Abstract
Background.
Nocardia is an opportunistic pathogen that can cause life-threatening disease. We aimed to characterize the epidemiological, microbiological, and clinical features of nocardiosis in the tropical north of Australia.
Methods.
We conducted a retrospective cohort study of nocardiosis diagnosed between 1997 and 2014. Population-based incidences were calculated using district population data.
Results.
Clinically significant nocardiosis was identified in 61 patients. The unadjusted population-based annual incidence of nocardiosis was 2.02 (95% confidence interval [CI], 1.55–2.60) per 100000 people and was 1.7 (95% CI, .96–2.90) fold higher in Indigenous compared with non-Indigenous persons (P = .027). Of 61 patients, 47 (77%) had chronic lung disease, diabetes, and/or hazardous alcohol consumption; 22 (36%) were immunocompromised; and 8 (13%) had no identified comorbidities. Disease presentations included pulmonary (69%; 42 of 61), cutaneous (13%; 8 of 61), and disseminated nocardiosis (15%; 9 of 61). The most commonly identified species were Nocardia asteroides and Nocardia cyriacigeorgica (each 11%). Linezolid was the only antimicrobial to which isolates were universally susceptible; 89% (48 of 54), 60% (32 of 53), and 48% (26 of 54) of isolates were susceptible to trimethoprim-sulfamethoxazole, ceftriaxone, and imipenem, respectively. Eighteen patients (30%) required intensive care unit (ICU) admission, and 1-year mortality was 31%.
Conclusions.
The incidence of nocardiosis in tropical Australia is amongst the highest reported globally. Nocardiosis occurs in both immunocompromised and immunocompetent hosts, and it is associated with high rates of ICU admission, 1-year mortality, and resistance to commonly recommended antimicrobials. Diagnosis should be considered in patients with consistent clinical features, particularly if they are Indigenous or have chronic lung disease.
Keywords. epidemiology, immunocompromised host, Nocardia, nocardiosis, treatment.
Nocardia is a ubiquitous environmental saprophyte that can cause life-threatening disease in humans [1, 2]. Molecular analyses have identified approximately 86 Nocardia species, of which half are implicated in human infections [3]. Inhalation is the primary route of entry, but infection can also occur after direct cutaneous inoculation. Subsequent haematogenous dissemination may lead to infection of almost any organ, most commonly the central nervous system (CNS) and cutaneous tissues [4]. Nocardiosis typically affects immunocompromised hosts, particularly those with defects in cell-mediated immunity [1, 4].
Nocardia case series are frequently restricted to specific groups, such as solid organ transplant recipients [5, 6] or cancer patients [7], or specific clinical presentations such as pulmonary nocardiosis [8, 9]. There are limited data on the demographics, clinical manifestations, treatment, and long-term outcomes in cohorts that include both immunocompromised and immunocompetent individuals, and very few studies report the population-based incidence of nocardiosis [10].
There are few reported series of clinical nocardiosis in Australia and none from the tropical north; the incidence of nocardiosis is unknown because the disease is not notifiable. We aimed to review the epidemiology, demographics, comorbidities, clinical presentation, microbiology, treatment, and outcomes of all Nocardia infections presenting to Royal Darwin Hospital (RDH) in the tropical north of Australia, over an 18-year period from 1997 to 2014.
METHODS
Design and Setting
We conducted a retrospective cohort study of nocardiosis managed at RDH, a 350-bed tertiary referral center that serves a well defined population of 170000 over an area of approximately 500000 km2 [11]. Ethics approval was provided by the Human Research Ethics Committee of the Northern Territory (NT) Department of Health (HREC 10–1354).
Study Population
All patients with clinically significant culture-positive Nocardia infection identified between January 1997 and December 2014 were eligible for inclusion. Potential cases were identified through the following: (1) the RDH microbiology isolate database; (2) hospital discharge summary coding data; and (3) the infectious diseases consultation database. All cases were reviewed by at least 2 investigators, and a consensus was reached to classify the patient as “colonized” or “infected”. Cases were classified as clinically significant (infected) when culture of Nocardia species was associated with clinical disease judged by an infectious diseases specialist as requiring treatment.
Infections were divided into 3 categories: primary pulmonary (infection confined to the lungs and pleural space), primary cutaneous (infection localized to the skin and soft tissues with no other organ involvement), and disseminated disease (involvement of ≥2 noncontiguous organs, disease of the CNS, or the presence of Nocardia species in blood samples). Hazardous alcohol consumption was defined according to the definition of “harmful drinking” in Australian Guidelines [12]. Individual patient consent was not required because only data collected in the course of routine clinical care were extracted.
Data Collection
Demographic, clinical, treatment, and outcome data were collected from medical records. Microbiological data (specimen type, Nocardia species, and antimicrobial susceptibility) were obtained from the laboratory database. Data were entered into a purpose-built database (Epidata 3.1, Odense, Denmark).
Microbiology Methods
Presumptive diagnosis of Nocardia species was made in the RDH microbiology laboratory based on Gram and/or modified acid-fast staining findings and colony morphology. Isolates were referred to a reference laboratory for species identification by sequencing of 16S ribosomal ribonucleic acid and secA1 housekeeping genes [3, 13, 14]. Antimicrobial susceptibility testing was performed by Clinical and Laboratory Standards Institute standardized broth microdilution methods [15].
Statistical Analysis
Data were analyzed using STATA (version 10; StataCorp, College Station, TX). Descriptive statistics are presented as means, medians, and proportions, and between group differences were assessed with the Fisher’s exact test and Wilcoxon rank-sum test, as appropriate for the data. Population-based incidence was calculated using health district population data from 1997 to 2014, collated from Australian Bureau of Statistics data by the Health Protection Division of the NT Department of Health [11].
RESULTS
Epidemiology and Demographics
Between January 1997 and December 2014, Nocardia was isolated from the clinical specimens of 75 patients. Sixty-one patients had isolates that were considered clinically significant and were included in the analysis. Fourteen patients were thought to be colonized with Nocardia and were excluded: all had Nocardia isolated from respiratory specimens, none met criteria for clinical nocardiosis, and none received specific treatment for nocardiosis. Follow-up data were available for 7 (50%) patients: patients were observed for a median of 12 months (range, 8–55 months) with 2 reported deaths: one attributed to metastatic cancer and the other to end-stage chronic lung disease.
The overall population-based incidence of Nocardia infections was 2.02 (95% confidence interval [CI], 1.55–2.60) per 100000 people per year and was 1.7 (95% CI, .96–2.90; P = .027) fold higher in Indigenous people (2.8 [95% CI, 1.83–4.32] per 100000/year) compared with non-Indigenous people (1.71 [95% CI, 1.21–2.35] per 100000/year). The most common place of residence was urban Darwin (46%; 28 of 61) (Supplemental Figure 1). The mean number of Nocardia cases per year was 3.4 (range, 0–9), and the yearly number of cases between 1997 and 2014 demonstrated an upward trend (1, 3, 1, 1, 3, 2, 0, 1, 3, 5, 4, 6, 5, 3, 2, 6, 6, 9).
Demographic features are described in Table 1. The median age was 57 years (range, 1–80); 34 (56%) were male and 23 (38%) were Indigenous. Indigenous patients were more likely to be female (65%; 15 of 23) compared with non-Indigenous patients (32%; 12); odds ratio = 2.07, 95% CI = 1.19–3.60, P = .016. The most frequently identified predisposing factor was chronic lung disease, which was present in 32 patients (52%), primarily related to chronic obstructive pulmonary disease. Other common predisposing factors were hazardous alcohol consumption (18 patients; 30%) and diabetes mellitus (13 patients; 21%). Fifty-three patients (87%) had at least 1 predisposing factor, and 22 patients (36%) were recognized to be immunocompromised. The most common immunocompromising factors identified were immunosuppressive drug therapy (15 patients; 25%) and active malignancy (12 patients; 20%). Other immunocompromising factors included solid organ transplant (3 patients; 5%) and human immunodeficiency virus (HIV) infection (2 patients; 3%). It is noteworthy that 39 (64%) patients were considered to be immunocompetent, and 8 patients (13%) had no documented predisposing or immunocompromising factors.
Table 1.
Demographic Features of 61 Patients
| Patient Characteristics | Number (%) |
|---|---|
| Age, median years (range) | 57 (range 1–80) |
| Male | 34 (56%) |
| Indigenous | 23 (38%) |
| Male | 8 (35%) |
| Female | 15 (65%) |
| Nonindigenous | 38 (62%) |
| Male | 26 (68%) |
| Female | 12 (32%) |
| Predisposing and immunocompromising factors | |
| Chronic lung disease | 32 (52%) |
| Diabetes mellitus | 13 (21%) |
| Hazardous alcohol consumption | 18 (30%) |
| Immunosuppressive medicationa | 15 (25%) |
| High-dose corticosteroid-based therapy in preceding 3 monthsb | 13 (21%) |
| Other immunosuppressive therapyc | 2 (3%) |
| Active malignancy | 12 (20%) |
| Cytotoxic chemotherapy in past 6 months | 5 (8%) |
| Solid organ transplantationd | 3 (5%) |
| HIV infection | 2 (3%) |
| Number of predisposing/immunocompromising factors (from above list) | |
| 0 | 8 (13%) |
| 1 | 26 (43%) |
| 2 or more | 27 (44%) |
| Immunocompromisede | 22 (36%) |
Abbreviations: HIV, human immunodeficiency virus.
aImmunosuppressive medications included corticosteroids, calcineurin inhibitors, mycophenolate mofetil, azathioprine, and methotrexate.
bDefined as ≥20 mg/day prednisolone or >2 mg/day dexamethasone for 1 month or >2 pulses of 1 gram intravenous methylprednisolone with or without other immunosuppressive agents.
cPatients receiving low-dose prednisolone (<20 mg/day) and at least 1 other immunosuppressive agent.
dRenal transplantation in all 3 patients.
eDefined as presence of any of the following: immunosuppressive medication, history of transplantation, HIV infection, active malignancy.
Clinical Characteristics
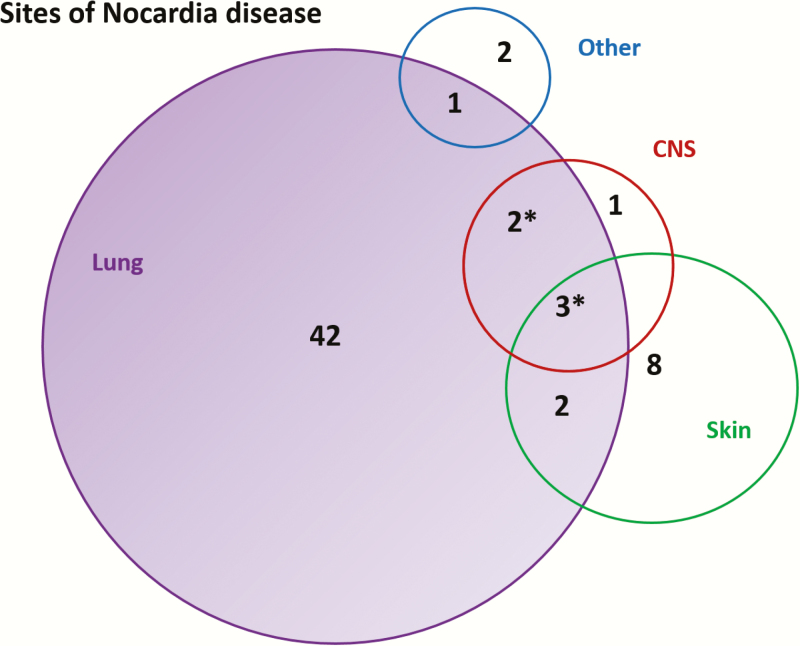
Sites of infection for all patients are shown in Figure 1. Forty-two patients (69%) had primary pulmonary, 8 (13%) had primary cutaneous, and 9 (15%) had disseminated disease. Six patients (67%) with disseminated disease had evidence of CNS disease. Two patients had localized infections at other sites: one was a peritoneal dialysis patient with Nocardia peritonitis, the other was a patient with metastatic colorectal cancer complicated by an ilio-vaginal fistula who had Nocardia isolated from a recurrent pelvic collection.
Figure 1.
Sites of infection for 61 patients with Nocardia. *These patients also had disease at an “other” site. Sites of Nocardia in patients with disseminated disease included lung (8 patients, 89%), skin (5 patients, 44%), central nervous system (CNS) (6 patients, 67%), bones or joints (3 patients, 33%), kidney (1 patient, 11%), and adrenal (1 patient, 11%). Venn diagram drawn and calculated using eulerAPE software (Micallef L, Rodgers P. eulerAPE: Drawing Area-Proportional 3-Venn Diagrams Using Ellipses. PLoS ONE, 2014; 9(7):e101717. http://www.eulerdiagrams.org/eulerAPE).
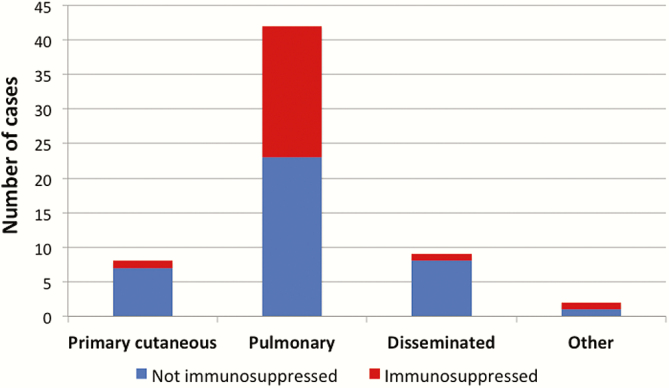
There was no clear association between clinical disease pattern and immune status (Figure 2). Of the 8 patients with primary cutaneous disease, only 1 was immunocompromised (history of active malignancy and autoimmune hepatitis on prednisolone and azathioprine). Nineteen of the 42 patients with pulmonary disease were immunocompromised, including the 3 solid organ transplant recipients and 2 HIV-infected patients. Of the 9 patients with disseminated disease, only 1 was immunocompromised (history of active malignancy and recent iatrogenic corticosteroids). Other predisposing factors for disseminated disease included hazardous alcohol (4 patients), chronic lung disease (4 patients), and diabetes (2 patients).
Figure 2.
Immune status and disease site. Immunosuppressive factors included are immunosuppressive medications, active malignancy, solid organ transplantation, and human immunodeficiency virus.
Microbiology and Susceptibility Profiles
Nocardia was isolated from 71 sites from the 61 patients. Most cases were diagnosed from noninvasive samples, including sputum (58%) and wound swabs (14%). Only 1 patient had Nocardia isolated from blood cultures.
Sixty-three Nocardia isolates were submitted for molecular identification. The 41 isolates identified to the species level represented 15 different species (Table 2). An additioinal 9 isolates were identified as belonging to the Nocardia asteroides complex, and 13 isolates were identified as Nocardia but species could not be further determined. No association between Nocardia species and clinical disease pattern was noted. Antimicrobial susceptibility testing results were available for 55 isolates, and significant variability in susceptibility was seen across different species. The only antimicrobial to which isolates were universally susceptible was linezolid (Table 2). Eighty-nine percent of tested isolates were susceptible to trimethoprim-sulfamethoxazole (TMP-SMX) and amikacin, respectively, 60% were susceptible to ceftriaxone, and only 48% were susceptible to imipenem.
Table 2.
Species Distribution and Susceptibility Profile of Nocardia Isolates
| Species | No. of Isolates (%Total) | No. of Isolates With AST | Percentage (Proportion) of Isolates Susceptible | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LZD | TMP-SMX | AMK | CEF | CLA | IMI | CIP | AUG | |||
| Nocardia asteroides sensu stricto | 7 (11%) | 7 | NP | 100% (7/7) | 100% (5/5)* | 100% (7/7) | 33% (1/3)* | 86% (6/7) | 29% (2/7) | 0% (0/7) |
| N asteroides complex† | 9 (14%) | 9 | 100% (3/3)* | 89% (8/9) | 89% (8/9) | 67% (6/9) | 78% (7/9) | 67% (6/9) | 33% (3/9) | 33% (3/9) |
| Nocardia beijingensis | 4 (6%) | 4 | 100% (3/3)* | 100% (4/4) | 100% (4/4) | 100% (4/4) | 100% (3/3)* | 50% (2/4) | 25% (1/4) | 0% (0/4) |
| Nocardia blacklockiae | 1 (2%) | 1 | 100% (1/1) | 100% (1/1) | 0% (0/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | 0% (0/1) |
| Nocardia brevicatena | 1 (2%) | 1 | 100% (1/1) | 100% (1/1) | 100% (1/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | 100% (1/1) | 0% (0/1) |
| Nocardia caviae | 3 (5%) | 1 | NP | 100% (1/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) |
| Nocardia cyriacigeorgica | 7 (11%) | 6 | 100% (4/4)* | 67% (4/6) | 100% (6/6) | 67% (4/6) | 0% (0/6) | 33% (2/6) | 0% (0/6) | 0% (0/6) |
| Nocardia cerradoensis | 1 (2%) | 0 | NP | NP | NP | NP | NP | NP | NP | NP |
| Nocardia elegans | 1 (2%) | 1 | 100% (1/1) | 100% (1/1) | 100% (1/1) | 0% (0/1) | NP | 0% (0/1) | NP | NP |
| Nocardia farcinica | 3 (5%) | 2 | 100% (1/1)* | 100% (1/1)* | 100% (2/2) | 0% (1/1)* | 0% (1/1)* | 0% (0/1)* | 100% (2/2) | 0% (0/1)* |
| Nocardia nova | 2 (3%) | 1 | NP | 100% (1/1) | 100% (1/1) | NP | 100% (1/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) |
| Nocardia otidiscaviarum | 3 (5%) | 3 | NP | 100% (3/3) | 100% (2/2)* | 0% (0/3) | 0% (0/2)* | 33% (1/3) | 33% (1/3) | 0% (0/3) |
| Nocardia pseudobrasiliensis | 2 (3%) | 2 | NP | 100% (2/2) | 50% (1/2) | 100% (2/2) | 100% (2/2) | 0% (0/2) | 100% (2/2) | 0% (0/2) |
| Nocardia seriolae | 1 (2%) | 1 | NP | 100% (1/1) | 100% (1/1) | 0% (0/1) | 100% (1/1) | 0% (0/1) | 0% (0/1) | 0% (0/1) |
| Nocardia transvalensis | 2 (3%) | 2 | 100% (1/1)* | 50% (1/2) | 0% (0/2) | 100% (2/2) | 0% (0/2) | 0% (0/2) | 50% (1/2) | 0% (0/2) |
| Nocardia veterana | 3 (5%) | 3 | NP | 100% (3/3) | 100% (3/3) | 67% (2/3) | 67% (2/3) | 67% (2/3) | 0% (0/2)* | 0% (0/3) |
| Nocardia sp (unspeciated) | 13 (21%) | 11 | 100% (7/7)* | 82% (9/11) | 91% (10/11) | 27% (3/11) | 60% (6/10)* | 45% (5/11) | 27% (3/11) | 10% (1/10)* |
| Total (all Nocardia isolates) | 63 | 55 | 22/22 (100%) | 48/54 (89%) | 46/52 (89%) | 32/53 (60%) | 25/46 (55%) | 26/54 (48%) | 16/53 (30%) | 4/52 (8%) |
Abbreviations: AMK, amikacin; AST, antimicrobial susceptibility testing; AUG, amoxicillin-clavulanate; CEF, ceftriaxone/cefotaxime; CIP, ciprofloxacin; CLA, clarithromycin; IMI, imipenem/meropenem; LZD, linezolid; NP, testing not performed; TMP-SMX, trimethoprim-sulfamethoxazole.
*Indicates that 1 or more isolates were not tested.
†The “N asteroides complex” is an old taxonomic classification referring to a group of Nocardia isolates that have now been separated into different species (including N nova, N transvalensis, and N farcinica).
Treatment
Information on treatment prescribed was available for 58 patients (95%). The remaining 3 patients did not receive treatment for nocardiosis; 1 was palliated, and the other 2 died before the diagnosis being made. Four patients did not commence treatment until after susceptibility results were available. The remaining 54 patients (89%) received empirical combination therapy. Empiric TMP-SMX was almost universal (52 patients; 96%), usually in combination with ceftriaxone (32 patients; 59%) and/or meropenem (26 patients; 48%). Amikacin (6 patients) and linezolid (3 patients) were uncommon in empiric regimens.
Drug susceptibility results were available for 46 of 54 patients who received empiric therapy. Of these, 93% (43 of 46) received empirical treatment with at least 1 antibiotic active against the corresponding isolate, and 57% (26 of 46) received 2 or more active antibiotics. Three patients (7%) did not receive effective empiric treatment: all 3 had isolates resistant to TMP-SMX, ceftriaxone, and imipenem. One of the 3 had disseminated nocardiosis and died before susceptibilities became available: he was treated with ceftriaxone, meropenem, and TMP-SMX but his Nocardia sp isolate only tested susceptible to amikacin and linezolid.
Among the 43 patients who completed treatment and whose susceptibility results were known, oral TMP-SMX was the most commonly prescribed antibiotic (37 patients; 86%), administered either alone (10 of 37) or in combination with other agents. Definitive regimens included ceftriaxone (17 patients), meropenem (8 patients), amikacin (6 patients), linezolid (6 patients), ciprofloxacin (5 patients), clarithromycin (3 patients), minocycline (3 patients), and amoxicillin-clavulanate (1 patient). Sixteen patients (28%) developed a drug-related adverse event requiring treatment modification; 13 (81%) were related to TMP-SMX and included the development of cytopenia, rash, and/or gastrointestinal upset. Two adverse events were related to amikacin: 1 case each of reversible acute kidney injury and reversible hearing loss. One patient receiving linezolid developed anemia after 2 weeks of therapy, but the drug was successfully reintroduced 2 weeks later without further complications.
In those who completed treatment, the mean duration of therapy was 7 months (range, 2–15 months; interquartile range [IQR], 5–9 months). The mean duration of intravenous (IV) therapy was 28 days (range, 0–5 months), and mean duration of oral therapy was 6 months (range, 1–14 months). Patients with primary cutaneous disease received shorter treatment courses, with a mean duration of 4.5 days IV (range, 0–14 days) and 3 months oral therapy (range, 2–6 months). In contrast, those with disseminated disease who survived beyond 30 days received a mean duration of 2 months IV (range, 1–4 months) and 9 months oral therapy (range, 3–15 months).
Outcome
Fifty-eight patients were hospitalized. Median length of stay was 20 days (IQR, 10–44 days). Patients were observed for a median of 461 days. Four patients were lost to follow up at 30 days, and 8 patients were lost to follow up at 1 year. No deaths or ICU admissions occurred in the primary cutaneous nocardiosis group. Of the 39 immunocompetent patients, 12 (31%) were admitted to ICU and 9 (27%) died within 1 year of diagnosis. Amongst the immunocompromised patients, 6 (27%) were admitted to ICU and 10 (45%) died within 1 year of diagnosis. Of the 42 patients with pulmonary disease, 12 (29%) were admitted to ICU, 30-day mortality was 13%, and 1-year mortality was 40%. Of the 9 patients with disseminated disease, 56% (5 of 9) were admitted to ICU and 30-day mortality was 33% (3 of 9); no additional deaths were recorded at 1 year. No statistically significant association (P < .05) was identified between any clinical risk factors and mortality or ICU admission (Table 3). High rates of ICU admission (34%) and 1-year mortality (31%) were seen in the chronic lung disease group.
Table 3.
Univariate Analysis of Death and ICU Admission*
| Variable | ICU Admission (N = 61) | Death 30 d (N = 57)† | Death 1 y (N = 53)† | |||
|---|---|---|---|---|---|---|
| Y (N = 18) Number (%) | N (N = 43) Number (%) | Y (N = 8) Number (%) | N (N = 49) Number (%) | Y (N = 19) Number (%) | N (N = 34) Number (%) | |
| Age >60 | 5 (28%) | 17 (40%) | 4 (50%) | 17 (35%) | 8 (42%) | 12 (35%) |
| Female gender | 7 (39%) | 20 (47%) | 2 (25%) | 25 (51%) | 6 (32%) | 20 (59%) |
| Diabetes | 4 (22%) | 9 (21%) | 2 (25%) | 11 (22%) | 4 (21%) | 9 (26%) |
| Hazardous alcohol | 7 (39%) | 11 (26%) | 3 (38%) | 15 (31%) | 4 (21%) | 12 (35%) |
| Malignancy | 3 (17%) | 9 (21%) | 2 (25%) | 8 (16%) | 6 (32%) | 4 (12%) |
| Chronic lung disease | 10 (56%) | 22 (51%) | 5 (65%) | 24 (49%) | 10 (53%) | 19 (56%) |
| Transplant | 1 (6%) | 2 (5%) | 0 (0%) | 3 (6%) | 0 (0%) | 3 (9%) |
| HIV | 0 (0%) | 2 (5%) | 0 (0%) | 2 (4%) | 0 (0%) | 2 (6%) |
| Immunosuppressive medication | 6 (33%) | 9 (21%) | 3 (38%) | 11 (22%) | 7 (37%) | 7 (21%) |
| Indigenous | 8 (44%) | 15 (35%) | 2 (25%) | 21 (43%) | 7 (37%) | 15 (44%) |
| Cutaneous disease | 0 (0%) | 8 (19%) | 0 (0%) | 8 (16%) | 0 (0%) | 3 (9%) |
| Pulmonary disease | 12 (67%) | 30 (70%) | 5 (65%) | 35 (71%) | 16 (84%) | 24 (71%) |
| Disseminated disease | 5 (28%) | 4 (9%) | 3 (38%) | 5 (10%) | 3 (16%) | 5 (15%) |
| Remote | 8 (44%) | 10 (23%) | 2 (25%) | 16 (33%) | 7 (37%) | 10 (29%) |
| Immunocompromised | 6 (33%) | 16 (37%) | 3 (38%) | 17 (35%) | 10 (53%) | 10 (29%) |
| Nocardia isolate resistant to TMP-SMX | 2 (11%) | 4 (9%) | 1 (13%) | 5 (10%) | 2 (11%) | 3 (9%) |
Abbreviations: CNS, central nervous system; HIV; human immunodeficiency virus; ICU, intensive care unit; TMP-SMX, trimethoprim-sulfamethoxazole.
*Associations between categorical variables measured using Fisher’s exact test.
†Numbers in these columns relate to cases where data was available. Four patients were lost to follow up by 30 days, and 8 patients were lost to follow up by 1 year. Note: P > .05 in each instance.
DISCUSSION
This large case series provides the first detailed account of the spectrum of nocardiosis in Australia’s tropical north. The annual incidence of nocardiosis was 2.02 per 100000 people, substantially higher than previously reported rates in Quebec, Canada [16], Madrid, Spain [10], and South Australia (ranging from 0.47 to 0.87 per 100000 people) [17].
We observed similar clinical presentations to those seen in other unrestricted case series. Pulmonary nocardiosis is described as the most common clinical presentation of Nocardia infection [4], and 82% of our cohort had pulmonary involvement. Fifteen percent of patients in our series had disseminated disease, slightly higher than the 6%–13% seen in other unrestricted case series [7, 18, 19] but lower than the 20%–21% reported in highly immunosuppressed populations such as solid organ transplant patients [5, 20]. The overall proportion of immunocompromised hosts in our population (36%) was lower than that reported in a previous review of the Nocardia literature [2].
Our findings highlight the importance of hazardous alcohol as a predisposing factor for nocardiosis. Hazardous alcohol use was reported in 30% of cases, including 44% of the patients with disseminated disease. In 13% of cases (8 of 61), hazardous alcohol use was the only reported predisposing factor for nocardiosis. These findings support previous studies that have suggested (1) an association between hazardous alcohol use and nocardiosis [19, 21–23], (2) that alcohol abuse may be a predisposing factor for dissemination of Nocardia to the CNS [21, 22, 24], and (3) that alcohol may be an important cofactor in predisposition to Nocardia infection due to its effect on macrophage function [25].
Chronic lung disease is well recognized as a predisposing factor for pulmonary nocardiosis, both in the presence and absence of immunosuppressive factors [9, 17, 24, 26]. Chronic lung disease was an important predisposing factor for nocardiosis in our population; approximately two fifths of these patients also had coexistent immunosuppressive factors. Furthermore, chronic lung disease was associated with high rates of ICU admission and 1-year mortality. The presence of clinical nocardiosis in this population may reflect very severe lung disease and heralds a poor prognosis.
Our study accords with previous reports of high mortality (31%–40%) and high rates of ICU admission in unrestricted studies of pulmonary and disseminated nocardiosis [10, 19, 26–28]. Severe disease was common, and fulminant presentations were seen in both immunocompetent and immunocompromised individuals. One third of those with pulmonary and disseminated nocardiosis were admitted to ICU, and 1-year mortality was 38%. It is worth noting that there was a wide range of clinical presentations among immunocompetent hosts, including disseminated disease, as has also been reported previously in Australia [19, 21, 22].
Previous case series of nocardiosis have reported a higher proportion of men compared with women [7–9]. This was not the case in our series, largely due to the overrepresentation of women amongst Indigenous patients with nocardiosis, a phenomenon seen with other infections in this population [29, 30]. A higher incidence of nocardiosis was seen in Indigenous people versus nonindigenous people (2.88 versus 1.71 incident cases per 100 000 people per year); similar findings have also been observed in the setting of other infections in the NT [29]. Possible explanations for the disproportionate burden of nocardiosis in the Indigenous population include a higher rate of predisposing factors such as diabetes, chronic lung disease [31], and hazardous alcohol use, as well as increased environmental exposure.
Randomized controlled trials investigating the treatment of Nocardia infection are extremely challenging given the low numbers of patients presenting at a single institution. Hence, existing recommendations are largely based on observational studies and expert opinion. Because susceptibility profiles vary widely among Nocardia species, combination therapy is frequently used. Current Australian guidelines [32] recommend the use of TMP-SMX plus at least 1 other agent. In our cohort, the most common empirical regimen was TMP-SMX plus ceftriaxone and/or meropenem, and the majority of cases (93%) received an empiric regimen containing at least 1 antibiotic that was active against the corresponding isolate. Rates of TMP-SMX resistance amongst isolates in our study were relatively low (11%), but ceftriaxone (40%) and imipenem (52%) resistance was common, and 4 Nocardia isolates included in our study were resistant to TMP-SMX, ceftriaxone, and imipenem, including 2 Nocardia cyriacigeorgica isolates. All 4 of these isolates were susceptible to linezolid.
Large antimicrobial susceptibility studies of Nocardia isolates have been performed in the United States, Canada, Spain, and Taiwan. Reported resistance rates to imipenem (25%–51%) and ceftriaxone (32%–86%) are high; whereas resistance to amikacin (0%–5%) and linezolid (0%–1%) is uncommon [16, 33–37]. There is considerable variability in reporting of TMP-SMX resistance, ranging from 2% to–43% across these studies. Although geographic variation in Nocardia isolates may account for some of the variability between studies, Brown-Elliott et al [36] (who report a 2% TMP-SMX resistance rate amongst their Nocardia isolates) hypothesize that the discrepancy may be associated with difficulty in the laboratory interpretation of in vitro minimum inhibitory concentrations and the lack of quality controls for Nocardia for TMP-SMX. Although reports of in vitro resistance TMP-SMX have increased, there have only been rare clinical reports describing treatment failure with TMP-SMX [36, 38]. It is notable that 1 patient in our series with TMP-SMX resistance had a fatal outcome.
All of the isolates presented here demonstrated in vitro susceptibility to linezolid. In vitro activity of linezolid against all clinically relevant species of Nocardia has previously been demonstrated [33, 37], and clinical success has been reported in case reports and series [38, 39]. Although linezolid has excellent bioavailability and crosses the blood-brain barrier, the barriers to its use are high cost and serious toxicities associated with prolonged administration, which include myelosuppression, lactic acidosis, peripheral neuropathy, and optic neuritis [40]. However, in light of its excellent in vitro profile against Nocardia, it is attractive as an option for empiric treatment of nocardiosis in patients with extensive or disseminated disease. Based on our experience, we would recommend an empiric regimen that includes TMP-SMX and linezolid in unwell patients pending susceptibilities.
There are some limitations to our study. Data were collected retrospectively and did not include potentially relevant comorbidities such as chronic liver disease and nondialysis-dependent chronic kidney disease. Species identification of Nocardia isolates occurred at the time of collection, and taxonomic changes have occurred over time. In addition, this is a single-center study from a geographically restricted area, and findings regarding species type and susceptibilities are not necessarily applicable to other settings.
CONCLUSIONS
The annual incidence rate of Nocardia infections in our setting is the highest reported globally to date. Chronic lung disease was the most common underlying condition. Most of the patients in our series did not have classic immunosuppressive risk factors, but hazardous alcohol use and diabetes were common. Severe disease was seen in both immunocompromised and immunocompetent hosts, and there were high rates of ICU admission and 1-year mortality. Linezolid and TMP-SMX demonstrated the best in vitro activity against the isolates studied and should be considered for inclusion in an empiric regimen for unwell patients with nocardiosis pending susceptibilities. The diagnosis of nocardiosis should be considered in patients presenting with consistent clinical features regardless of immunosuppression status, particularly if they have underlying chronic lung disease.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
Acknowledgments
We thank Dr. Peter Markey (Centre for Disease Control) for providing access to Health District Population Data, Dr. Alexandra Hofer and Jordan Amor-Robertson for assistance with data collection, and the laboratory staff at the Royal Darwin Hospital for providing provisional Nocardia results.
Financial support. N. M. A., A. P. R., J. S. D., and S. Y. C. T. are supported by Fellowships from the National Health and Medical Research Council of Australia. R. N. P. is supported by a Wellcome Trust Senior Fellowship.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 2006; 19:259–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev 1994; 7:213–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown-Elliott BA, Conville PS, Wallace RJ., Jr Current status of Nocardia taxonomy and recommended identification methods. Clin Microbiol Newsl 2015; 37:25–32. [Google Scholar]
- 4. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc 2012; 87:403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peleg AY, Husain S, Qureshi ZA, et al. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis 2007; 44:1307–14. [DOI] [PubMed] [Google Scholar]
- 6. Roberts SA, Franklin JC, Mijch A, Spelman D. Nocardia infection in heart-lung transplant recipients at Alfred Hospital, Melbourne, Australia, 1989–1998. Clin Infect Dis 2000; 31:968–72. [DOI] [PubMed] [Google Scholar]
- 7. Wang HL, Seo YH, LaSala PR, et al. Nocardiosis in 132 patients with cancer: microbiological and clinical analyses. Am J Clin Pathol 2014; 142:513–23. [DOI] [PubMed] [Google Scholar]
- 8. Amin A, Mahmood SF, Anis M, et al. Pulmonary nocardiosis: a comparative analysis of Nocardia asteroides and non-asteroides species. Trop Doct 2012; 42:94–6. [DOI] [PubMed] [Google Scholar]
- 9. Kurahara Y, Tachibana K, Tsuyuguchi K, et al. Pulmonary nocardiosis: a clinical analysis of 59 cases. Respir Investig 2014; 52:160–6. [DOI] [PubMed] [Google Scholar]
- 10. Minero MV, Marín M, Cercenado E, et al. Nocardiosis at the turn of the century. Medicine (Baltimore) 2009; 88:250–61. [DOI] [PubMed] [Google Scholar]
- 11. Health Gains Planning Branch HPD, Department of Health, Northern Territory Government. Health District Population Data for the Northern Territory. Darwin, Northern Territory; 2014. [Google Scholar]
- 12. National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol. Australian Government National Health and Medical Research Council. Canberra, Australia; 2009. [Google Scholar]
- 13. Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 1991; 173:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kong F, Wang H, Zhang E, et al. secA1 gene sequence polymorphisms for species identification of Nocardia species and recognition of intraspecies genetic diversity. J Clin Microbiol 2010; 48:3928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. CLSI. Susceptibility Testing of Mycobacteria, Nocardiae and Other Aerobic Actinomycetes; Approved Standard—Second Edition. CLSI document M24-A2. Wayne, PA; Clinical and Laboratory Standards Institute; 2011. [PubMed] [Google Scholar]
- 16. Tremblay J, Thibert L, Alarie I, et al. Nocardiosis in Quebec, Canada, 1988-2008. Clin Microbiol Infect 2011; 17:690–6. [DOI] [PubMed] [Google Scholar]
- 17. Hui CH, Au VW, Rowland K, et al. Pulmonary nocardiosis re-visited: experience of 35 patients at diagnosis. Respir Med 2003; 97:709–17. [DOI] [PubMed] [Google Scholar]
- 18. Liu WL, Lai CC, Ko WC, et al. Clinical and microbiological characteristics of infections caused by various Nocardia species in Taiwan: a multicenter study from 1998 to 2010. Eur J Clin Microbiol Infect Dis 2011; 30:1341–7. [DOI] [PubMed] [Google Scholar]
- 19. Georghiou PR, Blacklock ZM. Infection with Nocardia species in Queensland. A review of 102 clinical isolates. Med J Aust 1992; 156:692–7. [DOI] [PubMed] [Google Scholar]
- 20. Santos M, Gil-Brusola A, Morales P. Infection by Nocardia in solid organ transplantation: thirty years of experience. Transplant Proc 2011; 43:2141–4. [DOI] [PubMed] [Google Scholar]
- 21. Kennedy KJ, Chung KH, Bowden FJ, et al. A cluster of nocardial brain abscesses. Surg Neurol 2007; 68:43–9; discussion 49. [DOI] [PubMed] [Google Scholar]
- 22. Lee GY, Daniel RT, Brophy BP, Reilly PL. Surgical treatment of nocardial brain abscesses. Neurosurgery 2002; 51:668–71; discussion 671–2. [PubMed] [Google Scholar]
- 23. Crotty JM. Systemic mycotic infections in northern territory aborigines. Med J Aust 1965; 1:184–6. [DOI] [PubMed] [Google Scholar]
- 24. Martínez Tomás R, Menéndez Villanueva R, Reyes Calzada S, et al. Pulmonary nocardiosis: risk factors and outcomes. Respirology 2007; 12:394–400. [DOI] [PubMed] [Google Scholar]
- 25. Javaly K, Horowitz HW, Wormser GP. Nocardiosis in patients with human immunodeficiency virus infection. Report of 2 cases and review of the literature. Medicine (Baltimore) 1992; 71:128–38. [DOI] [PubMed] [Google Scholar]
- 26. Chen YC, Lee CH, Chien CC, et al. Pulmonary nocardiosis in southern Taiwan. J Microbiol Immunol Infect 2013; 46:441–7. [DOI] [PubMed] [Google Scholar]
- 27. Rosman Y, Grossman E, Keller N, et al. Nocardiosis: a 15-year experience in a tertiary medical center in Israel. Eur J Intern Med 2013; 24:552–7. [DOI] [PubMed] [Google Scholar]
- 28. Tuo MH, Tsai YH, Tseng HK, et al. Clinical experiences of pulmonary and bloodstream nocardiosis in two tertiary care hospitals in northern Taiwan, 2000–2004. J Microbiol Immunol Infect 2008; 41:130–6. [PubMed] [Google Scholar]
- 29. Davis JS, Cheng AC, McMillan M, et al. Sepsis in the tropical top end of Australia’s Northern Territory: disease burden and impact on Indigenous Australians. Med J Aust 2011; 194:519–24. [DOI] [PubMed] [Google Scholar]
- 30. Tong SY, Bishop EJ, Lilliebridge RA, et al. Community-associated strains of methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus in indigenous Northern Australia: epidemiology and outcomes. J Infect Dis 2009; 199:1461–70. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Y, Connors C, Wright J, et al. Estimating chronic disease prevalence among the remote Aboriginal population of the Northern Territory using multiple data sources. Aust N Z J Public Health 2008; 32:307–13. [DOI] [PubMed] [Google Scholar]
- 32. Antibiotic Expert Groups. Therapeutic Guidelines: Antibiotic, Version 15. Melbourne: Therapeutic Guidelines Limited; 2014. [Google Scholar]
- 33. Schlaberg R, Fisher MA, Hanson KE. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother 2014; 58:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larruskain J, Idigoras P, Marimón JM, Pérez-Trallero E. Susceptibility of 186 Nocardia sp. isolates to 20 antimicrobial agents. Antimicrob Agents Chemother 2011; 55:2995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai CC, Liu WL, Ko WC, et al. Antimicrobial-resistant Nocardia isolates, Taiwan, 1998-2009. Clin Infect Dis 2011; 52:833–5. [DOI] [PubMed] [Google Scholar]
- 36. Brown-Elliott BA, Biehle J, Conville PS, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol 2012; 50:670–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uhde KB, Pathak S, McCullum I, Jr, et al. Antimicrobial-resistant Nocardia isolates, United States, 1995–2004. Clin Infect Dis 2010; 51:1445–8. [DOI] [PubMed] [Google Scholar]
- 38. Moylett EH, Pacheco SE, Brown-Elliott BA, et al. Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis 2003; 36:313–8. [DOI] [PubMed] [Google Scholar]
- 39. Tanioka K, Nagao M, Yamamoto M, et al. Disseminated Nocardia farcinica infection in a patient with myasthenia gravis successfully treated by linezolid: a case report and literature review. J Infect Chemother 2012; 18:390–4. [DOI] [PubMed] [Google Scholar]
- 40. Jodlowski TZ, Melnychuk I, Conry J. Linezolid for the treatment of Nocardia spp. infections. Ann Pharmacother 2007; 41:1694–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.