Abstract
The current experiments use the Friend retrovirus model to demonstrate that vaccine-primed B cells are essential for sterilizing immunity, and the results indicate that the requisite function of these cells is the production of virus-neutralizing antibodies rather than priming or reactivation of T cells. B cell-deficient mice were poorly protected by vaccination, but adoptive transfer experiments showed that the T cells from B cell-deficient mice were primed as well as those from wild-type mice. Furthermore, passive transfer of virus-neutralizing antibodies completely compensated for B cell deficiency. The presence of virus-neutralizing antibodies at the time of infection was crucial for vaccine efficacy. Interestingly, virus-neutralizing antibodies worked synergistically with vaccine-primed T cells to provide a level of protection many orders of magnitude greater than either antibodies or immune T cells alone. Nonneutralizing antibodies also contributed to protection and acted cooperatively with neutralizing antibodies to reduce infection levels. These results emphasize the importance of inducing both T cell responses and virus-neutralizing antibody responses for effective retroviral vaccine protection.
Friend virus (FV) infection in mice has proven to be a useful model for determining the fundamental requirements for immunological protection from a retroviral infection. FV is an immunosuppressive retrovirus that infects adult mice of all strains tested. Genetically resistant strains of mice recover from acute infection, but the virus is never cleared and the mice maintain low-level persistent infections for life (1, 2). Rapid establishment of persistence is a common feature among retro-viruses that makes sterilizing immunity difficult to achieve. The only vaccine that has consistently provided sterilizing immunity against FV is a live attenuated virus (3, 4). Unfortunately, live attenuated retroviruses have the potential to mutate and recombine into virulent forms, making them unsafe for use in humans (5–8). Nevertheless, attenuated viruses are powerful tools for studying the basic requirements for vaccine protection.
Previous studies demonstrated that vaccine protection against FV required the involvement of all three major lymphocyte subsets: CD4+ T cells, CD8+ T cells, and B cells (9). The current study more closely examines the role of one of these subsets, the B cells, to determine what critical function they provide. The most obvious role of B cells is production of virus-specific antibody. It is well established that passive transfers of HIV-neutralizing antibodies can protect monkeys from subsequent infection with chimeric simian–human immunodeficiency virus (SHIV) (10–15). These experiments have proven the efficacy of antibodies in suppressing retroviral infection, but they do not directly address the issue of whether such antibodies are essential for effective vaccine protection. To date, HIV and SHIV vaccines have been decidedly poor at eliciting and maintaining virus-neutralizing antibody responses (16–21). This problem has led to speculation that vaccine-induced T cell responses alone might be sufficient for protection (22, 23). There has been some success in generating CD8+ T cell responses by prototype vaccines both in experimental animals and in phase I clinical trials (24–27), but T cell-based vaccines have not provided sterilizing immunity.
In addition to antibody production, B cells also have been shown to play important roles in the stimulation of T cell responses through mechanisms such as antigen presentation (28–32). Thus, the requirement for B cells in vaccine protection against FV that we previously observed could have been due to defective secondary T cell responses rather than the lack of virus-specific antibodies. In the current study, the contributions of virus-neutralizing antibodies and nonneutralizing antibodies, the priming of T cells, and cooperative effects between antibodies and T cells were investigated.
Materials and Methods
Mice. Experiments were conducted using female C57BL/6 (B6) and B cell-deficient (B6.UMT) mice (The Jackson Laboratory) (33). All of the mice were 12–24 weeks old at the beginning of the experiments and were treated in accordance with the regulations and guidelines of the Animal Care and Use Committee of the Rocky Mountain Laboratories and the National Institutes of Health.
Viruses, Vaccines, and Infections. The pathogenic FV stock used in these experiments was an uncloned stock of FV complex described in ref. 34. For challenge experiments, mice were injected i.v. with 1,500 spleen focus-forming units (ffu) of FV. Vaccinations were administered by i.v. injection of 10,000 ffu of N-tropic Friend murine leukemia virus (F-MuLV) (35). FBL-3 is an FV-induced tumor cell (36) that expresses the glycosylated form of the Gag protein (glycoGag) on its cell surface (37, 38). Our FBL-3 line expresses no detectable cell surface Env protein but produces cytoplasmic Env. It does not produce infectious virus particles (data not shown). Vaccinations were administered by intradermal implantation of 107 viable FBL-3 cells. For detection of tetramer responses from spleen cells, 5 × 106 FBL-3 cells were injected i.p.
Cell Surface Staining and Flow Cytometry. Cell surface staining was performed with Becton Dickinson/Pharmingen reagents (except where noted): FITC-anti-CD43 (1B11), FITC-anti-CD4(RM4-5), phycoerythrin (PE)-anti-CD19(1D3), and allo-phycocyanin (APC)-anti-CD8(53-6.7). Dead cells (propidium iodidehigh) were excluded from all cell surface analyses. Data were acquired on a flow cytometer (FACSCalibur, Becton Dickinson), and analyses were performed by using cellquest pro software (Version 4.0.1, Becton Dickinson).
Viremia Assays. For viremia assays, freshly frozen plasma samples were titrated by using focal infectivity assays (39) on susceptible Mus dunni cells pretreated with 4 μg/ml Polybrene. The cultures were incubated for 4 days, fixed with ethanol, and labeled first with F-MuLV-envelope-specific mAb 720 (40) and then with goat anti-mouse peroxidase-conjugated antisera (Cappel) followed by 3-amino-9-ethylcarbazole (Sigma) as substrate to detect foci.
Virus-Neutralizing Antibody and Infectious-Center (IC) Assays. Plasma samples were assayed for antibodies as described in ref. 41. The titer was defined as the dilution at which >75% of the input virus was neutralized. The IC assays were performed as described in ref. 42.
Tetramers and Tetramer Staining. For detection of Db-GagL-specific CD8+ T cells (43), nucleated spleen cells were dually stained with APC-labeled anti-CD8 and PE-labeled MHC class I H2-Db tetramers (Beckman Coulter) specific for FV GagL peptide (Db-GagL tetramers) constructed as described in ref. 44.
Enrichment of Lymphocyte Subsets and Adoptive Cell Transfers. The MidiMACS separation system (Miltenyi Biotec, Auburn, CA) was used for the purification of B and T lymphocyte subsets as described in ref. 9. Fraction purities were between 90% and 94% and fractions contained <5% dead cells. Adoptive transfers were done by i.v. injection of 1.9–2 × 107 purified cells as described in ref. 9.
Treatment of Mice with Antibodies. mAb 48 is a mouse IgG2a specific for the Env protein of F-MuLV (45). For in vivo treatments, 37 μg of mAb 48 was injected i.p., yielding a mean neutralizing titer of 1:48 vs. 1:40 after vaccination with live attenuated virus (see Fig. 2C). mAb 34, is a mouse IgG2b specific for the glycoGag protein (45), which is expressed on infected cells but present at extremely low levels in virion particles (38). For in vivo treatments, 42 μg of mAb 34 was injected i.p., resulting in a mean binding titer of 1:640, equivalent to the titer after vaccination with live attenuated virus (Fig. 2C). In vivo CD4+ T cell depletions were done as previously described (1) using mAb from clone 191.1 injected i.p. on days -7, -5, -3, 0, and 2 relative to FBL-3 inoculation. After depletion, splenic CD4+ T cell levels were all <0.5% in the B6.UMT mice and <5% in B6 mice at 8 days after FBL-3 inoculation. For passive transfers, 0.5 ml of neat immune serum was used. The serum was obtained from B6 mice immunized and boosted with 104 ffu of B-tropic F-MuLV. The day after passive transfer, mouse plasma contained a virus-neutralizing mean titer of 1:90 and an FBL-3-binding titer of 1:320.
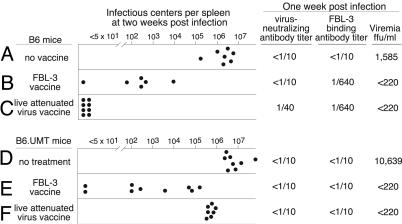
Fig. 2.
The effects of vaccination. Each dot represents results from a single mouse. The limit of detection for the spleen IC assays was 1 IC per 3 × 107 spleen cells. Antibody titers are log2 geometric means determined by the last doubling dilution that produced 75% neutralization or, for FBL-3 binding, the last doubling dilution that produced 50% of the maximum mean fluorescence intensity signal by flow cytometry. Viremia results are geometric means expressed as ffu/ml of plasma with a limit of detection of 220 per ml.
Results
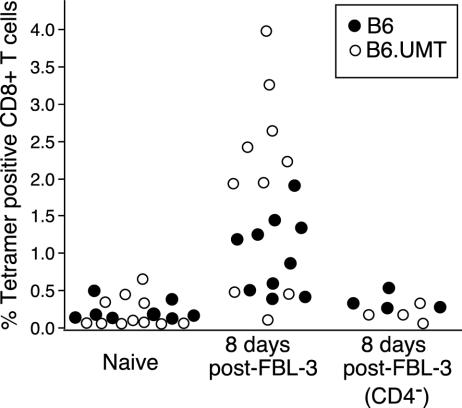
B Cells Are Critical for Vaccine Protection Against Spleen Infection. To analyze the importance of B cells and antibodies in protection against FV the responses of wild-type C57BL/6 and B6.UMT mice were studied after immunization with the FV-induced tumor cell, FBL-3. FBL-3 cells express a cell-surface form of the Gag protein (glycoGag) (37, 38) but very little or no detectable cell surface Env protein, and FBL-3 cells do not produce infectious virus particles (data not shown). We were interested in FBL-3 as a vaccine because lack of Env protein expression indicated that no virus-neutralizing antibody response would be elicited, and testing responses to this vaccine would help us examine the relative importance of virus-neutralizing and non-neutralizing antibodies. Immunization with FBL-3 tumor cells has previously been shown to induce both CD4+ (46) and CD8+ T cell responses (43, 47) in B6 mice, but B6.UMT mice had not been tested. To determine whether immunization with FBL-3 tumor cells also elicited CD8+ T cell responses in B6.UMT mice, tetramers were used to analyze spleen cells for the presence of CD8+ T cells specific for the Db-GagL epitope of FV (43, 44). At 8 days after vaccination with FBL-3, the percentage of tetramer-positive cells in the CD8+ T cell subset increased significantly above background in most mice of both strains (Fig. 1). However, the average tetramer response was significantly higher in the B6.UMT mice than in wild-type B6 (P < 0.05). Thus, for the immunodominant CD8+ T cell epitope of FBL-3 cells, the response was higher in B6.UMT than in B6. This increase could have been due to a mechanism to compensate for the lack of B cells. When the mice were depleted of CD4+ T cells before vaccination, no significant expansion of tetramer-positive CD8+ T cells occurred in either strain (Fig. 1). Thus, the expansion of FBL-3-specific CD8+ T cells was CD4-dependent.
Fig. 1.
Tetramer staining of CD8+ T cells. CD8+ T cells from spleens of B6 mice and B6.UMT mice were stained with Db-GagL tetramers. The CD4- group was depleted of CD4+ T cells before immunization with FBL-3 tumor cells. Statistical analysis by using ANOVA with a Tukey–Kramer multiple-comparisons posttest showed significant differences between naive and vaccinated mice of both strains (P < 0.01) and also between FBL-3-immunized B6 and B6.UMT mice (P < 0.05). There were no significant differences between naive and CD4-depleted, FBL-3-immunized mice. Each circle represents results from a single mouse.
At 1 month after vaccination, the mice were challenged with a high dose of FV. FBL-3-vaccinated B6 mice had high titers of FBL-3-binding antibodies at 1 week after challenge but no detectable virus-neutralizing antibody titers (Fig. 2B). FBL-3 vaccination protected against viremia and reduced the number of infected spleen cells at 2 weeks after infection but did not provide consistent sterile immunity. In contrast, vaccination with live attenuated virus induced virus-neutralizing antibodies and there were no infected spleen cells at 2 weeks after infection (Fig. 2C). Previous experiments demonstrated that vaccination with live attenuated virus produced sterilizing immunity as determined by the inability to passage infection into highly susceptible mice (3) or to detect ICs 2 months after infection (4, 9). Sterilizing immunity correlated well with the absence of infected spleen cells at 2 weeks after infection (9).
As expected, FBL-3-vaccinated B6.UMT mice did not develop any detectable antibody responses (Fig. 2E). Despite the absence of antibodies, the FBL-3-vaccinated B6.UMT mice were protected against viremia and had levels of virus-infected spleen cells similar to those of vaccinated B6 mice. Thus, protection from viremia was achieved via FBL-3-primed T cells in the absence of any antibody, but protection from spleen cell infection was quite variable and generally poor. There was no indication from this experiment that nonneutralizing antibodies enhanced protection, but virus-neutralizing antibodies may have done so.
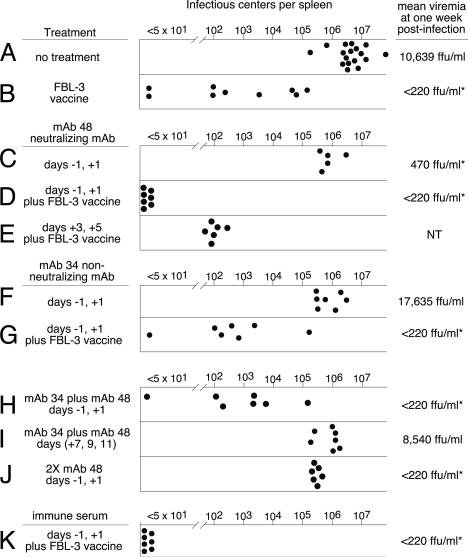
Virus-Neutralizing Antibodies Compensate for B Cell Deficiency. To determine whether virus-neutralizing antibodies could substitute for the absence of B cells in B6.UMT mice, passive transfer experiments were performed using virus-neutralizing mAb 48 specific for the FV Env protein (45, 49). Passive transfer of mAb 48 has been shown to reduce viremia levels in vivo without inducing antibody-dependent cellular cytotoxicity in infected cells (50). The amount of mAb 48 transferred was empirically determined to provide a virus-neutralizing titer in plasma similar to that after vaccination by live attenuated virus. By itself, passive transfer of mAb 48 into B6.UMT mice at days -1 and +1 relative to infection reduced plasma viremia by >20-fold at 1 week after infection (Fig. 3, compare A and C). There also was a slight but significant reduction in virus-infected spleen cells at 2 weeks after infection.
Fig. 3.
The effects of treatment on infection in B6.UMT mice. Each circle represents the results from a single mouse. For ease of comparison, 2-week data from Fig. 2 are included. Viremia results are log3 geometric means expressed as ffu/ml of plasma. The asterisks indicate significant differences in viremia from the no-treatment group (P < 0.05 by ANOVA). Passive antibody transfers were done on the indicated days relative to infection with FV. Vaccines were administered 1 month before FV infections. Groups H and I received a combination of mAb 34 and mAb 48, each at the normal dose. Group J received twice the normal dose of mAb 48 per injection. Statistical analyses were done by using Student's t test with Bonferroni correction where applicable. Groups C and F had significantly lower (P < 0.05) IC levels than did group A, and group D had significantly lower levels than did groups B and E. Group K received 0.5 ml of neat immune serum as described in Materials and Methods.
Most striking was the effect of passive transfer of mAb 48 into recipients previously vaccinated with FBL-3. The combination of neutralizing antibody and FBL-3-induced T cells produced a potent synergistic effect equivalent to wild-type mice vaccinated with live attenuated virus (compare Figs. 3D and 2C). Sterilizing immunity was not observed when passive transfers of virus-neutralizing antibody were begun at 3 days after infection (Fig. 3E). These results indicated that virus-neutralizing antibodies present at vaccine-inducible levels during the first few days of infection were able to compensate for the lack of B cells.
To determine the extent that virus-neutralizing antibodies had suppressed the initial infection, spleen ICs were assayed at 14 h after infection, before completion of the first round of virus replication. At this time, the untreated mice had an average of 127 infected spleen cells per 3 × 107 cells (±25 IC, n = 6). In contrast, one injection of mAb 48 on the day before infection reduced the level of infected spleen cells to <1 IC per 3 × 107 cells in all six mice tested. However, the effect was not sterilizing, as evidenced by virus outgrowth by 2 weeks after infection (Fig. 3C). These results indicated that the neutralization of free virus at the time of infection dramatically reduced but did not eliminate initial infection of the spleen. Further, these results demonstrate the high replicative capacity of FV that makes the assay for ICs at 2 weeks after infection a very sensitive assay for sterilizing immunity.
Nonneutralizing Antibodies also Contribute to Vaccine Protection. To more closely examine the contribution of nonneutralizing antibodies in vaccine protection, passive transfer experiments were performed with mAb 34, which reacts with the viral p15 Gag protein present within viral cores and also on the surface of infected cells as glycoGag. mAb 34 binds to infected cells but does not neutralize virus and does not induce antibody-dependent cellular cytotoxicity in vivo (50). Again, the dosage for passive transfers was empirically determined to match titers observed in B6 mice after vaccination with live attenuated virus. Passive transfer of mAb 34 into B6.UMT mice 1 day before and 1 day after FV infection produced a slight reduction in virus-infected spleen cells similar to the reductions observed after passive transfer of mAb 48 (Fig. 3F). However, unlike passive transfer of mAb 48, there was no reduction in viremia levels and no significantly enhanced effect when transfers were done into FBL-3-vaccinated mice (Fig. 3G). The most potent effect from passive transfer of mAb 34 was observed when it was transferred in combination with mAb 48 (Fig. 3H). This complementary effect was not simply due to greater antibody quantity, because transfer of twice as much mAb 48 did not reproduce the effect (Fig. 3J). When passive transfers of combined mAb 34/48 were begun at 1 week after infection, there was no significant decrease in virus-infected spleen cells (Fig. 3I). This result emphasized the difference between the presence of a standing titer of antibodies at the time of infection, as would be expected in a vaccine situation, and waiting for 1 week, the approximate time necessary for an antibody response to develop during the natural infection of a naive host. Furthermore, the strong complementary effect of combining mAb 34 with mAb 48 illustrated the importance of having antibodies specific for both infected cells and virus particles. To ensure that the effects observed were not due to a special characteristic of the monoclonal antibodies such as especially high affinity, passive transfer of immune serum from vaccinated B6 mice also was tested. The immune serum contained both neutralizing and nonneutralizing antibodies. Passive transfer of immune serum into FBL-3-vaccinated B6.UMT mice also produced complete protection when given before virus challenge (Fig. 3K).
B Cells Are Not Required for Vaccine Priming of T Cells. To further analyze the role of B cells in vaccine protection, we studied a second vaccine, a live attenuated virus (F-MuLV-N), that we previously showed to elicit anti-viral T cell responses and virus-neutralizing antibody responses (9, 51). Vaccination of B6 mice with live attenuated virus elicited both virus-neutralizing and FBL-3-binding antibodies and induced complete protection against both viremia and spleen infectious centers (Fig. 2C). Thus, protection by live attenuated virus vaccination was better than by FBL-3 vaccination, and better protection was associated with virus-neutralizing antibodies. In contrast to the complete protection of wild-type B6 mice, B cell-deficient mice vaccinated with the same vaccine had no detectable antibody responses and averaged only about a one log10 reduction in virus-infected spleen cells loads, compared with unvaccinated mice (Fig. 2F). Despite the high levels of virus-infected spleen cells in most of the vaccinated B cell-deficient mice, their viremia levels at 1 week after infection were markedly reduced, compared with unvaccinated B6.UMT mice. This was further evidence that T cell responses in the absence of antibodies could suppress viremia.
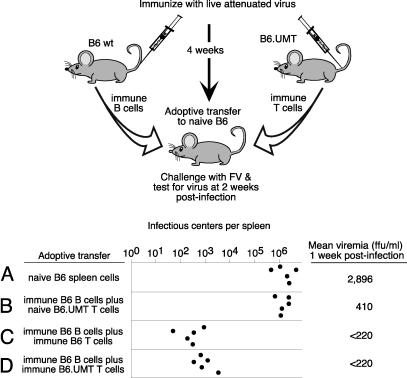
It has been reported that B cell-deficient mice may have defective T cell responses (28–30), so it was important to determine whether that defect could account for their poor protection. Our previous studies showed that vaccine-induced protection against FV could be adoptively transferred to naive mice, but only when both T and B cells from immune mice were adoptively transferred (9). Therefore, we used adoptive transfer experiments to test the protective capacity of the T cells coming from B6.UMT mice vaccinated with live attenuated virus. Co-transfer experiments were done with T cells from either vaccinated wild-type or B cell-deficient mice combined with B cells from vaccinated wild-type mice (Fig. 4). A control experiment in which the recipients received naive spleen cells showed no protection (Fig. 4A). Furthermore, adoptive transfers of naive T cells plus immune B cells demonstrated that immune B cells alone did not confer protection (Fig. 4B). However, when immune B cells were combined with T cells from vaccinated B cell-deficient mice, the protection was similar to that obtained when the immune T cells came from wild-type mice (Fig. 4 C and D). The incomplete protection following adoptive transfer of immune cells in this experiment was likely due to the lack of antiviral antibodies in the mice at the time of challenge because the challenge was performed the day after transfer. Regardless, vaccine failure in B6.UMT mice could not be attributed to lack of T cell priming but could be attributed to the absence of virus-specific antibodies.
Fig. 4.
Protection by adoptive transfer. B6 and B6.UMT mice were immunized with live attenuated virus. After 1 month, immune B cells from the B6 mice and immune T cells from the B6.UMT mice were purified from spleen and transferred to naive B6 mice. The next day, the recipient mice were challenged with FV. ICs were determined at 2 weeks after infection. Recipient mice were all wild-type B6 mice. Group A received 5 × 107 nucleated spleen cells from naive B6 mice. The other groups received 2 × 107 CD19+ B cells from immunized B6 mice and one of the following: group B, 2 × 107 T cells from naive B6.UMT mice; group C, 2 × 107 T cells from immune B6 mice; and group D, 1.9 × 107 T cells from immune B6.UMT mice. The mice were infected with FV the day after adoptive transfer of the B and T cells. The limit of detection of the assay was 1 IC per 3 × 107 spleen cells. Each dot represents results from a single mouse. The purities of the cell populations were all >90%. Groups C and D, but not B, were statistically different from group A (P < 0.01 by ANOVA with Dunnett multiple-comparisons posttest). Groups C and D were not statistically different by Mann–Whitney U test (P = 0.0556).
Discussion
Although B cells play a number of significant roles in adaptive immunity, the results presented here strongly argue that the most critical role of B cells in vaccine protection is the production of virus-neutralizing antibodies. The incomplete protection of wild-type mice by FBL-3 vaccination correlated with lack of virus-neutralizing antibodies, and full protection was completely restored in vaccinated B cell-deficient mice after adoptive transfer of virus-neutralizing antibodies or immune serum. In addition, we found that B cells were not required to prime protective T cell responses. For example, vaccination induced virus-specific CD8+ T cells from B cell-deficient mice to proliferate as well or better than CD8+ T cells from wild-type mice. Furthermore, T cells from B cell-deficient mice were equivalent to T cells from wild-type mice in their ability to adoptively transfer protection. These results strongly indicate that the main defect in B cell-deficient mice was lack of antibodies rather than defective antigen presentation to T cells.
The presence of virus-neutralizing antibodies at the time of infection suppressed the level of initially infected spleen cells to below our limit of detection, a reduction of more than two orders of magnitude. Despite this dramatic reduction of infection at 14 h, the virus was not completely eliminated as evidenced by the exponential outgrowth of spleen ICs by 2 weeks after infection. This result illustrates both the potency of antibodies in reducing infection and the remarkable ability of retroviruses to escape an immune response and establish an infection. The challenge virus in these experiments was not cloned, but a swarm stock was obtained from passage in mice. Thus, it is possible that antibody-escape variants preexisted in the stock. However, it also may be that antibodies at vaccine-induced titers cannot be expected to completely neutralize all challenge virus.
In situations where complete neutralization of challenge virus by antibodies is not achieved, the ability of the cell-mediated immune response to eliminate infected cells is critical. T cell responses primed by FBL-3 vaccination suppressed viremia and reduced the number of infected spleen cells, but most mice retained high levels of infection. Unlike vaccine-induced antibodies, which are fully active at the time of infection, vaccine-primed T cells require time to activate, proliferate and acquire effector function (52). Because retroviruses replicate and spread at an exponential rate, this lag time is a critical factor. Thus, the initial blunting of infection by virus-neutralizing antibodies provides the necessary time for the T cell responses to mature before the virus load becomes overwhelming. In addition to the complementary activities of neutralizing antibodies and T cells, antibodies also may potentiate cell-mediated responses. It has been reported in antitumor studies that antibodies can help recruit CD8+ T cells to sites of tumors via inflammatory signals from antibody/FcR-activated macrophages (53, 54). Rapid recruitment of CD8+ T cells could certainly impact viral infections as well.
In a recent chimeric simian–human immunodeficiency virus experiment in macaques that combined DNA vaccine-induced cell-mediated immunity and passive antibody transfer, no synergistic effect on protection was observed (55). Although this result was discouraging, it may be that the quality or quantity of the T cell responses induced by DNA vaccination was not high enough. For example, it was not clear whether CD4+ T cell responses were elicited, and the FV studies have shown that CD4+ T cell responses are critical for vaccine efficacy (4, 9, 56).
Nonneutralizing antibodies acted in combination with neutralizing antibodies to produce a potent cooperative effect that could be very important in vaccine protection. These results indicate that even though nonneutralizing antibodies alone may be unable to reduce viremia or cooperate with immune T cells to reduce infection levels, they are nonetheless important mediators of protection that should be considered in vaccine strategies.
The live attenuated vaccine in this study is a paradigm of how a retroviral vaccine should work. Our studies demonstrate that it is possible to achieve sterilizing immunity against a retrovirus that rapidly establishes persistence as long as multiple arms of the immune response are brought into play at the appropriate times. Unfortunately, there are unique aspects about HIV that make development of an equally efficacious vaccine difficult. For example, HIV can establish latent (transcriptionally silent) infections (57–60), whereas there is no evidence that FV does so (61). If HIV becomes latent very early after infection, then it will be extremely difficult to eradicate, although strong vaccine-induced immunity could still prevent latent HIV from spreading and causing pathology. Thus, it will be especially important for an HIV vaccine to blunt infection at the earliest time point. Another problem is that HIV has escape mechanisms that could diminish the efficacy of both CD4+ and CD8+ T cell-mediated immune responses (62–68). The antigenic variability of HIV also is of concern. Another obstacle to overcome in HIV vaccine development is that, unlike FV, it is extremely difficult to generate sustained virus-neutralizing antibodies against HIV. This problem is related to the poor antigenicity and immunogenicity of the heavily glycosylated HIV Env protein (17, 69–73). The implication of the current study is that it is unlikely that a retroviral vaccine will be protective in the absence of a virus-neutralizing antibody response. Solving the HIV-neutralizing antibody problem should be a major focus of research.
Abbreviations: FV, Friend virus; F-MuLV, Friend murine leukemia virus; ffu, focus-forming units; IC, infectious center.
References
- 1.Hasenkrug, K. J., Brooks, D. M. & Dittmer, U. (1998) J. Virol. 72, 6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasenkrug, K. J. (1999) J. Virol. 73, 6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittmer, U., Brooks, D. M. & Hasenkrug, K. J. (1999) J. Virol. 73, 3753-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dittmer, U. & Hasenkrug, K. J. (2000) Virology 272, 177-182. [DOI] [PubMed] [Google Scholar]
- 5.Gundlach, B. R., Lewis, M. G., Sopper, S., Schnell, T., Sodroski, J., Stahl-Hennig, C. & Uberla, K. (2000) J. Virol. 74, 3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba, T. W., Liska, V., Khimani, A. H., Ray, N. B., Dailey, P. J., Penninck, D., Bronson, R., Greene, M. F., McClure, H. M., Martin, L. N. & Ruprecht, R. M. (1999) Nat. Med. 5, 194-203. [DOI] [PubMed] [Google Scholar]
- 7.Baba, T. W., Jeong, Y. S., Pennick, D., Bronson, R., Greene, M. F. & Ruprecht, R. M. (1995) Science 267, 1820-1825. [DOI] [PubMed] [Google Scholar]
- 8.Kedzierska, K., Churchill, M., Maslin, C. L., Azzam, R., Ellery, P., Chan, H. T., Wilson, J., Deacon, N. J., Jaworowski, A. & Crowe, S. M. (2003) J. Acquired Immune Defic. Syndr. 34, 445-453. [DOI] [PubMed] [Google Scholar]
- 9.Dittmer, U., Brooks, D. M. & Hasenkrug, K. J. (1999) Nat. Med. 5, 189-193. [DOI] [PubMed] [Google Scholar]
- 10.Shibata, R., Igarashi, T., Haigwood, N., Buckler-White, A., Ogert, R., Ross, W., Willey, R., Cho, M. W. & Martin, M. A. (1999) Nat. Med. 5, 204-210. [DOI] [PubMed] [Google Scholar]
- 11.Parren, P. W., Marx, P. A., Hessell, A. J., Luckay, A., Harouse, J., Cheng-Mayer, C., Moore, J. P. & Burton, D. R. (2001) J. Virol. 75, 8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veazey, R. S., Shattock, R. J., Pope, M., Kirijan, J. C., Jones, J., Hu, Q., Ketas, T., Marx, P. A., Klasse, P. J., Burton, D. R. & Moore, J. P. (2003) Nat. Med. 9, 343-346. [DOI] [PubMed] [Google Scholar]
- 13.Mascola, J. R., Stiegler, G., VanCott, T. C., Katinger, H., Carpenter, C. B., Hanson, C. E., Beary, H., Hayes, D., Frankel, S. S., Birx, D. L. & Lewis, M. G. (2000) Nat. Med. 6, 207-210. [DOI] [PubMed] [Google Scholar]
- 14.Baba, T. W., Liska, V., Hofmann-Lehmann, R., Vlasak, J., Xu, W., Ayehunie, S., Cavacini, L. A., Posner, M. R., Katinger, H., Stiegler, G., et al. (2000) Nat. Med. 6, 200-206. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura, Y., Igarashi, T., Haigwood, N. L., Sadjadpour, R., Donau, O. K., Buckler, C., Plishka, R. J., Buckler-White, A. & Martin, M. A. (2003) Proc. Natl. Acad. Sci. USA 100, 15131-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore, J. P. & Burton, D. R. (1999) Nat. Med. 5, 142-144. [DOI] [PubMed] [Google Scholar]
- 17.Burton, D. R., Desrosiers, R. C., Doms, R. W., Koff, W. C., Kwong, P. D., Moore, J. P., Nabel, G. J., Sodroski, J., Wilson, I. A. & Wyatt, R. T. (2004) Nat. Immunol. 5, 233-236. [DOI] [PubMed] [Google Scholar]
- 18.McElrath, M. J., Corey, L., Montefiori, D., Wolff, M., Schwartz, D., Keefer, M., Belshe, R., Graham, B. S., Matthews, T., Wright, P., et al. (2000) AIDS Res. Hum. Retroviruses 16, 907-919. [DOI] [PubMed] [Google Scholar]
- 19.Earl, P. L., Sugiura, W., Montefiori, D. C., Broder, C. C., Lee, S. A., Wild, C., Lifson, J. & Moss, B. (2001) J. Virol. 75, 645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ourmanov, I., Brown, C. R., Moss, B., Carroll, M., Wyatt, L., Pletneva, L., Goldstein, S., Venzon, D. & Hirsch, V. M. (2000) J. Virol. 74, 2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doria-Rose, N. A., Ohlen, C., Polacino, P., Pierce, C. C., Hensel, M. T., Kuller, L., Mulvania, T., Anderson, D., Greenberg, P. D., Hu, S. L. & Haigwood, N. L. (2003) J. Virol. 77, 11563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMichael, A. & Hanke, T. (2002) Nat. Rev. Immunol. 2, 283-291. [DOI] [PubMed] [Google Scholar]
- 23.Baltimore, D. (2002) Science 296, 2297. [DOI] [PubMed] [Google Scholar]
- 24.Barouch, D. H., Santra, S., Schmitz, J. E., Kuroda, M. J., Fu, T. M., Wagner, W., Bilska, M., Craiu, A., Zheng, X. X., Krivulka, G. R., et al. (2000) Science 290, 486-492. [DOI] [PubMed] [Google Scholar]
- 25.Belshe, R. B., Gorse, G. J., Mulligan, M. J., Evans, T. G., Keefer, M. C., Excler, J. L., Duliege, A. M., Tartaglia, J., Cox, W. I., McNamara, J., et al. (1998) AIDS 12, 2407-2415. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari, G., Humphrey, W., McElrath, M. J., Excler, J. L., Duliege, A. M., Clements, M. L., Corey, L. C., Bolognesi, D. P. & Weinhold, K. J. (1997) Proc. Natl. Acad. Sci. USA 94, 1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiver, J. W., Fu, T. M., Chen, L., Casimiro, D. R., Davies, M. E., Evans, R. K., Zhang, Z. Q., Simon, A. J., Trigona, W. L., Dubey, S. A., et al. (2002) Nature 415, 331-335. [DOI] [PubMed] [Google Scholar]
- 28.Schultz, K. R., Klarnet, J. P., Gieni, R. S., HayGlass, K. T. & Greenberg, P. D. (1990) Science 249, 921-923. [DOI] [PubMed] [Google Scholar]
- 29.Homann, D., Tishon, A., Berger, D. P., Weigle, W. O., von Herrath, M. G. & Oldstone, M. B. (1998) J. Virol. 72, 9208-9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linton, P. J., Harbertson, J. & Bradley, L. M. (2000) J. Immunol. 165, 5558-5565. [DOI] [PubMed] [Google Scholar]
- 31.Martinez, C., Pereira, P., Toribio, M. L., Marcos, M. A., Bandeira, A., de la Hera, A., Marquez, C., Cazenave, P. A. & Coutinho, A. (1988) Immunol. Rev. 101, 191-215. [DOI] [PubMed] [Google Scholar]
- 32.Moulin, V., Andris, F., Thielemans, K., Maliszewski, C., Urbain, J. & Moser, M. (2000) J. Exp. Med. 192, 475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. (1991) Nature 350, 423-426. [DOI] [PubMed] [Google Scholar]
- 34.Lilly, F. & Steeves, R. A. (1973) Virology 55, 363-370. [DOI] [PubMed] [Google Scholar]
- 35.Lander, M. R. & Chattopadhyay, S. K. (1984) J. Virol. 52, 695-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoy, J. L., Fefer, A. & Glynn, J. P. (1967) Cancer Res. 27, 2267-2271. [PubMed] [Google Scholar]
- 37.Evans, L. H., Dresler, S. & Kabat, D. (1977) J. Virol. 24, 865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujisawa, R., McAtee, F. J., Favara, C., Hayes, S. F. & Portis, J. L. (2001) J. Virol. 75, 11239-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sitbon, M., Nishio, J., Wehrly, K., Lodmell, D. & Chesebro, B. (1985) Virology 141, 110-118. [DOI] [PubMed] [Google Scholar]
- 40.Robertson, M. N., Miyazawa, M., Mori, S., Caughey, B., Evans, L. H., Hayes, S. F. & Chesebro, B. (1991) J. Virol. Methods 34, 255-271. [DOI] [PubMed] [Google Scholar]
- 41.Morrison, R. P., Earl, P. L., Nishio, J., Lodmell, D. L., Moss, B. & Chesebro, B. (1987) Nature 329, 729-732. [DOI] [PubMed] [Google Scholar]
- 42.Dittmer, U., Brooks, D. M. & Hasenkrug, K. J. (1998) J. Virol. 72, 6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen, W., Qin, H., Chesebro, B. & Cheever, M. A. (1996) J. Virol. 70, 7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schepers, K., Toebes, M., Sotthewes, G., Vyth-Dreese, F. A., Dellemijn, T. A., Melief, C. J., Ossendorp, F. & Schumacher, T. N. (2002) J. Immunol. 169, 3191-3199. [DOI] [PubMed] [Google Scholar]
- 45.Chesebro, B., Wehrly, K., Cloyd, M., Britt, W., Portis, J., Collins, J. & Nishio, J. (1981) Virology 112, 131-144. [DOI] [PubMed] [Google Scholar]
- 46.Iwashiro, M., Kondo, T., Shimizu, T., Yamagishi, H., Takahashi, K., Matsubayashi, Y., Masuda, T., Otaka, A., Fujii, N., Ishimoto, A., et al. (1993) J. Virol. 67, 4533-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klarnet, J. P., Kern, D. E., Okuno, K., Holt, C., Lilly, F. & Greenberg, P. D. (1989) J. Exp. Med. 169, 457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parren, P. W., Moore, J. P., Burton, D. R. & Sattentau, Q. J. (1999) AIDS 13, Suppl. A, S137-S162. [PubMed] [Google Scholar]
- 49.Hasenkrug, K. J., Brooks, D. M. & Chesebro, B. (1995) Proc. Natl. Acad. Sci. USA 92, 10492-10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Britt, W. J. & Chesebro, B. (1983) J. Immunol. 130, 2363-2367. [PubMed] [Google Scholar]
- 51.Dittmer, U., Race, B. & Hasenkrug, K. J. (1999) J. Virol. 73, 8435-8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wherry, E. J., Teichgraber, V., Becker, T. C., Masopust, D., Kaech, S. M., Antia, R., von Andrian, U. H. & Ahmed, R. (2003) Nat. Immunol. 4, 225-234. [DOI] [PubMed] [Google Scholar]
- 53.Selenko, N., Majdic, O., Jager, U., Sillaber, C., Stockl, J. & Knapp, W. (2002) J. Clin. Immunol. 22, 124-130. [DOI] [PubMed] [Google Scholar]
- 54.Vasovic, L. V., Dyall, R., Clynes, R. A., Ravetch, J. V. & Nikolic-Zugic, J. (1997) Eur. J. Immunol. 27, 374-382. [DOI] [PubMed] [Google Scholar]
- 55.Mascola, J. R., Lewis, M. G., VanCott, T. C., Stiegler, G., Katinger, H., Seaman, M., Beaudry, K., Barouch, D. H., Korioth-Schmitz, B., Krivulka, G., et al. (2003) J. Virol. 77, 10348-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Super, H. J., Brooks, D., Hasenkrug, K. J. & Chesebro, B. (1998) J. Virol. 72, 9400-9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chun, T. W., Stuyver, L., Mizell, S. B., Ehler, L. A., Mican, J. A., Baseler, M., Lloyd, A. L., Nowak, M. A. & Fauci, A. S. (1997) Proc. Natl. Acad. Sci. USA 94, 13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pomerantz, R. J. (2002) Clin. Infect. Dis. 34, 91-97. [DOI] [PubMed] [Google Scholar]
- 59.Igarashi, T., Brown, C. R., Endo, Y., Buckler-White, A., Plishka, R., Bischofberger, N., Hirsch, V. & Martin, M. A. (2001) Proc. Natl. Acad. Sci. USA 98, 658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Embretson, J., Zupancic, M., Ribas, J. L., Burke, A., Racz, P., Tenner-Racz, K. & Haase, A. T. (1993) Nature 362, 359-362. [DOI] [PubMed] [Google Scholar]
- 61.Dittmer, U., He, H., Messer, R. J., Schimmer, S., Olbrich, A. R., Ohlen, C., Greenberg, P. D., Stromnes, I. M., Iwashiro, M., Sakaguchi, S., et al. (2004) Immunity 20, 293-303. [DOI] [PubMed] [Google Scholar]
- 62.Le Gall, S., Erdtmann, L., Benichou, S., Berlioz-Torrent, C., Liu, L., Benarous, R., Heard, J. M. & Schwartz, O. (1998) Immunity 8, 483-495. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz, O., Marechal, V., Le Gall, S., Lemonnier, F. & Heard, J. M. (1996) Nat. Med. 2, 338-342. [DOI] [PubMed] [Google Scholar]
- 64.Collins, K. L., Chen, B. K., Kalams, S. A., Walker, B. D. & Baltimore, D. (1998) Nature 391, 397-401. [DOI] [PubMed] [Google Scholar]
- 65.Norris, P. J., Moffett, H. F., Brander, C., Allen, T. M., O'Sullivan, K. M., Cosimi, L. A., Kaufmann, D. E., Walker, B. D. & Rosenberg, E. S. (2004) AIDS Res. Hum. Retroviruses 20, 315-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goulder, P. J., Brander, C., Tang, Y., Tremblay, C., Colbert, R. A., Addo, M. M., Rosenberg, E. S., Nguyen, T., Allen, R., Trocha, A., et al. (2001) Nature 412, 334-338. [DOI] [PubMed] [Google Scholar]
- 67.Aandahl, E. M., Michaelsson, J., Moretto, W. J., Hecht, F. M. & Nixon, D. F. (2004) J. Virol. 78, 2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douek, D. C., Brenchley, J. M., Betts, M. R., Ambrozak, D. R., Hill, B. J., Okamoto, Y., Casazza, J. P., Kuruppu, J., Kunstman, K., Wolinsky, S., et al. (2002) Nature 417, 95-98. [DOI] [PubMed] [Google Scholar]
- 69.Kwong, P. D., Doyle, M. L., Casper, D. J., Cicala, C., Leavitt, S. A., Majeed, S., Steenbeke, T. D., Venturi, M., Chaiken, I., Fung, M., et al. (2002) Nature 420, 678-682. [DOI] [PubMed] [Google Scholar]
- 70.Moore, J. P., Cao, Y., Qing, L., Sattentau, Q. J., Pyati, J., Koduri, R., Robinson, J., Barbas, C. F., III, Burton, D. R. & Ho, D. D. (1995) J. Virol. 69, 101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyatt, R., Kwong, P. D., Desjardins, E., Sweet, R. W., Robinson, J., Hendrickson, W. A. & Sodroski, J. G. (1998) Nature 393, 705-711. [DOI] [PubMed] [Google Scholar]
- 72.Mascola, J. R. & Montefiori, D. C. (2003) Nat. Med. 9, 393-394. [DOI] [PubMed] [Google Scholar]
- 73.Montefiori, D. C., Altfeld, M., Lee, P. K., Bilska, M., Zhou, J., Johnston, M. N., Gao, F., Walker, B. D. & Rosenberg, E. S. (2003) J. Immunol. 170, 3906-3914. [DOI] [PubMed] [Google Scholar]