Abstract
Salmonella carriage is an insidious problem for the poultry industry. While most Salmonella serotypes are avirulent in poultry, these bacteria can contaminate chicken meat during processing, leading to one of the most important food safety hazards. In this study, we examined the anti-Salmonella effects of Diamond V Original XPC™ (XPC) included in the finisher diet fed to commercial broilers. On 3 occasions between day one (D1) and D20, broilers were experimentally infected with multiple antibiotic-resistant Salmonella Typhimurium. After confirming that the chicks were shedding Salmonella in the feces on D21, broiler chicks were fed a diet containing XPC (n = 57 birds; 1.25 kg/MT) or an XPC-free control diet (CON) (n = 57 birds) to D49. Fecal samples were obtained weekly and subjected to selective culture for enumerating and determining the antibiotic resistance of the Salmonella. Salmonella isolates were then subjected to an in vitro virulence assay, which predicts the ability of Salmonella to cause illness in a mammalian host. Broilers were euthanized on D49 and a segment of the large intestine was removed and subjected to the same assays used for the fecal samples. When compared to the birds fed the CON diet, Salmonella fecal shedding, virulence (invasion and invasion gene expression), and antibiotic resistance were significantly decreased in birds fed XPC (5-fold, 7.5-fold, 6-fold, and 5.3-fold decreases, respectively). Birds fed XPC exhibited heavier body weight (BW) and greater BW gains than those fed the CON diet. The decrease in virulence was associated with a decreased expression of a genetic regulator of Salmonella invasion into cells (hilA), while the decrease in antibiotic resistance was due to a loss of an integron (SGI1) from the input strain. This study revealed that Original XPC™ inhibits the shedding, downstream virulence, and antibiotic resistance of Salmonella residing in broilers.
Keywords: Salmonella, XPC, virulence, antibiotic sensitivity, broiler chickens
INTRODUCTION
Most Salmonella, except for serovars Pullorum and Gallinarum (Wilson et al., 2000) and possibly other strains [e.g., S. Kentucky (Ogunleye and Carlson 2012)], are capable of asymptomatically residing in the intestinal tracts of poultry. Many Salmonella serotypes can be acquired by the fecal-oral route and then be shed into the feces (Traub-Dargatz et al., 2006). Many birds can be infected since the ingestion, colonization, and shedding events typically cause no harm to the bird and since Salmonella is ubiquitous in the environment. Salmonella can therefore contaminate poultry meat prior to (from fecal shedding) or during processing (from intestinal leakage), resulting in one of the leading causes of Salmonella infections in humans (CDC, 2015).
Recent studies have shown that the fermentation metabolites of Original XPC™ (XPC, Diamond V, Cedar Rapids, IA), and earlier product derivatives, enhance poultry health and performance (Jensen et al., 2008) by promoting immune functions such as inducing the production of anti-viral antibodies, enhancing serum lysozyme activity, increasing IgM, increasing T-lymphocytes, and increasing secretory IgA (Gao et al., 2008; Gao et al., 2009). Additional studies revealed that an XPC-like technology, available for use in humans (EpiCor®, Embria Health Sciences), increases NK cell activity and serum antioxidant protection (Jensen et al., 2011).
Furthermore, Salmonella suppressing effects have been shown in the gastrointestinal tract of the bird (Al-Homidan and Fahmy, 2007; Ibukic et al., 2012). Specifically, XPC fostered a significant reduction in both numbers and prevalence of Salmonella Heidelberg in broilers (Hofacre et al., 2015). These anti-Salmonella effects (decreased load and prevalence) also have been observed in calves fed XPC, where the product also protected the animal from various pathophysiologic consequences of the pathogen (Brewer et al., 2014).
Because of these potential food safety benefits, a study was designed to further examine the anti-Salmonella Typhimurium effects associated with XPC in chickens. The objectives of this study were to determine the effects of XPC on a multiple antibiotic resistant Salmonella Typhimurium in broiler chickens experimentally infected with the microbe: specifically, fecal shedding, large intestinal carriage, downstream virulence, and antibiotic resistance. The virulence aspect was examined since an XPC-like technology (EpiCor®, Embria Health Sciences) has been shown to increase intestinal butyrate production (Possemiers et al., 2013), and this short-chain fatty acid has been shown to inhibit Salmonella virulence mechanisms in vitro by down regulating 17 genes (including hilA) in the pathogenicity island of Salmonella (Gantois et al., 2006). This repression in hilA was most evident in S. Enteriditis where a 6-fold decrease in expression was observed through an unknown mechanism (Gantois et al., 2006). We also pursued the virulence studies since XPC can apparently suppress Salmonella virulence in cattle (Brewer et al., 2014).
Antibiotic resistance was examined since XPC can modulate the microbiome (Mullins et al., 2013; Price et al., 2010), which can alter the antibiograms of resident bacteria (Kirkup and Riley, 2004). To assess the parameters, birds were experimentally infected with integron-bearing multi-resistant Salmonella and fed XPC after which quantity, prevalence, virulence, and antibiotic resistance of Salmonella was examined. Integron-mediated resistance was chosen since our previous studies revealed that intestinal factors facilitate the expulsion of integrons from Salmonella (Brewer et al., 2013). Comparisons were made to Salmonella recovered from experimentally infected broilers fed a standard commercial diet.
MATERIALS AND METHODS
Experimental Design and Husbandry
An experiment was conducted at Iowa State University (Ames) using one-day-old Cobb broiler chicks that were obtained from Welp Hatchery (Bancroft, Iowa). Three separate and independent replications of this experiment were conducted using a total of 50 chicks per experiment (25 per treatment group), resulting in a total of 75 chicks per treatment. On day zero (D0), birds were housed in a BL-2 facility in pens (0.09 m2; 10 birds/pen) within rooms that were both humidity (∼40%) and temperature controlled (35°C for 3 d, then 28 to 31°C for the remainder of the study). On D14, birds were moved to elevated Tenderfoot-type decks (13.4 m2 per treatment group) for the remainder of each experiment. Feed was provided in a metal feed trough and water through a bell drinker.
All birds were fed a non-medicated starter diet (24% crude protein; Tractor Supply Company, Brentwood, TN) from D0 to 21. Birds were then randomly assigned on D21 to one of 2 feed treatment groups: 1) finisher control diet only (CON), or 2) finisher diet that contained 1.25 kg/MT XPC. From D21 to 49, the basal diet was a non-medicated finisher diet (18 to 19% crude protein; Solon Feed Mill, Solon, IA) and birds were allowed ad libitum access to feed and water. The photoperiod consisted of 12 h light and 12 h dark. All birds were individually weighed on D21 and then again at the end of the study on D49.
Each room held one treatment group to avoid inadvertently administering the wrong treatment within a room. Throughout the 3 consecutive studies, treatment groups were alternated in the 2 different rooms to avoid a potential room effect. The investigators at Iowa State University were blinded as to which birds received the CON or XPC diet during the entire study. The protocol used in these experiments was approved by the Institutional Animal Care and Use Committee at Iowa State University.
Salmonella Challenge
All birds were confirmed to be Salmonella-free by fecal culture upon arrival. Specifically, one to 5 g of freshly voided feces from each chick was diluted in 10 mL of Lennox L broth (Invitrogen, Carlsbad, CA). After settling for one to 2 h at room temperature, an aliquot (100 μL) of this mixture was streaked onto and then incubated overnight at 37°C on Xylose Lysine Deoxycholate (XLD) agar (Fisher Scientific, Pittsburgh, PA) that is selective for Salmonella, which appear as white colonies with black centers (Anderson et al., 2015). All pre-infection fecal samples were free of Salmonella.
On D2, 9, and 16, birds were orally inoculated with Salmonella Typhimurium strain LNWI (Wu et al., 2002; Anderson et al., 2015). The dose increased from 2 × 108 CFU/bird on D2 (Anderson et al., 2015) to 4 × 108 CFU/bird on D9 to 8 × 108 CFU/bird on D16, and this procedure was done to maximize the likelihood of large intestinal carriage. The Salmonella inoculum was prepared and dosed as previously reported (Xiong et al., 2011; Xiong et al., 2012; Xiong et al., 2013; Anderson et al., 2015). The inoculum was slowly introduced into the mouth of each bird using a pipette tip. Previous studies revealed that Salmonella is viable after incubation with either XPC (at the concentration equivalent to the dose used in this study) or the CON treatment (Anderson et al., 2015).
Assessment of Salmonella Fecal Shedding Prior to Treatments
On D6, 13, and 20, one to 5 g of freshly voided feces from each bird was diluted in 10 mL of Lennox L broth (Invitrogen, Carlsbad, CA). After settling for one to 2 h at room temperature, an aliquot (100 μL) of this mixture was streaked onto and then incubated overnight at 37°C on XLD agar. On D7 and 14, fecal samples were examined for the qualitative presence of Salmonella colonies on XLD agar. On D21, Salmonella were enumerated on XLD agar and shedding was determined quantitatively as number of colonies x 100 (i.e., the dilution factor) divided by the grams of feces in the sample. Any non-shedding individual birds (as determined by fecal culture, n = 6 per group per each of the 3 separate trials) were euthanized and removed from the study on D21. The remaining birds were assigned to either treatment group based on body weight and Salmonella shedding, using a serpentine assignment format that mathematically redistributes birds in order to prevent a weight bias between groups. Specifically, each bird was ranked based on weight and the bird with the lowest weight was grouped (e.g., Treatment Group A) with the bird with the highest weight; the bird with the second lowest weight was placed in the other group (Treatment Group B) along with the bird with the second highest weight; the bird with the third lowest weight was placed in Group A along with the bird with the third highest weight; the bird with the fourth lowest weight was placed in Group B along with the bird with the fourth highest weight, and so forth. As an illustrative example using 36 birds segregated into 2 treatment groups (either XPC or CON), the following body weight-based rankings would be used in each group: Treatment Group A, birds 1, 36, 3, 34, 5, 32, 7, 30, 9, 28, 11, 26, 13, 24, 15, 22, 17, and 21; Treatment Group B, birds 2, 35, 4, 33, 6, 31, 8, 29, 10, 27, 12, 25, 14, 23, 16, 21, 18, and 19. Fecal shedding was also factored into the assignments for birds with identical weights or when an odd number of birds was available for segregation into the 2 groups. That is, the fecal shedding data were considered, when necessary, in order to make the average fecal shedding equivalent between the groups.
Assessment of Salmonella shedding during treatments
On D21, treatments began for each group of birds (n = 19 to 22 per group after removing non-shedders in each experiment). On D28, 35, and 42, approximately 0.5 g of freshly voided feces (from each bird) was briefly vortexed in 10 mL of Lennox L broth (Invitrogen, Carlsbad, CA). An aliquot of this mixture (100 μL) was incubated overnight at 37°C on XLD agar. The following d, white colonies with black centers were enumerated and CFU/g of feces was calculated based on a dilution factor equal to 100.
Assessment of Large Intestinal Carriage by Salmonella
On D49, all remaining birds were euthanized and a 5 cm section (approximately 3 g) of distal intestine (between the cloaca and ceca) was aseptically removed from each bird and cut longitudinally. Each section was placed in 10 mL Lennox L broth (Invitrogen, Carlsbad, CA) and briefly vortexed to dislodge the Salmonella. An aliquot (100 μL) of this mixture was then dispersed onto XLD agar plates that were incubated overnight at 37°C. The following d, white colonies with black centers were enumerated and CFU/g of intestine was calculated based on a dilution factor equal to 100.
Assessment of the Invasiveness of Salmonella Recovered from Broiler Chickens
On D21, 28, 35, 42, and 49, Salmonella recovered from broiler chickens were subjected to a mammalian tissue culture invasion assay. After enumeration of colonies on XLD agar plates, approximately 30% of recovered colonies were immediately inoculated en masse into LB broth that was used in a standard gentamicin protection-based invasion assay using Human Epithelial Type 2 cells (Carlson et al., 2000; Carlson et al., 2007), with a multiplicity of infection equal to at least one. Bacteria were allowed to adhere and invade tissue culture cells for one h, after which the extracellular (i.e., non-invasive) were killed with 50 μg/ml gentamicin. Tissue culture cells were then lysed with 1% Triton and the lysates were plated on XLD agar and grown overnight at 37°C. The next d, colonies were counted and percent invasion was calculated as 100 x (number of Salmonella recovered from tissue culture wells/number of Salmonella incubated with tissue culture wells). Invasion assays were performed in triplicate for both groups (XPC and CON) in each of the 3 separate experiments. Bacteria isolated from XLD were done so immediately to prevent changes in invasion gene expression.
Assessment of Invasion Gene Expression in Salmonella Recovered from Broiler Chickens
Approximately 20% of Salmonella recovered from the birds were subjected to a semi-quantitative RT-PCR that assesses the expression of hilA (Carlson et al., 2007), the global regulator of Salmonella invasion (Bajaj et al., 1995). RNA was isolated and subjected to the semi-quantitative RT-PCR assay in which the number of PCR cycles (5 to 40) required to visualize an amplicon on agarose gel electrophoresis is documented (Carlson et al., 2007).
RNA Isolation.
RNA was isolated from a group of colonies (n > 40 colonies) picked directly from XLD plates and placed into PBS. RNA was isolated using the RNEasy kit (Qiagen) as per the manufacturer's protocol.
Semi-quantitative RT-PCR.
RNA (50 ng/assay) was subjected to the semi-quantitative RT-PCR assay in which the number of PCR cycles (5 to 40) required to visualize an amplicon on agarose gel electrophoresis is documented (Carlson et al., 2007). PCR conditions and the hilA primers are described previously (Carlson et al., 2007). The rpoS primers are 5′-ATGAGTCAGAATACGCTGAA-3′ and 5′-TTACTCGCGGAACAGCGCTT-3′, representing the forward and reverse primers, respectively.
Gene Analysis and Expression.
Subsets of reactions are removed every 5 cycles and resolved on 2% agarose gels run for 30 min., and amplicons are visualized under UV light. Expression is then calculated as percent of CON, i.e., 100 x (lowest number of cycles required to visualize an amplicon for CON samples/lowest number of cycles required to visualize and amplicon for XPC samples). Invasion gene expression assays were performed in triplicate for both groups (XPC and CON) in each of the 3 separate experiments, with rpoS used as the housekeeping gene control whose expression does not change significantly. That is, rpoS amplicons are typically observed at 25 to 30 cycles whereas hilA amplicons were typically observed at a wider range (10 to 35) of cycles. Data were pooled in order to calculate the Mean ± SEM for three experiments performed separately.
Assessment of the Antibiotic Resistance of Salmonella Recovered from Broiler Chickens
On D21, 28, 35, 42, and 49, approximately 20% of Salmonella recovered from broiler chickens were assessed for resistance to chloramphenicol at the breakpoint concentration (32 μg/mL; CLSI, 2011). Chloramphenicol was chosen since resistance to this antibiotic is encoded by the SGI1 integron present in the input Salmonella isolate (Wu et al., 2002). Individual black-centered colonies from XLD plates (n = 96/treatment group) were inoculated into an individual well of a 96-well dish containing 200 μL of LB broth. Bacteria were grown statically overnight at 37°C to an OD600 equal to approximately 0.3, which corresponds to a concentration of 3 × 108 CFU/mL. Approximately 3 μL of the growth was pin-replicated into a fresh 96-well dish in which each well contained 32 μg/mL of chloramphenicol in 200 μL of LB broth. Percent chloramphenicol resistance was calculated as 100 x (number of wells in which Salmonella grew in the presence of chloramphenicol/96). Chloramphenicol susceptibility assays were performed for both groups (XPC and CON) in each of the 3 separate experiments.
Assessment of the Presence of the Antibiotic Resistance-encoding Integron in Salmonella Recovered from Broiler Chickens
To determine if the changes in chloramphenicol resistance were due to loss of the SGI1 (Salmonella genomic island 1) integron from the input strain, a PCR assay was performed to determine the qualitative presence of the SGI1 integron. Recovered Salmonella colonies were individually inoculated into LB broth in 96-well dishes in the absence of chloramphenicol. Bacterial growth was then subjected to a qualitative PCR assay developed and previously described by Carlson et al. (1999). Percent SGI1(+) was calculated as 100 x (number of wells in which Salmonella yielded an SGI1-specific amplicon visualized using agarose gel electrophoresis/96). SGI1 prevalence was determined for both groups (XPC and CON) in each of the 3 separate experiments, with the input strain used as a positive control.
Statistical Analysis
For data in which assessments were performed on multiple d (antibiotic resistance, invasion, and fecal shedding), statistical comparisons were made using a repeated measures analysis of variance with Tukey's ad hoc test for multiple comparisons (GraphPad Prism, Version 6, La Jolla, CA). For data involving single measurements from each group (large intestinal carriage), statistical comparisons were performed using a student's t test (GraphPad). Body weight data were analyzed by the general linear model procedure of SAS software (Version 8.02, SAS Institute, Cary, NC) with Treatment (CON or XPC), Trial, and the interaction Treatment x Trial considered as the main effects. Absolute weight data were transformed to common logarithms prior to analysis. Mean separations were accomplished using LSMEANS with Tukey's correction. For all variables under analysis, significant differences were defined at P ≤ 0.05. Statistical trends were consistent when the 3 sets of experiments were examined independently (data not shown).
RESULTS
Three individual and independent replications of this experiment were conducted and no significant Trial*Treatment or Trial effects were observed, so data were combined for analysis. Data presented in Table 1 and Figures 1A–B, 2A–B, 3, 4, 5, and 6 represent the Mean ± SEM for 3 experiments performed separately.
Table 1.
Body weights (kg), body weight gains (kg), and statistical probabilities for broilers fed with and without Diamond V Original XPC™ and challenged with Salmonella Typhimurium.
| Treatment | |||
|---|---|---|---|
| Variable1,2 | CON3 | XPC3,4,5 | P-value |
| BW D21 | 0.896 ± 0.018 | 0.881 ± 0.017 | 0.5077 |
| BW D49 | 3.243 ± 0.045 | 3.504 ± 0.044 | <0.0001 |
| BW Gain (D21 to 49) | 2.343 ± 0.045 | 2.613 ± 0.043 | 0.0122 |
1Means ± SEM.
2Means across rows within the same variable column with no common superscript differ significantly (P < 0.05).
3Sample size: CON (n = 57) and XPC (n = 57).
4Diamond V Original XPC™ inclusion rate was 1.25 kg/MT for all diets in the XPC treatment group.
5Dietary treatment was applied on Day 21.
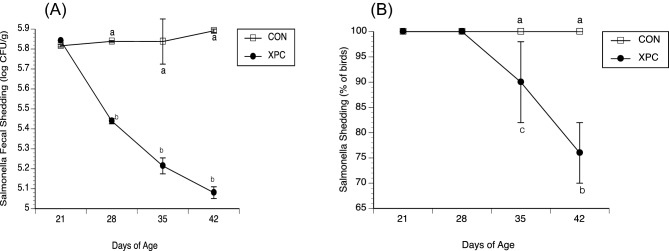
Figure 1.
(A) Salmonella fecal shedding (CFU/g) in broilers fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Birds were orally inoculated with multiple antibiotic-resistant Salmonella Typhimurium on D2, 9, and 16. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21 and Salmonella were isolated from feces (using XLD agar) on D21, 28, 35, and 42. Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01. (B) Prevalence of Salmonella fecal shedding in broilers fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Birds were orally inoculated with multiple antibiotic-resistant Salmonella Typhimurium on D2, 9, and 16. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21 and Salmonella were isolated from feces (using XLD agar) on D21, 28, 35, and 42. Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01, a,c0.01 < P < 0.05.
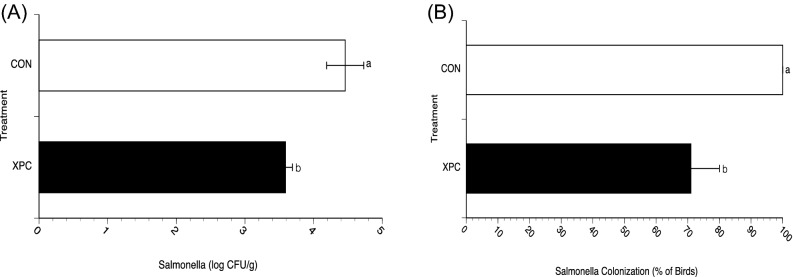
Figure 2.
(A) Large intestinal colonization by Salmonella on D49 in broilers fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21. On D49, all birds were euthanized and a section of the large intestine was removed and selectively and enumeratively cultured for Salmonella using XLD agar. Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01. (B) Prevalence of large intestinal colonization by Salmonella on D49 in broilers fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21. On D49, all birds were euthanized and a section of the large intestine was removed and selectively and enumeratively cultured for Salmonella using XLD agar. Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01.
Figure 3.
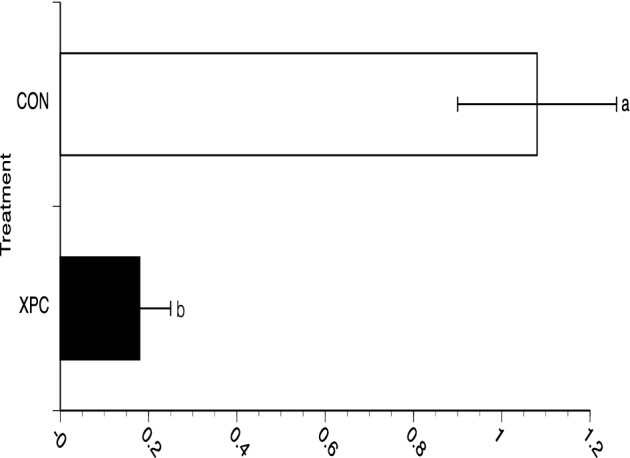
Tissue culture invasiveness of Salmonella recovered from broilers challenged with Salmonella Typhimurium and fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21 and continued until D49 when intestinal samples were taken and approximately 30% of the Salmonella recovered (approximately 105 CFU) were subjected to the invasion assay using mammalian tissue culture cells and a multiplicity of infection equal to at least one. Percent invasion is calculated as 100 x (number of Salmonella recovered from within the tissue culture wells/number of Salmonella added to the tissue culture wells). Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01
Figure 4.
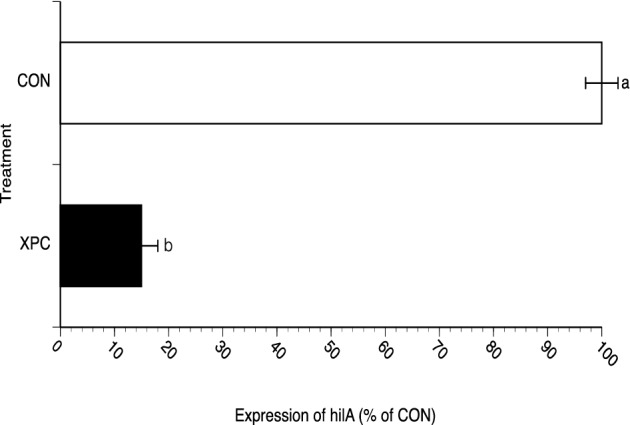
Expression of hilA in Salmonella recovered from feces of broilers challenged with Salmonella Typhimurium and fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21 and continued until D49 when intestinal samples were taken and approximately 20% of the Salmonella recovered were subjected to the RNA isolation and semi-quantitative RT-PCR as previously described (Carlson et al., 2007). Expression was then calculated as percent of CON, i.e., 100 x (number of cycles required to visualize an amplicon for CON samples/number of cycles required to visualize an amplicon for XPC samples). Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01
Figure 5.
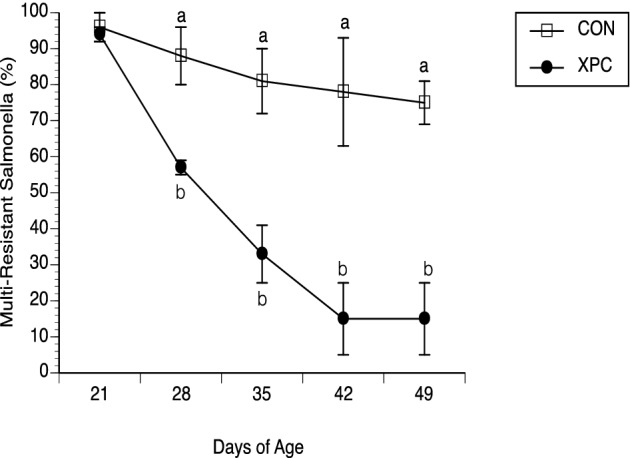
Chloramphenicol resistance of Salmonella recovered from the feces (D21, 28, 35, and 42) or intestines (D49) of broilers challenged with Salmonella Typhimurium and fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21. On D21, 28, 35, 42, and 49, approximately 20% Salmonella recovered from broiler chickens were assessed for resistance to chloramphenicol at the breakpoint concentration (32 μg/mL; CLSI, 2011). Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01
Figure 6.
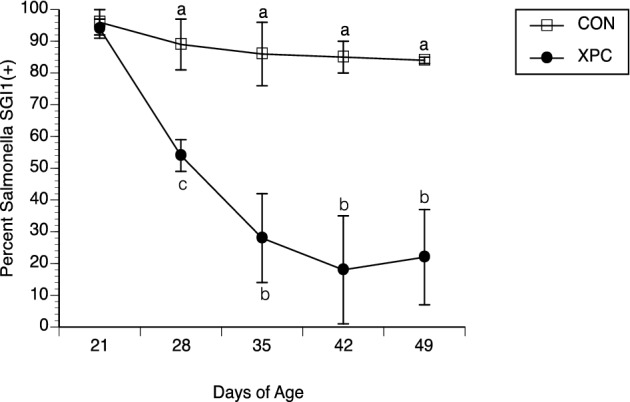
Presence of SGI1 in Salmonella recovered from the feces (D21, 28, 35, and 42) or intestines (D49) of broilers challenged with Salmonella Typhimurium and fed with and without Diamond V Original XPC™ at an inclusion rate of 1.25 kg/MT. Dietary treatments, CON (n = 57) or XPC (n = 57), were applied on D21. Recovered Salmonella colonies were individually inoculated into LB broth in 96-well dishes in the absence of chloramphenicol. Bacterial growth was then subjected to a PCR assay developed and previously described by Carlson et al. (1999). Percent SGI1(+) was calculated as 100 x (number of wells in which Salmonella yielded an SGI1-specific amplicon/96). Data represent the Mean ± SEM for 3 experiments performed separately. a,bP < 0.01, a,c0.01 < P < 0.05
Fecal shedding of Salmonella is a potential source of this pathogen for humans who consume poultry products. On D2, 9, and 16, birds were orally inoculated with Salmonella Typhimurium and on D21 they were assigned to either the CON or XPC dietary treatment. As expected, no significant differences were observed for fecal shedding of Salmonella between chicks assigned to CON and XPC on D21 (6.5 × 105 versus 6.9 × 105 CFU/g, respectively), as dietary treatments did not start until D21 (Figure 1A). Significant differences (P < 0.05) in fecal shedding were observed between CON and XPC on D28, 35, and 42 with lower fecal shedding of Salmonella in birds fed XPC when compared to the CON-fed birds (Figure 1A). On D28, the XPC-fed group had a 2.4-fold decrease (0.38 on a log10 scale) in shedding compared to CON-fed birds (2.7 × 105 versus 6.9 × 105 CFU/g, respectively). The greatest difference was observed on D42, with an approximately 7.5-fold decrease (0.88 on a log10 scale) in shedding for chicks fed XPC (7.7 × 105 versus 1.2 × 105 CFU/g, respectively). The relative prevalence of fecal shedding on D49 (as indirectly measured by large intestinal carriage) was significantly less (P < 0.05) in birds fed XPC than CON (76% vs. 100%, respectively) (Figure 1B).
Since Salmonella carriage in the large intestine can be a source of contamination for poultry meat, the Salmonella load in each bird was determined at the end of the study. On D49, large intestinal sections were excised and subjected to Salmonella culture and enumeration that quantifies the intestinal load of Salmonella. As shown in Figure 2A, large intestinal carriage was significantly less (P < 0.05) in birds fed XPC compared to CON (3,875 vs. 29,023 CFU/g of intestine, respectively; 0.88 log10 reduction). The relative prevalence of large intestinal carriage was significantly less (P < 0.05) in birds fed XPC versus CON (71% vs. 100%, respectively) (Figure 2B).
To determine if the treatment had an effect on broiler performance as previously reported after challenge with a different Salmonella serovar (McIntyre, 2013), broilers in each group were individually weighed on D21 and 49 and these data are presented in Table 1. No significant 2-way interaction between Treatment and Trial were observed for any of the measurements, therefore only Treatment effects are presented in Table 1. Because of the serpentine assignment format, no significant differences in body weight (BW) were observed between birds assigned to XPC and CON groups on D21. By D49, XPC-fed birds were significantly heavier than CON-fed birds (3.504 vs. 3.243 kg, respectively). Birds fed XPC from D21 to 49 exhibited significantly heavier weight gain than CON-fed birds (2.613 vs. 2.343 kg, respectively).
At all 5 time points in which Salmonella were recovered and quantitated from the birds (D21, 28, 35, 42 and 49), presumptive colonies were collected by group and then subjected to a tissue culture invasion assay. This approach was taken since a similar technology (EpiCor®, Embria Health Sciences) has been reported to increase butyrate levels in the intestine (Possemiers et al., 2013) and butyrate can decrease Salmonella invasion gene expression in vitro (Gantois et al., 2006). No significant differences were observed for invasiveness of Salmonella on D21 (1.06 vs. 1.03%), as dietary treatments did not start until D21. Significant differences (P < 0.05) in invasiveness were observed between CON and XPC (1.08% vs. 0.18%, respectively; i.e., a 0.78 reduction on a log10 scale) on D49 with Salmonella exhibiting decreased invasiveness following isolation from birds fed XPC when compared to the CON-fed birds (Figure 3). This decrease in invasiveness of Salmonella coincided with a decrease in the expression of hilA (Figure 4), a major regulator of Salmonella virulence for mammalian hosts (Bajaj et al., 1995).
Salmonella recovered from birds were subjected to a chloramphenicol susceptibility assay. This line of study was pursued since the input strain bears a chloramphenicol resistance-encoding genetic structure (SGI1) that can be dislodged from Salmonella based on previous studies (Brewer et al., 2013). Figure 5 reveals a similar prevalence of resistant Salmonella on D21 in both groups (96 vs. 94%) followed by a decrease in the prevalence of chloramphenicol resistance (P < 0.05) in Salmonella recovered from broilers fed XPC on D28, 35, 42 and 49, as compared to Salmonella recovered from CON fed birds (57 vs. 88%, 33 vs. 81%, 15 vs. 78%, and 15 vs. 75%, respectively). This reduction in chloramphenicol resistance was likely due to egress of the SGI1 integron, as presented in Figure 6, in which about 80% of the isolates from the CON-fed birds retained SGI1 yet only 10 to 20% of isolates retained SGI1 in birds fed XPC.
DISCUSSION
Salmonella is an insidious problem for the poultry industry, and this problem represents a critical food safety hazard. In addition, there are reports of non-typhoidal serovars causing salmonellosis in chickens (Ogunleye and Carlson 2012; Gong et al., 2016). Therefore, identifying mitigation strategies is of paramount importance to the poultry industry.
In this study, the anti-Salmonella effects of XPC were examined and 2 critical indicators of Salmonella contamination (shedding and large intestinal carriage) were significantly reduced by XPC. Both shedding and large intestinal carriage are dependent upon pathogen burden, and it appears that Salmonella may be less efficient at maintaining an attachment to the intestinal tract in the presence of XPC. The diminished shedding was observed at all 3 time points including just 7 d following the initiation of feeding XPC. In the CON-fed broilers, a higher number of Salmonella were present in the large intestinal tract, which ultimately increases the risk of pathogen transmission to humans that ingest the poultry meat. These results are consistent with a previous study in which XPC reduced intestinal colonization (Ibukic et al., 2012). Additionally, the current studies revealed a performance benefit for feeding XPC, which is consistent with previous reports (Nsereko et al., 2013; Hofacre et al., 2015).
McIntyre (2013) reported that broilers that were orally gavaged on D0 with a nalidixic acid resistant strain of Salmonella Heidelberg and fed a diet with and without XPC, at an inclusion rate of 1.25 kg/MT, did not differ in BW gain from D0 to 35 or D0 to 42. However, birds fed XPC did exhibit statistically lower cumulative feed conversions to D35 and 42 and better livability as compared to CON-fed birds (McIntyre, 2013). In the present study, birds were fed the CON diet until they were sorted into dietary treatment groups on D21, which was based on BW and Salmonella shedding using a serpentine assignment format. Therefore, it was not unexpected to find no significant differences for BW on D21 between the XPC- and CON-fed birds. However, the addition of XPC in the diet, starting on D21, resulted in improved BW on D49 and for BW gain from D21 to 49, suggesting that the addition of XPC can result in an improvement in a commercially important trait such as BW.
Other significant and unique findings in this study are the reduction of tissue culture invasiveness and SGI1-based antibiotic resistance in the Salmonella recovered from broilers fed XPC. The reduced virulence was manifested by diminished tissue culture invasion concordant with a reduction in the expression of hilA, the master regulator of Salmonella invasion (Bajaj et al., 1995). It is unclear how these virulence parameters were improved by feeding XPC, but previous studies revealed that XPC increases the gastrointestinal production of butyrate (Broomhead et al., 2012; Nsereko et al., 2013), which is an established repressor of hilA expression (Durant et al., 2000). Nonetheless, the observed magnitude of decreased invasiveness is likely to increase the infectious dose for a human as evidenced by a prior study in which this level of diminished invasiveness altered the murine LD50 by 10-fold (Carlson et al., 2000).
For the reduction in antibiotic resistance, this effect was correlated with a loss of the SGI1 integron from the input Salmonella strain. While we have no hypothesis for the mechanism by which XPC mediates this expulsion event, previous work revealed that SGI1 can be lost from Salmonella while in the intestinal tract (Brewer et al., 2013).
In summary, broilers were significantly less likely to harbor large intestinal Salmonella and subsequently shed the pathogen in birds that were fed XPC. It is unclear if this effect is due to decreased attachment or increased clearance of the microbe. Additionally, feeding XPC reduced the virulence and antibiotic resistance of the input Salmonella strain. Ultimately, these varying yet beneficial effects will have a marked positive effect on food safety in the poultry industry and future mechanistic studies will uncover the molecular bases for these effects.
Acknowledgments
The authors thank Dale Hinderaker, Alan Elsberry, Angelina Troutwine, and Diane McDonald for animal husbandry. We also would like to thank Drs. Victor Nsereko, Hilary Pavlidis, and Stuart Reeves for technical support.
REFERENCES
- Al-Homidan A., Fahmy M. O. The effect of dried yeast (Saccharomyces cerevisiae) supplementation on growth performance, carcass chemical analysis, immunity, ileum villi heights, and bacterial counts of broiler chickens. Egypt Poult. Sci. 2007;27:613–623. [Google Scholar]
- Anderson K., Brewer M., Rasmussen M., Carlson S. Identification of heritage chicken breeds with diminished susceptibility to intestinal colonization by multiple antibiotic-resistant Salmonella spp. Livestock Sci. 2015;182:34–37. [Google Scholar]
- Bajaj V., Hwang C., Lee C. A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- Brewer M., Xiong N., Anderson K., Carlson S. Effects of subtherapeutic concentrations of antimicrobials on gene acquisition events in Yersinia. Proteus. Shigella, and Salmonella recipient organisms in isolated ligated intestinal loops of swine. Am. J. Vet Res. 2013;74:1078–1083. doi: 10.2460/ajvr.74.8.1078. [DOI] [PubMed] [Google Scholar]
- Brewer M., Anderson K., Yoon I., Scott M., Carlson S. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet Microbiol. 2014;172:248–255. doi: 10.1016/j.vetmic.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Broomhead J., Severson D., Butler J., Frank J. Effects of a Saccharomyces cerevisiae fermentation product on volatile fatty acid production and growth of Salmonella in a complex fecal microbial population in vitro. Poult. Sci. 2012;91:132. Suppl.1. [Google Scholar]
- Carlson S., McCuddin Z., Rasmussen M., Franklin S., Sharma V. Involvement of a Salmonella genomic island 1 gene in the rumen protozoan-mediated enhancement of invasion for multiple-antibiotic-resistant Salmonella enterica Serotype Typhimurium. Infect. Immun. 2007;72:792–800. doi: 10.1128/IAI.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S. A., Bolton L. F., Briggs C. E., Hurd H. S., Sharma V. K., Fedorka-Cray P., Jones B. D. Detection of Salmonella typhimurium DT104 using multiplex and fluorogenic PCR. Mol. Cell. Probes. 1999;13:213–222. doi: 10.1006/mcpr.1999.0240. [DOI] [PubMed] [Google Scholar]
- Carlson S. A., Browning M., Ferris K. E., Jones B. D. Identification of diminished tissue culture invasiveness among multiple antibiotic resistant Salmonella typhimurium DT104. Micro. Pathog. 2000;28:37–44. doi: 10.1006/mpat.1999.0322. [DOI] [PubMed] [Google Scholar]
- CDC . Reports of Salmonella Outbreak Investigations from 2015. 2015. http://www.cdc.gov/salmonella/outbreaks-2015.html. [Google Scholar]
- CLSI . Performance standards for antimicrobial disk and dilution susceptibility tests. 2011. Wayne, PA. [Google Scholar]
- Durant J., Corrier D., Ricke S. Short-chain volatile fatty acids modulate the expression of the hilA and invF genes of Salmonella typhimurium. J. Food Prot. 2000;63:573–578. doi: 10.4315/0362-028x-63.5.573. [DOI] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Hautefort I., Thompson A., Hinton J., Van Immerseel F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Yu S. H., Wu S. G., Yoon I., Quigley J., Gao Y. P., Qi G. H. Effects of yeast culture in broiler diets on performance and immune-modulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Wu S. G., Yu S. H., Yoon I., Moore D., Gao Y. P., Yan H. J., Qi G. H. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 2009;88:2141–2151. doi: 10.3382/ps.2009-00151. [DOI] [PubMed] [Google Scholar]
- Gong J., Wang C., Shi S., Bao H., Zhu C., Kelly P., Zhuang L., Lu G., Dou X., Wang R., Xu B., Zou J. “Highly drug-resistant Salmonella enterica Serovar Indiana clinical isolates recovered from broilers and poultry workers with diarrhea in China.”. Antimicrob. Ag. Chemother. 2016;60:1943–1947. doi: 10.1128/AAC.03009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacre C., Berghaus R., McIntyre D., Smith D. Field studies: Preharvest Salmonella control using the immune modulator Original XPC in broilers and commercial turkeys. Poult. Sci. 2015;94 Suppl. 1. [Google Scholar]
- Ibukic M., Trampel D., Frana T., Logue C. M., Broomhead J. Evaluation of Diamond V Original XPC for reducing cecal colonization by Salmonella Enteriditis in layer pullets. Proc. 93rd Conf. Res. Work. Anim. Dis. 2012 Abstract 051P. Accessed at: http://www.abstractsonline.com/Plan/ViewAbstract.aspx. [Google Scholar]
- Jensen G. S., Patterson K. M., Yoon I. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immun. Microbiol. Infect. Dis. 2008;31:487–500. doi: 10.1016/j.cimid.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Jensen G. S., Redman K. A., Benson K. F., Carter S. G., Mitzner M. A., Reeves S., Robinson L. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: Results of a placebo-controlled double-blinded crossover pilot study. J. Med. Food. 2011;14:1002–1010. doi: 10.1089/jmf.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup B. C., Riley M. A. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature. 2004;428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- McIntyre D. R. Salmonella Heidelberg prevalence is reduced in Original XPC-fed broilers. Poultry Advisor. 2013:1–5. December. [Google Scholar]
- Mullins C., Mamedova L., Carpenter A., Ying Y., Allen M., Yoon I., Bradford B. Analysis of rumen microbial populations in lactating dairy cattle fed diets varying in carbohydrate profiles and Saccharomyces cerevisiae fermentation product. J. Dairy Sci. 2013;96:5872–5881. doi: 10.3168/jds.2013-6775. [DOI] [PubMed] [Google Scholar]
- Nsereko V. L., Broomhead J. N., Butler J., Weigand T., Gingerich E. Effects of Original XPC on growth of Salmonella Arizonae and Heidelberg in a complex fecal microbial population. Poult. Sci. 2013;92:130. Suppl. 1. [Google Scholar]
- Ogunleye A. O., Carlson S. A. Emergence of an SGI1-bearing Salmonella enterica serotype Kentucky isolated from septic poultry in Nigeria. J. Infect. Dev. Ctries. 2012;6:438–488. doi: 10.3855/jidc.1988. [DOI] [PubMed] [Google Scholar]
- Possemiers S., Pinheiro I., Verhelst A., Van den Abbeele P., Maignien L., Laukens D., Reeves S., Robinson L., Raas T., Schneider Y., Van de Wiele T., Marzorati M. A dried yeast fermentate selectively modulates both the luminal and mucosal gut microbiota and protects against inflammation, as studied in an integrated in vitro approach. J. Agricul. Food Chem. 2013;61:9380–9392. doi: 10.1021/jf402137r. [DOI] [PubMed] [Google Scholar]
- Price K., Totty H., Lee H., Utt M., Fitzner G., Yoon I., Ponder M., Escobar J. Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J. Anim. Sci. 2010;88:3896–3908. doi: 10.2527/jas.2009-2728. [DOI] [PubMed] [Google Scholar]
- Traub-Dargatz J., Ladely S., Dargatz D., Fedorka-Cray P. Impact of heat stress on the fecal shedding patterns of Salmonella enterica Typhimurium DT104 and Salmonella enterica Infantis by 5-week-old male broilers. Foodborne Pathog. Dis. 2006;3:178–183. doi: 10.1089/fpd.2006.3.178. [DOI] [PubMed] [Google Scholar]
- Wilson R., Elthon J., Clegg S., Jones B. Salmonella enterica serovars gallinarum and pullorum expressing Salmonella enterica serovar typhimurium type 1 fimbriae exhibit increased invasiveness for mammalian cells. Infect. Immun. 2000;63:4782–4785. doi: 10.1128/iai.68.8.4782-4785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. T., Carlson S. A., Meyerholz D. K. Cytopathic effects observed upon expression of a repressed collagenase gene present in Salmonella and related pathogens: mimicry of a cytotoxin from multiple antibiotic-resistant Salmonella enterica serotype Typhimurium phagetype DT104. Microb. Pathogen. 2002;32:1–9. doi: 10.1006/mpat.2002.0535. [DOI] [PubMed] [Google Scholar]
- Xiong N., Brewer M., Anderson K., Weeks K., Carlson S. Expression of a collagenase that enables blood-brain barrier penetration for Salmonella implicated in bovine encephalopathies. Microb. Pathog. 2011;51:230–232. doi: 10.1016/j.micpath.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Xiong N., Brewer M., Anderson K., Watrous G., Weeks K., Barnhill A., Day T., Kimber M., Carlson S. Beta-lactam antibiotics prevent Salmonella-mediated bovine encephalopathy regardless of the beta-lactam resistance status of the bacteria. The Vet. J. 2012;192:535–537. doi: 10.1016/j.tvjl.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Xiong N., Brewer M., Anderson K., Carlson S. Non-typhoidal Salmonella encephalopathy involving lipopolysaccharide in cattle. Vet. Microbiol. 2013;162:285–287. doi: 10.1016/j.vetmic.2012.08.007. [DOI] [PubMed] [Google Scholar]