were excluded. Patients who had another IV antibiotic therapy preceding the inclusion dates were not included when calculating incidence if traditional severe sepsis, sepsis-3, or septic shock (traditional sepsis or sepsis-3 criteria) was already present.
Data were collected regarding basic demography including mortality, the department at which the antibiotic therapy was prescribed, most abnormal physiological parameters ±12 hours, and diagnostic and microbiological data for the sepsis- causing infection. Two experienced physicians (L. M. and Å. L.) reviewed the medical records. To validate the review, 2.5% of the included patients were randomly selected and also reviewed by a second physician (A. L. or L. M.), regarding presence of infection, focus, and sepsis-induced organ dysfunction and organ failure. The reviewers were blinded to the others’ result.
Definitions
Traditional severe sepsis and traditional septic shock were defined based on the 1991 and 2001 conferences for sepsis definitions and Surviving Sepsis Campaign [1, 2, 16], and sepsis-3 and septic shock were defined according to the sepsis-3 [5] for separate estimations of incidence. Traditional severe sepsis was defined as hypotension, hypoperfusion, or organ dysfunction induced by sepsis (see Supplementary Data for details) [1, 2, 16]. The requirement of fulfilling 2 SIRS criteria was not applied due to its lack of sensitivity [3]. Septic shock was defined as traditional severe sepsis with hypotension refractory to ≥2000 mL fluid resuscitation or the need for vasopressors [1, 2]. Sepsis-3 was defined as organ dysfunction characterized by a rise in total SOFA ≥2 due to a dysregulated host response to infection, and septic shock as, despite adequate fluid resuscitation, a lactate >2 mmol/L and vasopressors needed for mean arterial pressure ≥65 mmHg [5, 17].
Partial pressure of oxygen in arterial blood (PaO2) was preferably used. When not available, we used the oxygen saturation by pulse oximetry (SpO2) for values <96% and calculated PaO2 by the Ellis equation. This substitution correlates with PaO2, but it has a tendency to overestimate it [18–20]. Reaction level scale (RLS 85) was used because it is applied in clinical practice in Sweden, although its association with Glasgow Coma Scale has only been evaluated for APACHE II [21].
For assessment of sepsis incidence, infections were defined as described by Calandra et al [22]. The definitions are aimed for trials in ICU. To adapt the definitions to patients on the ward, the following modifications were permitted. The criteria for pneumonia were changed regarding possible pneumonia, where high clinical suspicion without an abnormal chest radiograph was included.
In urinary tract infections, 2 of the following were required: symptoms, urine culture with ≥107 colony-forming unit/L uropathogens or radiographic evidence of infection. Because bone and joint, central nervous system, ear-nose-throat, reproductive tract infections and gastroenteritis are not included in the definitions, we used the Centers for Disease Control and Prevention/National Healthcare Safety Network Surveillance Definitions for Specific Types of Infections [23]. For evaluation of SIRS’s and qSOFA’s ability to identify traditional severe sepsis and sepsis-3, clinical suspicion was the only requirement for infection.
The presence of the following comorbid conditions was registered: congestive heart failure (New York Heart Association class III–IV), history of acute myocardial infarction, liver cirrhosis, cerebrovascular disease, chronic obstructive pulmonary disease or asthma, diabetes mellitus with ongoing treatment for glucose control, chronic renal failure (serum creatinine >177 μmol/L), malignancy diagnosed in the last 5 years, autoimmune disease, and immunodeficiency including human immunodeficiency virus.
The ICD-10 codes for sepsis at hospital discharge were registered. The ICD-10 codes for sepsis were as follows: R65.1, R57.2, A02.1, A22.7, A26.7, A32.7, A40.0–A40.3, A40.8–A409, A41.0–A41.5, A41.8–A419, and B37.7. The study protocol was approved by the local ethics committee (decision number 2015/285).
Statistical Analysis
Incidence rates of patients starting antibiotic therapy and fulfilling sepsis definitions were calculated, and their 95% CIs from the incidence ±1.96 times the square root of the incidence divided by person years at risk (i ± 1.96 √I⁄T). Person-years at risk were calculated from the regions population by dividing it by 365 days and multiplied by 4 days. All missing data were regarded as within the normal range for calculation of incidence. For continuous variables, medians are shown. Categorical values are expressed as percentages. Comparisons are made by McNemar’s test for incidence between different sepsis definitions. For demographic differences between the patients identified with the different sepsis definitions, P values are calculated by χ2 or Fishers exact test. P values are shown when significant. Sensitivity, specificity, positive predictive values, and negative predictive values with 95% CIs were calculated using Wilson’s score interval. Patients with missing SIRS or qSOFA parameters were excluded when comparing SIRS’s and qSOFA’s ability to identify sepsis.
Cohen’s κ statistics were used to measure the interrater agreement regarding presence of infection and whether organ failure/organ dysfunction was due to sepsis.
IBM SPSS statistics 23.0 and Graph-Pad Prism 6.0 were used for the calculations.
RESULTS
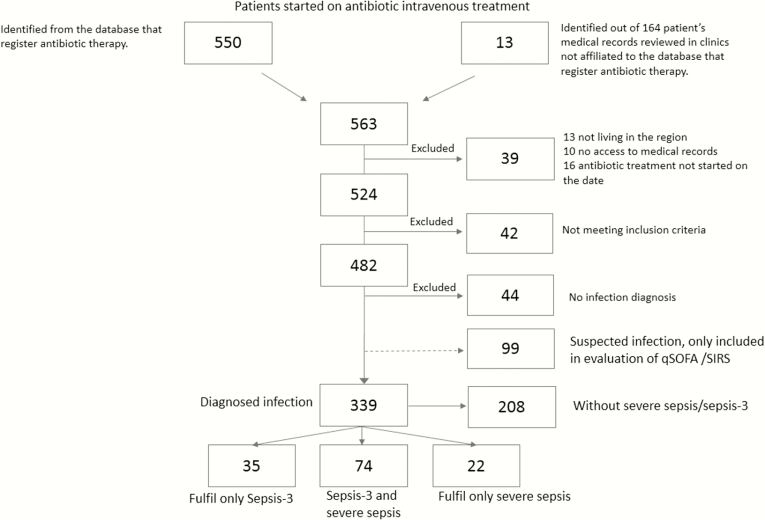
Five hundred sixty-three patients living in the regions started IV antibiotic treatment on the dates of the survey. Four hundred eighty-two patients were included in the study, and 44 of those patients had no infection diagnosis. An additional 99 patients had a suspected infection, but they did not fulfill the criteria for infection diagnosis and were excluded. This left 339 patients with a diagnosed infection: 96 (28.3%) of these patients had traditional severe sepsis, and 109 patients (32.3%) had sepsis-3 (see Figure 1 for details). Seventy-four (21.8%) of these patients fulfilled both traditional severe sepsis and sepsis-3. This is equivalent to an annual incidence of patients receiving IV antibiotics for a diagnosed infection of 2425/100000 persons (95% CI, 2167–2683).
Figure 1.
Flow chart of patient recruitment and sepsis groups.
The annual incidence of traditional severe sepsis was 687/100000 persons (95% CI, 549–824), and according to the new sepsis-3 definition the annual incidence was 780/100000 persons (95% CI, 633–926). The incidences of traditional severe sepsis and sepsis-3 for different 10-year age strata increased with increasing age; however, the actual numbers were small (Table 1).
Table 1.
Demography and Distribution of Organ Failure, Organ Dysfunction, and Infection in Patients With Traditional Severe Sepsis and Sepsis-3. Patients Can Be Represented in Both Columns
| Variables | Traditional Severe Sepsis | Sepsis-3 | PValue |
|---|---|---|---|
| N = 131 | 96 | 109 | |
| Age, median | 78 | 80 | |
| 18–24 | 1 (1.0%) | 1 (0.9%) | |
| 25–34 | 1 (1.0%) | 0 | |
| 35–44 | 3 (3.1%) | 1 (0.9%) | |
| 45–54 | 3 (3.1%) | 1 (0.9%) | |
| 55–64 | 6 (6.3%) | 10 (9.2%) | |
| 65–74 | 22 (22.9%) | 25 (22.9%) | |
| 75–84 | 29 (30.2%) | 32 (29.4%) | |
| 85–94 | 29 (30.2%) | 36 (33.0%) | |
| ≥95 | 2 (2.1%) | 3 (2.8%) | |
| Comorbidities | |||
| None | 23 (24.0%) | 20 (18.3%) | |
| Heart disease | 30 (31.3%) | 40 (36.7%) | |
| Malignancy | 23 (24.0%) | 26 (23.9%) | |
| Asthma, COPD | 22 (22.9%) | 19 (17.4%) | |
| Diabetes mellitus | 18 (18.8%) | 22 (20.2%) | |
| Cerebrovascular disease | 17 (17.7%) | 22 (20.2%) | |
| Chronic renal failure | 10 (10.4%) | 11 (10.1%) | |
| Cirrhosis | 2 (2.1%) | 1 (0.9%) | |
| Immunodeficiency | 0 | 0 | |
| Connective tissue disorder | 9 (9.4%) | 11 (10.1%) | |
| Ward at Initiation of Antibiotis | |||
| Emergency Department | 65 (67.7%) | 67 (61.5%) | |
| Nonsurgical ward | 19 (19.8%) | 31 (28.4%) | |
| Surgical ward | 10 (10.4%) | 8 (7.3%) | |
| Intensive care unit | 2 (2.1%) | 3 (2.8%) | |
| Organ Dysfunction | |||
| Cardiovascular | 50 (52.1%) | 41 (37.6%) | .037 |
| CNS | 30 (31.3%) | 28 (25.7%) | |
| Hepatic | 0 | 7 (6.4%) | .015 |
| Renal | 29 (30.2%) | 37 (33.9%) | |
| Respiratory | 27 (28.1%) | 87 (79.8%) | <.01 |
| Hematologic | 7 (7.3%) | 11 (10.1%) | |
| Hypoperfusion | 24 (25.0%) | ||
| Chock | 7 (7.3%) | 4 (3.7%) | |
| Number of Organs Dysfunction | |||
| 1 | 49 (51.0%) | 40 (36.7%) | .038 |
| >1 | 47 (49.0%) | 69 (63.3%) | |
| Site of Infection | |||
| Respiratory tract | 40 (41.7%) | 55 (50.5%) | |
| Urinary tract | 17 (17.7%) | 19 (17.4%) | |
| Abdominal | 14 (14.6%) | 12 (11.0%) | |
| Skin or soft tissue | 7 (7.3%) | 5 (4.6%) | |
| Other | 18 (18.8%) | 18 (16.5%) | |
Abbreviations: COPD, chronic obstructive pulmonary disease; CNS, central nervous system.
Among the 96 patients with traditional severe sepsis, 51 (53.1%) were female, 23 (24.0%) had bacteraemia, 15 (15.6%) had any sepsis diagnose code (ICD-10) at hospital discharge, and the inhospital mortality and mortality <90 days were 19 (19.8%) and 30 (31.3%), respectively. Among the 106 patients with sepsis-3, 59 (54.1%) were female, 21 (19.3%) had bacteraemia, 17 (15.6%) had any sepsis diagnose code (ICD-10) at hospital discharge, and the inhospital mortality and mortality <90 days were 19 (17.4%) and 28 (25.7%), respectively. There were no significant differences between the groups in these regards. Enterobacteriaceae were most frequently found in blood cultures, followed by Staphylococcus aureus and streptococci in both groups.
There were no significant seasonal variations in when the patients were observed with sepsis, neither in the patients who fulfilled the traditional severe sepsis criteria (January 23 [24.0%], April 24 [25.0%], July 18 [18.8%], October 31 [32.3%]) nor in those who fulfilled the sepsis-3 criteria (January 29 [26.6%], April 26 [23.9%], July 18 [16.5%], October 36 [33.0%]). For details on demography, distribution of organ failure/dysfunction, and infection, see Table 1. The interrater agreement for the reviewers was 87% (κ = 0.82; 95% CI, 0.73–0.95).
To evaluate the SIRS’s and qSOFA’s ability to identify traditional severe sepsis and sepsis-3, 339 patients with a diagnosed infection and 99 patients with clinically suspected infection were judged to be eligible. Sixty-six patients were excluded due to missing data, rendering 372 patients with diagnosed or suspected infection for evaluation of qSOFA and SIRS.
For distribution of SIRS and qSOFA in relation to traditional severe sepsis and sepsis-3, see Table 2. For SIRS’ and qSOFA’s sensitivity, specificity, positive predictive value, and negative predictive value, see Table 3. Of the 66 patients with missing SIRS or qSOFA parameters, 13 had traditional severe sepsis and 21 had sepsis-3.
Table 2.
Contingency Table of SIRS, qSOFA, and Traditional Severe Sepsis and Sepsis-3 (N = 372)
| Variables | Traditional Severe Sepsis | Sepsis-3 | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| <2 SIRS | 11 | 60 | 10 | 61 |
| ≥2 SIRS | 98 | 203 | 106 | 195 |
| <2 qSOFA | 49 | 246 | 67 | 228 |
| ≥2 qSOFA | 60 | 17 | 49 | 28 |
Abbreviations: qSOFA, quick Sequential (Sepsis-Related) Organ Failure Assessment; SIRS, systemic inflammatory response syndrome.
Table 3.
Sensitivity, Specificity, Positive Predictive Value, and Negative Predictive Value of SIRS and qSOFA for Predicting Traditional Severe Sepsis and Sepsis-3
| Parameters | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | Traditional Severe Sepsis | Sepsis-3 | Traditional Severe Sepsis | Sepsis-3 | Traditional Severe Sepsis | Sepsis-3 | Traditional Severe Sepsis | Sepsis-3 |
| 2 SIRS | 89.9 (82.7–94.9) | 91.4 (84.7–95.8) | 22.8 (17.9–28.4) | 23.8 (18.7–29.5) | 32.6 (27.3–38.2) | 35.2 (29.8–40.9) | 84.5 (74.0–92.0) | 85.9 (75.6–93.0) |
| 2 qSOFA | 55.0 (45.2–64.6) | 42.2 (33.1–51.8) | 93.5 (89.9–96.2) | 89.1 (84.6–92.6) | 77.9 (67.0–86.6) | 63.6 (51.9–74.3) | 83.4 (78.6–87.5) | 77.3 (72.1–81.9) |
Abbreviations: qSOFA, quick Sequential (Sepsis-Related) Organ Failure Assessment; SIRS, systemic inflammatory response syndrome.
DISCUSSION
Our study suggests a high incidence of traditional severe sepsis (687/100000), and the incidence was even somewhat higher using the newly proposed sepsis-3 definitions (780/10000) but without a significant difference. A substantial portion of patients with sepsis fulfilled only 1 of the sepsis definitions (traditional severe sepsis or sepsis-3). The qSOFA showed poor sensitivity but good specificity in predicting traditional severe sepsis and sepsis-3.
Comparison to Similar Studies
The incidence of traditional severe sepsis was higher than in most preceding studies, which is not surprising because many studies are based on discharge diagnoses and have proven to fail to register many episodes [11]. Our study also differs from other studies in that the study cohort was older; however, a recent study from Prescott et al [24] shows similar results.
The incidence found in the present study was also higher than in studies based on clinical findings [12]. This is probably partly due to different definitions; for example, we did not apply the requirement of fulfilling 2 SIRS criteria, which probably increased the incidence. In addition, earlier studies have been restricted to certain wards or EDs [12, 25].
In this study, we screened all adult patients who were started on IV antibiotic treatment in all parts of all hospitals in 2 entire regions; therefore, we identified both community-acquired and nosocomial sepsis cases. Even if our study includes a smaller number of patients, the CIs are well above the previously proposed incidences.
The magnitude of our data is illustrated by our recorded incidence of 2425/100000 adults receiving IV antibiotics for a diagnosed infection, which corresponds to more than 200000 Swedish patients each year. Approximately 1 of 4 of these patients receiving IV antibiotics had or developed organ dysfunction. The burden of antibiotic resistance is still relatively low in Sweden, indicating that the incidence of traditional severe sepsis and sepsis-3 may be higher in other parts of the world, and with a growing antimicrobial resistance it will likely increase [26].
Strengths and Limitations
We applied restrictive criteria of infection, demanding both clinical and objective signs of infection except for pneumonia where high clinical suspicion was sufficient for inclusion. The definitions for traditional severe sepsis are based on interpretations of consensus criteria, and the definitions can therefore vary from other studies. We believe that the incidence of patients with sepsis-related organ dysfunction is not overestimated, and we suspect that it might be underestimated. It is probable that several patients with sepsis were unobserved due to a lack of clinical suspicion and diagnostic measures or oral antibiotic treatment, avoidance of treatment, or sudden death. Organ dysfunction may have occurred before or after the infection is suspected and treatment initiated, and outside of the time window we screened for organ dysfunction. Other limitations to this study are the small size, only adults included, and the retrospective design.
The strengths of this study are the comprehensive inclusion of all patients with IV antibiotic therapy in a well defined population, the manual chart reviews, and the use of 2 different definitions. The outcomes and the features of the patients with only traditional severe sepsis or sepsis-3 would be interesting to characterize further, but the numbers are too small in the present study.
The qSOFA has been validated against inhospital mortality and ICU stay, among patients receiving treatment. However, a large proportion of patients with sepsis and multiorgan dysfunctions never reach the ICU and are hence treated in the wards [27, 28].
In our study, in patients with clinically suspected infection with traditional severe sepsis and sepsis-3 as outcomes, the sensitivity and the negative predictive value were unsatisfactory. A prospective evaluation of qSOFA with organ dysfunction in hospitalized patients as outcome might be enlightening.
CONCLUSIONS
The proposed criteria for sepsis-3 will identify a partially different cohort of patients, who are not obviously at higher risk for death or organ failure than those fulfilling the criteria for traditional severe sepsis. The epidemiological estimates of sepsis incidence will not be vastly affected by the definition change—at least to a lesser extent than from different methodology in different incidence studies.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Acknowledgments
Author contributions. A. L., L. M., and P. L. made contributions to conception and design. L. M., S. W., and Å. L. acquired data. L. M., S. W., and A. L. analyzed and interpreted data. L. M., A. L., S. W., Å. L., B. C., and P. L. drafted or revised the article. L. M., A. L., S. W., Å. L., B. C., and P. L. provided final approval of the manuscript.
Disclaimer. The funders were not involved in any part of the study or the preparation of the manuscript.
Financial support. This work was supported by Swedish Government Research Grant, Skane University Hospital Foundations, Southern Health Care Region Grant, Svenska Lakaresallskapet, Grochinsky Foundation, Lundgrens Foundation, and Osterlunds Foundation.
Potential conflicts of interest. All authors report no conflicts of interests that the editors consider relevant to the content of this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–55. [DOI] [PubMed] [Google Scholar]
- 2. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003; 29:530–8. [DOI] [PubMed] [Google Scholar]
- 3. Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Eng J Med 2015; 372:1629–38. [DOI] [PubMed] [Google Scholar]
- 4. Gille-Johnson P, Hansson KE, Gardlund B. Severe sepsis and systemic inflammatory response syndrome in emergency department patients with suspected severe infection. Scand J Infect Dis 2013; 45:186–93. [DOI] [PubMed] [Google Scholar]
- 5. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 29:1303–10. [DOI] [PubMed] [Google Scholar]
- 7. Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Eng J Med 2003; 348:1546–54. [DOI] [PubMed] [Google Scholar]
- 8. Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007; 35:1244–50. [DOI] [PubMed] [Google Scholar]
- 9. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41:1167–74. [DOI] [PubMed] [Google Scholar]
- 10. Wilhelms SB, Huss FR, Granath G, et al. Assessment of incidence of severe sepsis in Sweden using different ways of abstracting International Classification of Diseases codes: difficulties with methods and interpretation of results. Crit Care Med 2010; 38:1442–9. [DOI] [PubMed] [Google Scholar]
- 11. Wang HE, Addis DR, Donnelly JP, et al. Discharge diagnoses versus medical record review in the identification of community-acquired sepsis. Crit Care 2015; 19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henriksen DP, Laursen CB, Jensen TG, et al. Incidence rate of community- acquired sepsis among hospitalized acute medical patients-a population-based survey. Crit Care Med 2015; 43:13–21. [DOI] [PubMed] [Google Scholar]
- 13. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Statistikdatabasen [cited 2016 May 5]. Available at: http://www.statistikdatabasen.scb.se.
- 15.Public Health Agency of Sweden. Swedres-Swarm 2014, Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. Solna/Uppsala: Public Health Agency of Sweden;2015:34–5. [cited 2016 August 26]. Available at: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/s/Swedres-Svarm-2014/. [Google Scholar]
- 16. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of intensive care medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 18. Brown SM, Grissom CK, Moss M, et al. Non-linear imputation of PaO2/FiO2 from SpO2/FiO2 among patients with acute respiratory distress syndrome. Chest 2016; 150:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanz F, Dean N, Dickerson J, et al. Accuracy of PaO2/FiO2 calculated from SpO2 for severity assessment in ED patients with pneumonia. Respirology 2015; 20:813–8. [DOI] [PubMed] [Google Scholar]
- 20. Wilson BJ, Cowan HJ, Lord JA, et al. The accuracy of pulse oximetry in emergency department patients with severe sepsis and septic shock: a retrospective cohort study. BMC Emerg Med 2010; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walther SM, Jonasson U, Gill H. Comparison of the Glasgow Coma Scale and the reaction level scale for assessment of cerebral responsiveness in the critically ill. Intensive Care Med 2003; 29:933–8. [DOI] [PubMed] [Google Scholar]
- 22. Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005; 33:1538–48. [DOI] [PubMed] [Google Scholar]
- 23. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 24. Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: propensity matched cohort study. BMJ 2016; 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Esteban A, Frutos-Vivar F, Ferguson ND, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med 2007; 35:1284–9. [DOI] [PubMed] [Google Scholar]
- 26. ECDC. European Centre for Disease Prevention and Control. Surveillance Report: Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm 2015:8–65. [Google Scholar]
- 27. Rohde JM, Odden AJ, Bonham C, et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med 2013; 8:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prescott HC, Langa KM, Liu V, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med 2014; 190:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.