Abstract
Background.
Both human immunodeficiency virus (HIV) infection and antiretroviral therapy (ART) may increase cardiovascular disease (CVD) risk. Vascular function assessments can be used to study CVD pathogenesis. We compared the effect of immediate versus deferred ART initiation at CD4 counts >500 cells/mm3 on small arterial elasticity (SAE) and large artery elasticity (LAE).
Methods.
Radial artery blood pressure waveforms were recorded noninvasively. Small arterial elasticity and LAE were derived from analysis of the diastolic pulse waveform. Randomized treatment groups were compared with linear models at each visit and longitudinal mixed models.
Results.
Study visits involved 332 participants in 8 countries: mean (standard deviation [SD]) age 35 (10), 70% male, 66% nonwhite, 30% smokers, and median CD4 count 625 cells/mm3 and 10-year Framingham risk score for CVD 1.7%. Mean (SD) SAE and LAE values at baseline were 7.3 (2.9) mL/mmHg × 100 and 16.6 (4.1) mL/mmHg × 10, respectively. Median time on ART was 47 and 12 months in the immediate and deferred ART groups, respectively. The treatment groups did not demonstrate significant within-person changes in SAE or LAE during the follow-up period, and there was no difference in mean change from baseline between treatment groups. The lack of significant differences persisted after adjustment, when restricted to early or late changes, after censoring participants in deferred group who started ART, and among subgroups defined by CVD and HIV risk factors.
Conclusions.
Among a diverse global population of HIV-positive persons with high CD4 counts, these randomized data suggest that ART treatment does not have a substantial influence on vascular function among younger HIV-positive individuals with preserved immunity.
Keywords. antiretroviral therapy, arterial elasticity, cardiovascular disease, HIV infection, vascular dysfunction.
Human immunodeficiency virus (HIV)-positive persons are at increased risk for premature cardiovascular disease (CVD) [1], which is now a leading cause of morbidity and mortality among patients treated with antiretroviral therapy (ART) [2]. The underlying pathology of CVD among HIV-positive persons includes atherosclerotic disease, as well as associated vascular abnormalities such as endothelial dysfunction and hypertension [3, 4]. Factors contributing to excess CVD risk among HIV-positive persons include a greater prevalence of traditional risk factors (eg, smoking, lipid abnormalities) and consequences of HIV disease—such as HIV replication, immune deficiency, and exposure to certain antiretroviral medications [5–8]. Recent data also emphasize the central importance of systemic inflammation, which itself may improve but persist at elevated levels with ART [9, 10].
Given the potential for ART initiation to both increase (via drug toxicity) and decrease (via HIV viral suppression) CVD risk, it is important to study this complex pathophysiology in the context of randomized comparisons. The Strategic Timing of AntiRetroviral Therapy (START) trial is a randomized controlled clinical trial of immediate initiation of ART (“immediate” group) versus deferral of ART initiation until clinical symptoms develop or CD4+ counts decline to <350 cells/mm3 (“deferred” group), among patients naive to ART with CD4+ counts >500 cells/m3 at baseline. The START is an ideal study design to compare CVD risk between untreated and ART-treated HIV infection in a controlled fashion.
Prognostic markers of vascular disease are useful tools to detect early CVD. Carotid artery intima media thickness (CIMT) and coronary artery calcified plaque (CAC) are structural markers for CVD. In contrast, functional markers for CVD include arterial stiffness (the inverse of elasticity) or endothelial dysfunction (eg, assessed via brachial artery flow-mediated dilation). Functional markers have value for early detection of CVD before changes in structural markers are detected [11]. We have previously reviewed noninvasive techniques for assessing vascular function and the rationale for utilizing blood pressure (BP) waveform analysis in the START trial [12]. Our approach estimating arterial elasticity is responsive to interventions (eg, statins or angiotensin receptor blockers) [13, 14], is highly reproducible with repeated measures [15], and has been independently associated with subsequent coronary heart disease, stroke, congestive heart failure, and incident hypertension in the general population [16–18]. In this study, we report the primary results of the START Arterial Elasticity Substudy.
METHODS
Design and Sample Size Considerations
The design and primary findings from the START trial have been described previously [19, 20], and the experimental randomized intervention is described above. The primary hypothesis was that immediate initiation of ART would increase (improve) both small artery elasticity (SAE) and large artery elasticity (LAE), respectively, compared with deferred ART initiation. After informed consent, participants were randomized and study data collection occurred at baseline, at months 4, 8, and 12, and then annually thereafter. The coprimary outcome measures were changes from baseline in SAE and LAE, over an average of all follow-up visits. The study was designed to coenroll at least 300 participants. Assuming standard deviations (SDs) of 3.0 mL/mmHg × 100 for SAE and 5.0 mL/mmHg × 10 for LAE, the substudy had 80% power to detect a difference between groups of 1.2 mL/mmHg × 100 in SAE and 1.9 mL/mmHg × 10 in LAE. Based on epidemiologic data from 6235 participants in the Multi-Ethnic Study of Atherosclerosis (MESA), a 1.2-unit change in SAE corresponds to an effect size of 0.4 SD units for men and 0.5 SD units for women [21]; corresponding differences in absolute CVD event risk would then be 11% for men and 15% for women [16]. The study protocol was approved by ethics committees at the University of Minnesota and all participating clinical centers.
Clinical and Laboratory Data
Demographic and clinical data were collected as part of the main START trial [20]. Human immunodeficiency virus ribonucleic acid (RNA) level and CD4 count were ascertained at every visit, and blood cholesterol levels were obtained annually. Ten-year clinical CVD risk was estimated using the following: (1) Framingham Risk Score (FRS) for a CVD event [22], and (2) the pooled cohort risk assessment for atherosclerotic CVD (ASCVD) event [23]. Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.
Blood Pressure Waveform Analysis to Estimate Arterial Elasticity
Radial artery BP waveforms were recorded using the HDI/PulseWave CR-2000 (Hypertension Diagnostics, Inc., Eagan, MN). A solid-state pressure transducer array (tonometer) was placed over the radial artery of the dominant arm to record the BP contour. A 30-second analog tracing of the radial BP waveform, constituting pressure changes during diastole, was digitalized at 200 samples per second. An automated, oscillatory BP measurement was ascertained at the brachial artery of the contralateral arm. Arterial elasticity was estimated by modeling the diastolic BP waveform as a decaying exponential function plus a sinusoidal function dampened by a decaying exponential, as previously described [24]. This approach treats the circulatory system as a modified Windkessel model, considering the arterial system a network of vessels designed to convert intermittent flow from the heart to a continuous and steady flow across the capillaries. The model then characterizes capacitance and resistance factors to estimate 2 parameters (Supplemental Figure A): (1) the distal arterial compliance, or oscillatory compliance (C2), referred to as SAE; and (2) the proximal arterial compliance, or capacitance compliance (C1), referred to as LAE.
The procedures for obtaining a BP waveform are consistent with established protocols using this technique in large CVD cohorts [16, 18, 21, 25]. Participants are instructed to avoid caffeine, alcohol, nicotine, antihistamines, and nonsteroidal anti-inflammatory drugs before the study visit. Research staff were trained to measure BP waveform, and they were certified based on the quality of waveform measures from 3 separate volunteers as assessed by 2 independent reviewers. Research staff were recertified on BP waveform procedures annually. During study visits, waveform measures were performed in triplicate to reduce short-term biologic variability, and averages from each visit were used for analyses. The BP waveform data from each study visit was evaluated (blinded to treatment group) for waveform quality, with focus on appropriate morphology. Only waveforms passing qualitative review were included in analyses. To assess intraoperator repeatability of arterial elasticity measurements, study staff performed duplicate BP waveform assessments, each on a single volunteer separated by 30–120 minutes. The difference and correlation between repeatability measures were summarized.
Statistical Methods
Analyses for the coprimary endpoint (change in SAE and LAE) were by intention to treat, comparing the immediate and deferred ART arms. All participants with an acceptable baseline BP waveform were included in the analyses. Baseline and demographic summaries are presented as percentages and medians with 25th and 75th percentiles. General linear models were used to compare the treatment arms for change in arterial elasticity at each visit, and a longitudinal mixed model with an unstructured covariance matrix was used to estimate the overall change from baseline. The prespecified level of significance was set at alpha <0.025 (2-sided), given the 2 coprimary endpoints. Overall changes from baseline were also estimated with longitudinal mixed models for predefined subgroups, including age, gender, ethnicity, current smoking status, body mass index (BMI), systolic BP (SBP), diastolic BP (DBP), Framingham CVD risk score, CD4 cell counts, HIV RNA levels, and duration of HIV infection. All models for change in SAE and LAE were adjusted for baseline value.
RESULTS
Quality and Repeatability of Arterial Elasticity Measures
Among 2021 study visits involving 332 participants, 1991 (99%) visits had ≥1 BP waveform pass qualitative review. Among the 1961 study visits with ≥2 acceptable waveforms, the average within-person SD was 0.87 mL/mmHg × 100 (CV = 0.11) for SAE measurements and 1.67 mL/mmHg × 10 (CV = 0.10) for LAE measurements. Of 27 study staff who performed duplicate BP waveform assessments on the same volunteer, separated by 30–120 minutes, the mean (±SD) difference between the duplicate measurements was 0.05 ± 1.64 mL/mmHg × 100 for SAE and −0.10 ± 2.55 mL/mmHg × 10 for LAE. The correlation between the duplicate measurements was 0.72 and 0.71 for SAE and LAE measurements, respectively.
Participant Characteristics
Of 431 participants randomized to the main START study after the Arterial Elasticity Substudy opened, 336 participants (78%) from 21 sites and 10 countries were coenrolled and randomized into the substudy from 2009 to 2012. Of these, 332 participants had baseline BP waveforms that passed qualitative review and are included in the analysis cohort. Baseline demographics are shown (Table 1). This diverse study sample was at very low risk for CVD. The median values for BMI, BP, and blood cholesterol levels were within normal ranges. By chance, there were more participants in the deferred versus immediate ART group taking lipid-lowering therapy (5.2% vs 0.6%, respectively; P = .01) and with a prior history of CVD (2.6% vs 0.0%, respectively; P = .03). Several of the other traditional CVD risk factors were slightly higher among deferred group participants, including the FRS and ASCVD pooled cohort 10-year risk score (P = .05). Finally, among this substudy of START, there was 1 CVD event (in the deferred group) during follow-up.
Table 1.
Baseline Characteristics of START Arterial Elasticity Substudy (N = 332)
| Participant Characteristics | Immediate | Deferred |
|---|---|---|
| Number of Participants | 178 | 154 |
| Demographics | ||
| Female gender, N (%) | 54 (30.3%) | 46 (29.9%) |
| Age, median years (IQR) | 33 (27–40) | 34 (29–43) |
| Race/Ethnicity, N (%) | ||
| White | 57 (32.0%) | 56 (36.4%) |
| Black | 45 (25.3%) | 35 (22.7%) |
| Asian | 63 (35.4%) | 58 (37.7%) |
| Latino/Hispanic | 9 (5.1%) | 5 (3.2%) |
| Other | 4 (2.2%) | 0 (0.0%) |
| Clinical History | ||
| Sexual risk factor, N (%) same-sex exposure | 103 (57.9%) | 83 (53.9%) |
| Hepatitis B or C, N (%) | 15 (8.4%) | 12 (7.8%) |
| Diabetes, N (%) | 3 (1.7%) | 4 (2.6%) |
| Smoking, N (%) | 52 (29.2%) | 47 (30.5%) |
| Prior CVD, N (%) | 0 (0.0%) | 4 (2.6%) |
| BP-lowering therapy, N (%) | 8 (4.5%) | 9 (5.8%) |
| Lipid-lowering therapy, N (%) | 1 (0.6%) | 8 (5.2%) |
| Duration HIV positive, median years (IQR) | 1.2 (0.4–3.0) | 1.3 (0.4–3.5) |
| Clinical Data | ||
| BMI, median kg/m2 (IQR) | 23 (21–26) | 23 (21–26) |
| Systolic BP, median mmHg (IQR) | 119 (112–129) | 121 (113–133) |
| Diastolic BP, median mmHg (IQR) | 74 (68–80) | 75 (68–81) |
| 10-yr CVD risk (FRS), median (IQR) | 1.5 (0.4–4.4) | 1.9 (0.7–6.1) |
| 10-yr ASCVD risk, median (IQR) | 0.8 (0.2–2.4) | 1.0 (0.3–2.9) |
| eGFR, median mL/min/1.732 (IQR) | 113 (100–123) | 111 (98–121) |
| CD4 cell count, median cells/mm3 (IQR) | 616 (563–730) | 634 (562–726) |
| HIV RNA, median log10 copies/mL (IQR) | 4.2 (3.7–4.7) | 4.2 (3.6–4.7) |
| SAE, median mL/mmHg ×100 (IQR) | 7.6 (5.5–9.4) | 6.8 (5.0–8.8) |
| LAE, median mL/mmHg ×10 (IQR) | 16.9 (13.7–19.5) | 15.7 (13.3–18.3) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FRS, Framingham Risk Score; HIV, human immunodeficiency virus; IQR, interquartile range; LAE, large arterial elasticity; N, number; RNA, ribonucleic acid; SAE, small arterial elasticity; START, Strategic Timing of AntiRetroviral Treatment.
Baseline Small and Large Artery Elasticity Measurements
Median measures of SAE and LAE were 7.2 (interquartile range [IQR], 5.2–9.3) mL/mmHg × 100 and 16.3 (IQR, 13.6–19.3 mL/mmHg × 10), respectively (Table 1). Baseline distributions of SAE and LAE and associations with traditional CVD risk factors have been previously reported [12]. At baseline, both SAE and LAE were lower (impaired) with increased age, female gender, and increased SBP and DBP; SAE was lower for those with a history of CVD and a higher 10-year CVD risk by FRS [12]. Neither HIV RNA level nor CD4+ count were associated with baseline SAE or LAE [12].
Antiretroviral Therapy Treatment and Follow-up
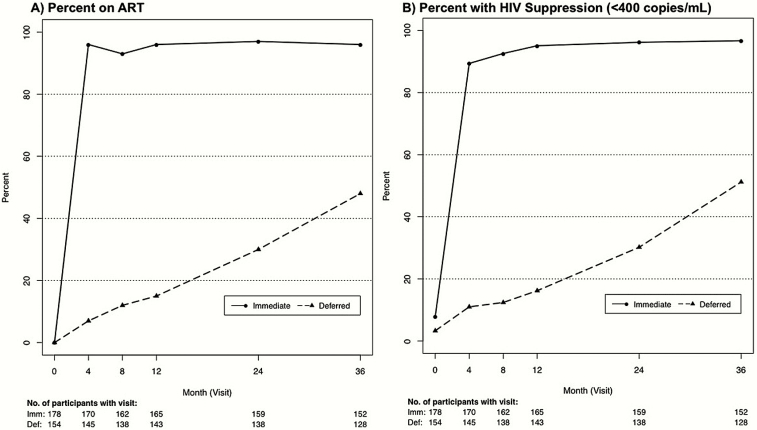
Median duration of follow-up was 48 (IQR, 40–59) months. Six percent of study participants (n = 20; 11 in immediate and 9 in deferred group) withdrew or were lost to follow-up, and 84% (n = 280) of participants completed a month 36 study visit (36% completed a month 48 visit). Median time on ART was 47 (IQR, 39–59) and 12 (IQR, 0–31) months in the immediate and deferred ART groups, respectively. The percentage of those on ART among immediate and deferred groups were 96% and 18% at month 12, 97% and 30% at month 24, and 96% and 48% at month 36, respectively.
Figure 1 plots HIV viral suppression and visit attendance by group over follow-up, indicating that 97% of immediate and 51% of deferred participants achieved an HIV RNA level <400 copies/mL by 36 months. Among participants in the immediate group, the initial ART regimen included tenofovir disoproxil fumarate (TDF) in 99%, efavirenz in 74%, a protease inhibitor ([PI] predominantly darunavir or atazanavir) in 15%, and an integrase strand transfer inhibitor ([INSTI] all consisting of raltegravir) in 7%. Among deferred group participants who started ART, the corresponding initial regimens included TDF in 98%, efavirenz in 60%, a PI in 15%, and an INSTI in 14%.
Figure 1.
Antiretroviral therapy (ART) use and viral suppression by treatment group over follow-up. Plots present the percentage of substudy participants in each treatment group that are on ART (A) and have a human immunodeficiency virus (HIV) ribonucleic acid level <400 copies/mL (B) at each visit over follow-up. The numbers of participants from each treatment group that attended each study visit are also listed below the x-axis.
Small Arterial Elasticity
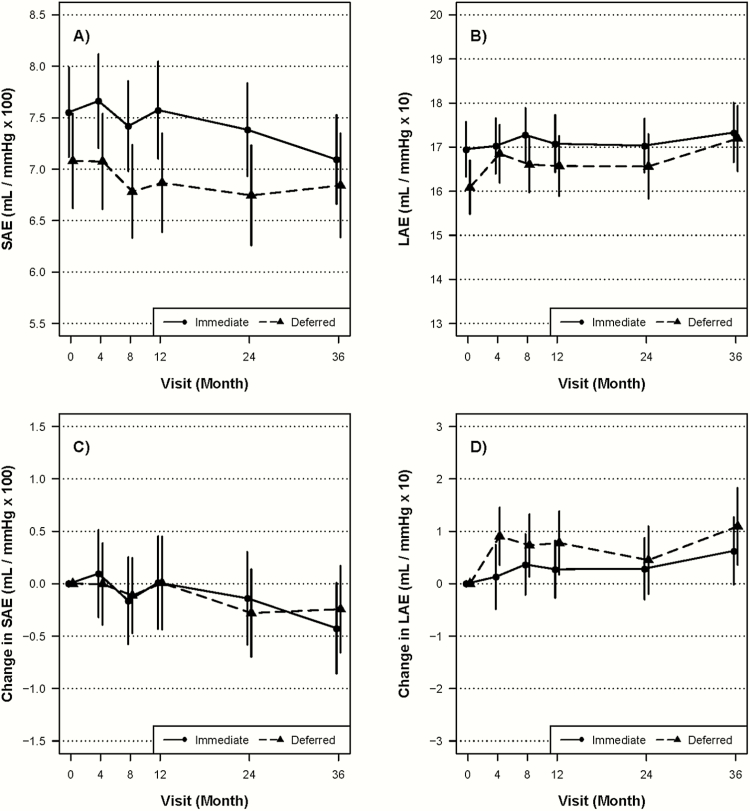
Figure 2 and Table 2 present the differences between treatment groups in change from baseline SAE values. The distribution of SAE values at each follow-up is included in a Supplemental Table. There was no significant difference between immediate and deferred ART groups in the change from baseline SAE, either over all of follow-up or at any of the individual follow-up visits. The lack of differences persisted when examining changes before or after 12 months, when censoring participants in the deferred ART group who started ART or when adjusting for CVD risk factors (eg, FRS, use of lipid-lowering therapy, use of BP-lowering therapy, or smoking) and other demographics (age, race, and gender) (data not shown). Neither treatment group demonstrated within participant changes in SAE over time, including analyses that focused on changes among deferred group participants after they started ART. Subgroup analyses, examining the treatment effect on changes from baseline in SAE, were examined, and there was no significant treatment by subgroup interaction (see Supplemental Figure B).
Figure 2.
Small and large arterial elasticity over time by treatment group in Strategic Timing of Antiretroviral Treatment (START). Plots present mean and change in small arterial elasticity ([SAE] A and C, respectively) and large arterial elasticity ([LAE] B and D, respectively) over time for both immediate and deferred groups in START. Confidence bounds represent 95% confidence intervals.
Table 2.
Estimates of Change in Small and Large Arterial Elasticity at Study Visits and Overall
| Follow-up Visit | Small Artery Elasticity | Large Artery Elasticity | ||
|---|---|---|---|---|
| Differencea | P Value | Differencea | P Value | |
| At month 4 | 0.29 (−0.24, 0.81) | 0.28 | −0.38 (−1.13, 0.38) | 0.33 |
| At month 8 | 0.22 (−0.27, 0.72) | 0.38 | 0.10 (−0.64, 0.84) | 0.79 |
| At month 12 | 0.30 (−0.26, 0.87) | 0.30 | −0.15 (−0.91, 0.61) | 0.70 |
| At month 24 | 0.35 (−0.19, 0.90) | 0.20 | 0.10 (−0.69, 0.89) | 0.80 |
| At month 36 | 0.00 (−0.53, 0.54) | >0.99 | −0.19 (−1.06, 0.69) | 0.68 |
| All available datab | 0.25 (−0.13, 0.63) | 0.20 | −0.12 (−0.67, 0.42) | 0.66 |
aTreatment group differences in small artery elasticity and large artery elasticity adjusted for baseline value. Positive values reflect an improvement in arterial elasticity in the early (compared with deferred) antiretroviral therapy group.
bRepeated measure analysis using unstructured covariance matrix and adjusted for baseline value.
Large Arterial Elasticity
Figure 2 and Table 2 also present the data for change from baseline in LAE over time. There was no difference between immediate and deferred ART groups in the change from baseline in LAE values. The lack of differences persisted in secondary and subgroup analyses (data not shown; analyses conducted analogous to those presented for SAE). Neither treatment group demonstrated within participant changes in LAE over time.
Antiretroviral Regimen Effects
Among the immediate ART group, there were no significant differences for change in SAE or LAE by initial ART regimen (ie, efavirenz containing versus not, PI containing versus not, or raltegravir containing versus not) (data not shown).
DISCUSSION
The arterial elasticity substudy of the START trial is the first randomized comparison of ART initiation on vascular function, when compared with deferral of ART (ie, untreated HIV infection). We did not observe a significant influence of ART initiation on SAE or LAE. These findings challenge prior reports, based on cross-sectional and uncontrolled data, suggesting that ART treatment may improve (or impair) vascular function [26–34], as well as clinical outcome data from the Strategic Management of AntiRetroviral Therapy trial showing that continuous ART treatment was associated with lower CVD event rates when compared with episodic ART [35, 36]. Strategic Timing of AntiRetroviral Therapy is unique with respect to studying HIV-related CVD risk, both in terms of the target population (with relative immune preservation and at very low CVD risk overall) and the prospective controlled study design (ie, experimental intervention of ART initiation). Therefore, findings from this study both address limitations from prior studies and raise important questions about CVD risk among contemporary patients who initiate ART very early during HIV disease.
Several potential explanations exist for why our findings do not show changes in arterial elasticity with ART initiation. Antiretroviral therapy initiation may influence CVD risk, positively or negatively, through biology not directly assessed. Arterial elasticity does not directly assess nitric oxide-mediated endothelial dysfunction, which has been shown to improve after ART initiation [29, 37]. Arterial elasticity estimation through BP waveform analysis reflects a more mixed pathology including both structural and functional changes throughout the vasculature. Furthermore, arterial elasticity measurements do not directly assess atherosclerotic disease per se. Some longitudinal HIV studies of imaging techniques that do assess atherosclerotic lesions (eg, ultrasound estimates of CIMT or carotid plaque prevalence, or computed tomography assessments of CAC) have demonstrated changes in disease progression that are influenced by ART use [38–41]. However, it is important to note that none of these data on ART-related changes in CVD assessments included a randomized comparison with untreated HIV infection.
Additional factors that may have influenced our findings include that participants were at low risk for CVD risk, had a median duration of HIV diagnosis of only 1.3 years, and were observed for a relatively modest duration. All of these factors may have influenced the degree to which HIV- or ART-related vascular damage occurred. Our ability to study the effects of specific antiretrovirals was also limited. Specifically, the ART regimen used by the immediate group included TDF in 99% and efavirenz in 74%, and neither drug has been associated with excess risk for CVD when compared with other antiretrovirals [42, 43]. In addition, this study did not include measures of plasma cytokines, monocyte activation, and/or T-cell activation—all shown to be potentially important for HIV-related CVD pathogenesis [9, 10, 44]. Further characterization of the effects of ART initiation on systemic inflammation during very early HIV disease will be an important focus of future research involving START. Finally, it is worth emphasizing that both HIV infection and ART may exert offsetting influences on vascular function [45, 46], such that the finding of no detectable treatment effect may be the net result of ART toxicity concurrent with declines in HIV-specific effects.
Our study also has risk for type-2 statistical error given our sample size; ie, there may be a small magnitude difference between immediate and deferred ART that we were unable to detect. However, we were able to rule out what would likely be a clinically meaningful difference in SAE—which is associated with a broad spectrum of CVD events in the MESA cohort [16]. If the estimate for an SAE treatment effect in our study was found to be significant in a larger sample (ie, an effect of ART initiation resulting in an improvement of SAE by 0.25 mL/mmHg × 100), data from MESA suggest this would correspond to an approximate 2%–3% absolute difference in CVD event risk (or a 5%–8% difference at our upper confidence limit of 0.63 SAE units).
Despite these limitations, our findings provide compelling randomized data suggesting that ART initiation may not affect CVD risk to a clinically meaningful degree among HIV-postive patients with preserved immunity. Indeed, the primary findings from START report 12 and 14 CVD events in the immediate and deferred ART groups, respectively, corresponding to a very low overall CVD event rate of 0.19/100 person-years (among n = 4685 participants over a mean 3.0 years of follow-up) [20]. The relative immune preservation and short duration of HIV infection in START are particularly important considerations. Much of the data demonstrating greater CVD risk at lower CD4 counts and at higher HIV viral loads, factors that improve with ART, involve studies of participants with a much greater degree of HIV disease progression [1, 47]. Analyses of CVD risk based on the level of immune depression (ie, current and nadir CD4 count) were conducted in the D:A:D (Data collection on Adverse events of anti-HIV Drugs) cohort and demonstrated very weak associations overall that were only present at lower CD4+ counts [8]. Cumulatively, these observations and our results support a hypothesis that excess CVD risk attributable to HIV infection may simply not be apparent in early disease, given that the effects of HIV infection on CVD pathogenesis occur over the long term, whether from immune depletion, inflammation, and/or other disease-specific factors. Thus, strategies such as ART to mitigate HIV-related CVD risk may only have a modest benefit that will take many years to manifest.
CONCLUSIONS
In conclusion, we have shown that immediate ART initiation did not significantly improve SAE or LAE, when compared with randomization to ART deferral (ie, largely untreated HIV infection), among a diverse global population of HIV-positive persons with recent HIV infection and high CD4+ counts. These data suggest that early ART treatment does not influence vascular function to a clinically meaningful extent among HIV-positive persons with preserved immunity.
Supplementary Data
Supplementary material is available at Open Forum Infectious Diseases online.
START Arterial Elasticity Study Team Credit Roster
Copenhagen: B. Aagaard, Á. H. Borges, P. O. Jansson, B. Riis Neilsen; London: A. Arenas-Pinto, N. B. Atako, A. G. Babiker, E. Dennis, S. Forcat, F. Hudson, B. Jackson, D. Maas, C. Purvis, C. Russell; Sydney: C. Carey, M. Clewett, S. Emery, S. Jacoby; Washington: E. B. Finley, A. Sánchez, M. J. Vjecha.
Site Investigators by Country by Enrollment (n = number of participants enrolled)
Thailand (n = 107): A. Avihingsanon, P. Chetchotisakd, S. Kiertiburanakul, W. Prasithsirikul, W. Ratanasuwan, P. Rerksirikul, K. Ruxrungtham, A. Tiyabut; Uganda (n = 67): J. Lutaakome, P. Munderi; United States (n = 41): G. Culbert, R. Givot, K. Henry, M. K. Jain, L. H. Makohon, N. P. Markowitz, R. M. Novak, C. Rosario, M. Santos, J. Shuter; Germany (n = 40): A. Jessen, H. Jessen, V. Müller, C. Stephan, K. Tillmann, T. Wolf; Argentina (n = 27): W. H. Belloso, J. Bruguera, M. del Lujan Sanchez, M. L. Doldan, G. R. Loria, M. H. Losso, A. Moricz; Greece (n = 17): O. Anagnostou, V. Gioukari, I. Katsarolis, K. Protopapas, G. Touloumi; India (n = 15): F. Beulah, N. Kumarasamy; Denmark (n = 11): T. Benfield, A.-M. Lehbech, I. H. Mathiesen, F. Rønsholdt; Australia (n = 9): C. Carey, M. Clewett, D. A. Cooper, S. Emery, S. Jacoby, S. L. Pett; United Kingdom (n = 3): B. Gazzard, A. Jackson.
Supplementary Material
Acknowledgments
We thank the participants in the Strategic Timing of AntiRetroviral Therapy (START) Arterial Elasticity Substudy.
Disclaimers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. The START study (NCT00867048) and the START Arterial Elasticity Substudy (NCT01776151) are registered at clinicaltrials.gov. The START study is primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number UM1-AI068641, the Department of Bioethics at the NIH Clinical Center and 5 NIH institutes: the National Cancer Institute, the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, and the National Institute of Arthritis and Musculoskeletal Disorders. Additional support for Arterial Elasticity Substudy data collection was provided by the NHLBI/NIH. Additional financial support for START was also provided by the French Agence Nationale de Recherches sur le SIDA et les Hépatites Virales, the German Ministry of Education and Research, the European AIDS Treatment Network, the Australian National Health and Medical Research Council, and the UK Medical Research Council and National Institute for Heath Research. Six pharmaceutical companies (AbbVie, Inc., Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, LLC, and Merck Sharp and Dohme Corp.) donated antiretroviral drugs to START.
Potential conflicts of interest. The University of Minnesota, the sponsor of START, receives royalties from the use of abacavir, one of the HIV medicines that can be used in START.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller CJ, Baker JV, Bormann AM, et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 2014; 9:e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seaberg EC, Muñoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005; 19:953–60. [DOI] [PubMed] [Google Scholar]
- 4. Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis 2006; 42:1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 6. Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007; 356:1723–35. [DOI] [PubMed] [Google Scholar]
- 7. Savès M, Chêne G, Ducimetière P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis 2003; 37:292–8. [DOI] [PubMed] [Google Scholar]
- 8. Sabin CA, Ryom L, De Witt S, et al. Associations between immune depression and cardiovascular events in HIV infection. AIDS 2013; 27:2735–48. [DOI] [PubMed] [Google Scholar]
- 9. Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker JV, Hullsiek KH, Singh A, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS 2014; 28:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duprez DA, Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep 2007; 9:139–44. [DOI] [PubMed] [Google Scholar]
- 12. Baker JV, Engen NW, Huppler Hullsiek K, et al. Assessment of arterial elasticity among HIV-positive participants with high CD4 cell counts: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med 2015; 16(Suppl 1):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duprez DA, Florea ND, Jones K, Cohn JN. Beneficial effects of valsartan in asymptomatic individuals with vascular or cardiac abnormalities: the DETECTIV Pilot Study. J Am Coll Cardiol 2007; 50:835–9. [DOI] [PubMed] [Google Scholar]
- 14. Leibovitz E, Hazanov N, Zimlichman R, et al. Treatment with atorvastatin improves small artery compliance in patients with severe hypercholesterolemia. Am J Hypertens 2001; 14:1096–8. [DOI] [PubMed] [Google Scholar]
- 15. Zimlichman R, Shargorodsky M, Boaz M, et al. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: Reliability, repeatability, and establishment of normal values for healthy European population–the seven European sites study (SESS). Am J Hypertens 2005; 18:65–71. [DOI] [PubMed] [Google Scholar]
- 16. Duprez DA, Jacobs DR, Jr, Lutsey PL, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2011; 174:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duprez DA. Arterial stiffness/elasticity in the contribution to progression of heart failure. Heart Fail Clin 2012; 8:135–41. [DOI] [PubMed] [Google Scholar]
- 18. Peralta CA, Adeney KL, Shlipak MG, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010; 171:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials 2013; 10:S5–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. INSIGHT Start Study Group , Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duprez DA, Jacobs DR, Jr, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity–results of the multi-ethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–50. [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991; 121:293–8. [DOI] [PubMed] [Google Scholar]
- 23. Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 24. Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens Suppl 1992; 10:S11–4. [PubMed] [Google Scholar]
- 25. Valappil NI, Jacobs DR, Duprez DA, et al. Association between Endothelial Biomarkers and Arterial Elasticity in Young Adults - The CARDIA Study. J Am Soc Hypertens 2008; 2:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS 2010; 24:1897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ngatchou W, Lemogoum D, Ndobo P, et al. Effects of antiretroviral therapy on arterial stiffness in Cameroonian HIV-infected patients. Blood Press Monit 2013; 18:247–51. [DOI] [PubMed] [Google Scholar]
- 28. Sevastianova K, Sutinen J, Westerbacka J, et al. Arterial stiffness in HIV-infected patients receiving highly active antiretroviral therapy. Antivir Ther 2005; 10:925–35. [PubMed] [Google Scholar]
- 29. Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008; 52:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balsam P, Mikuła T, Peller M, et al. Evaluation of endothelial function and arterial stiffness in HIV-infected patients: a pilot study. Kardiol Pol 2015; 73:344–51. [DOI] [PubMed] [Google Scholar]
- 31. Eira M, Bensenor IM, Dorea EL, et al. Potent antiretroviral therapy for human immunodeficiency virus infection increases aortic stiffness. Arq Bras Cardiol 2012; 99:1100–7. [DOI] [PubMed] [Google Scholar]
- 32. Charakida M, Loukogeorgakis SP, Okorie MI, et al. Increased arterial stiffness in HIV-infected children: risk factors and antiretroviral therapy. Antivir Ther 2009; 14:1075–9. [DOI] [PubMed] [Google Scholar]
- 33. van Vonderen MG, Hassink EA, van Agtmael MA, et al. Increase in carotid artery intima-media thickness and arterial stiffness but improvement in several markers of endothelial function after initiation of antiretroviral therapy. J Infect Dis 2009; 199:1186–94. [DOI] [PubMed] [Google Scholar]
- 34. Lekakis J, Ikonomidis I, Palios J, et al. Association of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virus. Am J Hypertens 2009; 22:828–34. [DOI] [PubMed] [Google Scholar]
- 35. El-Sadr WM, Lundgren J, Neaton JD, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med 2008; 149:289–99. [DOI] [PubMed] [Google Scholar]
- 36. El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 37. Baker JV, Neuhaus J, Duprez D, et al. HIV replication, inflammation, and the effect of starting antiretroviral therapy on plasma asymmetric dimethylarginine, a novel marker of endothelial dysfunction. J Acquir Immune Defic Syndr 2012; 60:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, Overton ET, Budoff M, Hammer J, Carpenter CC, Hodis HN, Brooks JT. for the SUN Study Investigators. Progression of Carotid Intima-Media Thickness in a Contemporary HIV Cohort. Clinical Infectious Diseases 2011;53(8):826–835. (PMC3174096). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanna DB, Post WS, Deal JA, et al. HIV infection is associated with progression of subclinical carotid atherosclerosis. Clin Infect Dis 2015; 61:640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volpe GE, Tang AM, Polak JF, et al. Progression of carotid intima-media thickness and coronary artery calcium over 6 years in an HIV-infected cohort. J Acquir Immune Defic Syndr 2013; 64:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stein JH, Ribaudo HJ, Hodis HN, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenblatt L, Farr AM, Johnston SS, Nkhoma ET. Risk of cardiovascular events among patients initiating efavirenz-containing versus efavirenz-free antiretroviral regimens. Open Forum Infect Dis 2016; 3:ofw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis 2010; 201:318–30. [DOI] [PubMed] [Google Scholar]
- 44. Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baker JV, Duprez D, Rapkin J, et al. Untreated HIV infection and large and small artery elasticity. J Acquir Immune Defic Syndr 2009; 52:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta SK, Shen C, Moe SM, et al. Worsening endothelial function with efavirenz compared to protease inhibitors: a 12-month prospective study. PLoS One 2012; 7:e45716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med 2008; 149:289–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.