Abstract
The effects of stocking density on the performance, egg quality, leukocyte concentration, blood biochemistry, corticosterone levels, bone mineral density, and noxious gas emission of laying hens were investigated. Eight hundred 34-week-old Hy-Line Brown laying hens (Gallus gallus domesticus) were randomly assigned to one of 4 treatments, each of which was replicated 4 times. Four stocking densities, including 5, 6, 7, and 10 birds/m2, were compared. A commercial-type basal diet was formulated to meet or exceed nutrient recommendations for laying hens from the National Research Council. The diet was fed to the hens ad libitum for 8 wk. Results indicated that hen-day egg production, egg mass, and feed intake were less for (P < 0.01) 10 birds/m2 stock density than other stock densities. Production rate of floor and broken eggs and eggshell strength were greater (P < 0.01) for 10 birds/m2 stock density than other stock densities. There were no significant differences in the level of leukocytes among densities. However, heterophils and the H/L ratio were greater (P < 0.01) for 10 birds/m2 than in stock density of 6 or 7 birds/m2. Serum corticosterone was greater (P < 0.01) 10 birds/m2 than stock density than other stock densities. Litter moisture and gas emission (CO2 and NH3) were greater (P < 0.01) for 10 birds/m2 than stock density than 6 and 7 birds/m2 stock density. Bone mineral content was not influenced by increasing stock density. However, bone mineral density was less (P < 0.05) for 10 m2 stock density than other stock densities. These results indicate that increasing the density beyond 5 birds/m2 elicits some negative effects on laying performance of Hy-Line brown laying hens.
Keywords: bone mineral density, corticosterone, laying performance, stock density, Hy-line Brown laying hens, animal welfare
INTRODUCTION
The welfare of laying hens raised in standard commercial cages has been placed under intense scrutiny. The traditional housing of egg-type chickens in conventional cages, long perceived as the most efficient method of housing laying hens, is now widely considered to have a negative effect on their welfare (Appleby, 1993; Appleby et al., 1993; Craig and Swanson, 1994). The limited environmental complexity and confinement in conventional cages physically restrict hens and eliminate many of their natural behaviors such as nesting, roosting, and scratching (Nicol, 1987; Baxter, 1994; Tactacan et al., 2009). The use of non-cage housing systems for laying hens increased after the 2012 EU ban on conventional cages was implemented. The European Union Council Directive 1999/74/EC (European Communities, 1999) outlined minimum standards for the welfare of laying hens after 1 January 2012, allotting a maximum stock density of 9 birds/m2. However, for those organizations that began applying these standards on 3 August 1999, a stock density of 12 birds/m2 was allowed where the usable area corresponded to the available ground surface (until 2012, when the new standards were applied). The Korean Council Directive 2012–68 (APQA, 2012), which lays down minimum standards for the welfare of laying hens in Korea, imposes a maximum stock density of 7 birds/m2. Stocking rate has been examined as a factor in a number of epidemiological surveys of laying hen behavior (Gunnarsson et al., 1999). There have been numerous studies demonstrating the negative effect of cage or floor density on egg production, egg weight, feed intake, feather pecking, and plumage scores (Bell, 1981; Roush et al., 1984; Anderson et al., 1989; Sandoval et al., 1991; Anderson et al., 2004; Onbasilar and Aksoy, 2005; Jalal et al., 2006; Nicol et al., 2006). Furthermore, the ratio of heterophils and lymphocytes (H/L) (an indicator of stress in birds) has been shown to increase from 0.5 in control birds to 2.76 in stock-density stressed birds. Craig et al. (1986) reported that increased stock density increased plasma corticosterone concentrations. Additionally, as stocking density increases, the amount of caked litter in the pens increases. The presence of caked litter could serve as a seal on the litter, altering the production of ammonia. Caked litter corresponds to high litter moisture and areas of anaerobic activity, which suppresses ammonia volatilization (Carr et al., 1990). To the best of our knowledge, limited research has been published on the effect of stock density on the welfare of laying hens in floor pens. Therefore, this study was conducted to investigate the effect of stock density on laying performance, leukocyte concentration, blood biochemistry, corticosterone concentrations levels, bone mineral density, and noxious gas emission of laying hens.
MATERIALS AND METHODS
The protocol for this experiment was reviewed and approved by the Institutional Animal Care and Welfare Committee of the National Institute of Animal Science, Rural Development Administration, Republic of Korea.
Birds and Experimental Design
Eight-hundred 34-week-old Hy-Line Brown laying hens (Gallus gallus domesticus) were randomly assigned to one of 4 dietary treatments with each treatment replicated 4 times. Four stocking rates of 5 (n = 50), 6 (n = 50), 7 (n = 50), and 10 (n = 50) birds/m2 in floors with deep litter of rice hulls were used for each replicate, respectively. A commercial-type basal diet was formulated to meet or exceed the nutrient recommendations for laying hens issued by the National Research Council (NRC, 1994) (Table 1 ). During the 8-wk experimental period, hens were provided with feed and water ad libitum and were exposed to a 16 h:8 h (light:dark) schedule. The temperature and humidity of the laying house were maintained at 20 ± 3°C and 65 to 70% respectively.
Table 1.
Composition and nutrient content of experimental diet.
| Ingredients (g/kg) | |
|---|---|
| Corn | 411.5 |
| Wheat | 150.0 |
| Soybean meal | 250.0 |
| DDGS | 50.0 |
| Canola meal | 20.0 |
| Tallow | 5.0 |
| Molasses | 5.0 |
| Dicalcium phosphate | 7.0 |
| Limestone | 97.0 |
| Sodium chloride | 2.0 |
| Vitamin premix1 | 1.5 |
| Mineral premix2 | 1.0 |
| Total | 1,000.0 |
| Energy and nutrient content3 | |
| MEn, MJ/kg | 11.4 |
| Crude protein, g/kg | 142.0 |
| Calcium, g/kg | 40.0 |
| Available P, g/kg | 3.3 |
| Lysine, g/kg | 7.5 |
| Methionine, g/kg | 3.6 |
1Provided per kilogram of the complete diet: vitamin A (vitamin A acetate), 12,500 IU; vitamin D3, 2,500 IU; vitamin E (DL-α-tocopheryl acetate), 20 IU; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B2, 5 mg; vitamin B6, 3 mg; vitamin B12, 18 μg; calcium pantothenate, 8 mg; folic acid, 1 mg; biotin, 50 μg; niacin, 24 mg.
2Provided per kilogram of the complete diet: Fe (FeSO4·7H2O), 40 mg; Cu (CuSO4·H2O), 8 mg; Zn (ZnSO4·H2O), 60 mg; Mn (MnSO4·H2O) 90 mg; Mg (MgO) as 1,500 mg.
3Nutrient contents in all diet were calculated.
Laying Performance
Hen-day egg production rate, floor eggs, broken egg production rate, and egg weight were recorded daily, whereas feed intake and the feed conversion ratio were recorded weekly. Egg mass was calculated as per Hayat et al. (2009):
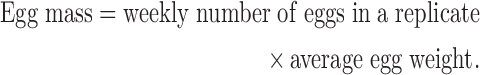 |
Determination of Egg Quality Parameter
Ten eggs per replicate were randomly collected at the end of each week. Eggshell strength, eggshell thickness, egg yolk color, and Haugh units (HU) were measured. Eggshell strength was measured by the Texture Systems Compression Test Cell (model T2100C, Food Technology Co., Ltd., Rockville, MD) and expressed as units of compression force exposed to units of eggshell surface area (kg/cm2). Eggshell thickness is defined as the mean value of measurements at 3 different locations on the egg (air cell, equator, and sharp end) and was measured with a dial pipe gauge (model 7360, Mitutoyo Co. Ltd., Kawasaki, Japan) and calculated using the following formula (Yannakopouls and Tserveni-Gousi 1986):
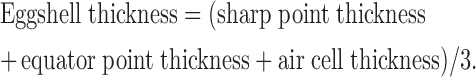 |
Egg yolk color was evaluated by the Roche Yolk Color Fan (Hoffman-La Roche Ltd., Basel, Switzerland; 15 = dark orange; 1 = light pale). Haugh unit values were calculated using a micrometer (model S-8400, Ames, Waltham, MA) with the following formula described by Eisen et al. (1962):
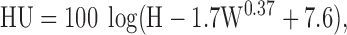 |
where W is egg weight, and H is albumen height.
Hematological Analysis
At the end of the 8-wk feeding trial, 2 birds/replicate (i.e., 8 birds per treatment) with a body weight near the mean were selected to be were euthanized by cervical dislocation. Immediately after death, a 5 mL blood sample was collected from the jugular vein of each bird using EDTA-treated and non-EDTA treated vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). The whole blood samples were kept on ice and used immediately for hematological analysis. Leukocytes (white blood cells, heterophils, lymphocytes, monocytes, eosinophils, and basophils) from the blood samples were analyzed using the Hemavet® Multi-species Hematology System (Drew Scientific Inc., Oxford, CT). The H/L ratios were determined by dividing the number of heterophils by the number of lymphocytes. Serum samples were obtained by centrifuging the samples for 20 min at 25,000 × g and 4°C and were stored at –15°C. Total cholesterol, triglyceride, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and calcium in the serum were quantified using an ADVIA 1650 Chemistry System (Bayer Diagnostic, Puteaux, France). Serum corticosterone concentration was measured using a commercial EIA kit (No. 500655, Cayman Chemical, Ann Arbor, MI) according to the instructions of the manufacturer.
Litter Moisture and Gas Emissions
Litter samples were collected at 4 wk from 4 predetermined points in each pen, and moisture content was measured as described in AOAC method 934.01 (AOAC, 1990). To determine gas emission, litter gas was sampled using a Gastec Gas Sampling Pump (Model GV-100; Gastec Corp., Japan, Gastec Detector Tube No. 3 M and 3 La for ammonia; No. 4LL and 4LK for hydrogen sulphide).
Bone Mineral Density (BMD)
At the end of experiment, 5 birds per treatment were selected and killed by cervical dislocation at the end of experiment for bone analysis. The bone mineral density and bone mineral content of the left tibia of each intact bird was measured using a dual energy x-ray absorptiometry x-ray densitometer (DEXA; GE Healthcare, Lunar Prodigy Advance PA+130472, Small Animal software, Diegem, Belgium). Scanning began at the proximal end of the bone and lasted for approximately 10 min.
Statistical Analysis
All data were analyzed by one-way analysis of variance as a completely randomized design using the PROC MIXED procedure (SAS Institute Inc., Cary, NC). Outlier data were identified by the UNIVARIATE procedure of SAS, but no outliers were found. Least squares means were calculated and the means among treatments were compared by the PDIFF option with the Tukey adjustment. Significance was set at P < 0.05.
RESULTS AND DISCUSSION
Laying Performance
Over the course of the entire experiment (34 to 41 wk) hen-day egg production, egg mass, and feed intake were lower for (P < 0.01) 10 birds/m2 stock density than other stock densities. Floor eggs and broken egg production rate were greater for (P < 0.01) 10 birds/m2 stock density than other stock densities (Table 2). In this study, laying performance was shown to decline in response to increased stocking density. This finding supports previous studies that have shown that decreasing egg production is attributable to a reduction in the amount of feeding area per hen (Hester and Wilson, 1986; Craig and Milliken, 1989; Lee and Moss, 1995; Suto et al., 1997) and increased stocking density (Adams and Craig, 1985). Anderson et al. (2004) found that higher stocking density in Hy-Line W36 and Dekalb XL commercial layer genotypes decreased hen-day egg production. Similarly, Onbasilar and Aksoy (2005) determined hen-day egg production to be 94.1%, 89.3%, and 78.5% at 968, 655, and 393.8 cm2 per hen respectively. Wells (1972), Carey (1987), and Shanawany (1988) all reported a decrease in feed intake with increasing stock density. Furthermore, the increased occurrence of floor or broken eggs in the highest stocking density may be secondary to increased competition for nest space.
Table 2.
Effects of stock density on laying performance of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| Hen-day egg production, % | 78.6a | 78.2a | 77.9a | 75.7b | 0.69 | 0.01 |
| Floor eggs, % | 1.34b | 1.06b | 1.30b | 3.86a | 0.555 | <0.01 |
| Broken egg production rate, % | 0.77b | 0.56b | 0.52b | 3.77a | 0.230 | <0.01 |
| Egg weight, g | 61.9 | 61.8 | 62.4 | 61.6 | 0.24 | 0.70 |
| Egg mass, egg weight, g/eggs | 48.7a | 48.3a | 48.6a | 46.7b | 0.51 | 0.04 |
| Feed intake, g/bird | 115.9a | 116.2a | 115.8a | 112.4b | 0.44 | <0.01 |
| Feed conversion ratio | 2.39 | 2.41 | 2.39 | 2.45 | 0.03 | 0.20 |
a,bValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 4 observations per treatment.
2Pooled error of mean.
Egg Quality Parameters
There were no significant differences in egg quality among stocking density treatments (eggshell thickness, egg yolk color, and Haugh unit; Table 3). However, eggshell strength was less (P < 0.05) for 10 birds/m2 than stock density than other stock densities. Eggshell strength of 4.31, 4.39, 4.41, and 4.11 kg/cm2 were measured for the respective stocking densities of 5, 6, 7, and 10 birds/m2.
Table 3.
Effects of stock density on egg quality of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| Eggshell strength, kg/cm2 | 4.31a,b | 4.39a | 4.41a | 4.11b | 0.032 | 0.04 |
| Eggshell thickness, μm | 350.0 | 363.0 | 354.0 | 365.0 | 12.29 | 0.59 |
| Eggyolk color | 8.6 | 8.6 | 8.3 | 8.6 | 0.02 | 0.20 |
| Haugh unit | 90.5 | 89.2 | 89.7 | 89.8 | 0.49 | 0.21 |
a,bValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 10 observations per treatment.
2Pooled error of mean.
Hematological Analysis
There were no significant differences in the level of leukocytes (Table 4). However, heterophils and the H/L ratio were greater (P < 0.01) for 10 birds/m2 than stock density than 6, and 7 birds/m2 stock density. Mean H/L ratio was 0.34, 0.37, 0.37, and 0.52 for the 5, 6, 7, and 10 birds/m2 treatments, respectively. There were no significant differences in the serum biochemistry (Table 5 ). During the initial (34 to 37 wk) and final 4 wk of the experiment (38 to 41 wk), serum corticosterone was greater (P < 0.01) 10 birds/m2 than stock density than other stock densities (Table 6). Blood parameters are good indicators of the physiological, pathological, and nutritional status of an animal, and changes in hematological parameters have the potential to be used to elucidate the impact of nutritional factors and additives supplied in the diet of any living creature. For example, leukocytes are known to increase sharply when infection occurs as they are one of the first lines of defense of the body (Ganong, 1999; Alzawqari et al., 2011; Masoudi et al., 2011). Leukocyte count has also been used as a measure of immune function in birds (Johnson and Zuk, 1998). Many factors, such as exposure to various microbes and chemicals, can cause changes in both granulocytic white blood cells (Lucas and Jamroz, 1961). The lack of adequate data on the role of increasing stock density in altering blood parameters in poultry requires further research.
Table 4.
Effects of stock density on blood parameter of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| Leukocytes3 | ||||||
| WBC, K/μL | 15.90 | 15.66 | 18.86 | 21.26 | 2.23 | 0.83 |
| HE, K/μL | 3.59b,c | 3.01c | 4.48a,b | 5.48a | 0.37 | <0.01 |
| LY, K/μL | 11.20 | 8.29 | 12.74 | 11.24 | 1.05 | 0.35 |
| SI, HE:LY | 0.34b | 0.37b | 0.37b | 0.52a | 0.06 | 0.04 |
| MO, K/μL | 1.51 | 0.94 | 1.80 | 1.49 | 0.24 | 0.46 |
| EO, K/μL | 0.21 | 0.07 | 0.22 | 0.16 | 0.09 | 0.99 |
| BA, K/μL | 0.04 | 0.01 | 0.02 | 0.02 | 0.02 | 0.48 |
a–cValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 4 observations per treatment.
2Pooled error of mean.
3Leukocytes: WBC = white blood cells; HE = heterophils; LY = lymphocytes; MO = monocytes; EO = eosinophils; BA = basophils.
Table 5.
Effects of stock density on blood biochemistry of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| Total cholesterol, mg/dL | 160.9 | 163.9 | 161.7 | 168.6 | 20.63 | 0.82 |
| Triglyceride, mg/dL | 1,033.9 | 1,382.9 | 1,254.8 | 1,550.5 | 279.42 | 0.25 |
| Glucose, mg/dL | 177.1 | 173.5 | 154.0 | 159.5 | 14.97 | 0.31 |
| Calcium, mg/dL | 3.36 | 3.40 | 3.89 | 3.17 | 0.401 | 0.57 |
| AST, U/L | 7.28 | 6.63 | 8.05 | 7.11 | 0.761 | 0.80 |
| ALT, U/L | 222.9 | 222.2 | 216.4 | 246.1 | 11.82 | 0.26 |
1Data are least squares means of 4 observations per treatment.
2Pooled error of mean.
Table 6.
Effects of stock density on corticosterone of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| – – – – – – – – – pg/mL– – – – – – – – – | ||||||
| 4 wk | 27.91b | 28.95b | 27.55b | 31.68a | 0.667 | <0.01 |
| 8 wk | 30.70b | 30.67b | 29.91b | 34.70a | 0.272 | <0.01 |
a,bValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 4 observations per treatment.
2Pooled error of mean.
The H/L ratio has proved to be a valuable measurement in stress-related research in poultry (Post et al., 2003; Zulkifli et al., 2003). Evaluation of the higher H/L ratio indicates increased stress in poultry with increasing stock density (Martrenchar et al., 1997; Feddes et al., 2002). El-Lethey et al. (2000) also reported that the H/L ratio was influenced by housing conditions (e.g. stock density).
Blood corticosterone concentration has been widely used as a measure of environmental stress in poultry (McFarlane and Curtis, 1989; Zulkifli et al., 2003). Craig et al. (1986) reported that increased stock density increased total plasma corticosteroids in some experiments. Hocking et al. (2001) also reported that mean corticosterone concentration in broiler breeders at 6 wk of age was 0.5 ng/mL under usual stocking density (9 birds/m2). Cheng and Muri (2004) reported that laying hens showed significantly lower plasma corticosterone levels in single-bird cages (525 cm2/bird) than in the 10-bird cages (419 cm2/bird), indicating that social stressors could be a factor in higher production of corticosterone in hens. Later work showed that serum corticosterone levels increased when population density was increased due to birds being forced to compete for feeding and watering space (Petsi and Howarth, 1983; Mashly et al., 1984; Craig et al., 1986).
Litter Moisture and Gas Emissions
Over the course of the experiment, litter moisture, gas emission (CO2 and NH3) were greater (P < 0.01) for 10 birds/m2 than stock density than in 5, 6, and 7 birds/m2 stock density (Table 7). Additionally, as stocking density increased, the amount of caked litter in the pens also increased. Higher stock density increases nitrogen and moisture level in the litter and thus favors microbial activity. Sorensen et al. (2000) reported increased litter moisture of broilers as stocking density increased from 622 to 455 cm2/birds. As stocking density increased, the amount of caked litter in the pens also increased, and the presence of caked litter could have served as a seal, altering the production of ammonia. Also, caked litter corresponds to high litter moisture or areas where litter becomes anaerobic, which suppresses ammonia volatilization (Carr et al., 1990). The wetter the litter, the more likely it will promote the proliferation of pathogenic bacteria and moulds. Litter condition is governed by the type of material, depth, friability, and moisture as well as housing, technical equipment, and management. From a welfare point of view, stock densities that are too high may create various problems such as increased air ammonia and heat produced from the birds, which can lead to stressful conditions and cause the death of individual hens.
Table 7.
Effects of stock density on litter moisture and gas emission of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| Litter moisture, % | 27.8b | 23.6b | 25.8b | 67.5a | 2.02 | <0.01 |
| Gas emission | ||||||
| NH3, ppm | 8.11b | 6.33b | 7.11b | 12.89a | 1.36 | <0.01 |
| CO2, ppm | 522.2b | 555.6b | 533.3b | 677.8a | 19.25 | <0.01 |
a,bValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 4 observations per treatment.
2Pooled error of mean.
Bone Mineral Density
Bone mineral content was not influenced by increasing stock density (Table 8). However, bone mineral density was less (P < 0.05) for 10 m2 stock density than other stock densities. One of the main welfare concerns in cage is the hen's inability to exercise, which leads to development of bone weakness and at peak production can easily result in skeletal damage (Webster, 2004). In the previous research, the bone mineral density of the tibia and humerus was higher in birds housed in enriched cages (Tactacan et al., 2009). Knowles and Broom (1990) and Newman and Leeson (1998) found that bone strength in cage was lower compared with alternatives such as aviary systems or floor pens. Hughes and Appleby (1989) found that bone strength can be increased by providing perches. Also, it is well known that bone mineralization is sensitive to calcium consumption. For example, bone mineral density and bone mineral contents of the tibias of laying hens, as measured by DEXA, increased linearly as hens consumed increasing levels of dietary calcium (Schreiweis et al., 2003).
Table 8.
Effects of stock density on bone mineral density of laying hens.1
| Stock density (birds/m2) | ||||||
|---|---|---|---|---|---|---|
| Items | 5 | 6 | 7 | 10 | SEM2 | P-value |
| BMC3, g | 3.76 | 3.85 | 3.85 | 3.18 | 0.491 | 0.50 |
| BMD4, g/cm2 | 0.28a | 0.28a | 0.29a | 0.22b | 0.030 | 0.03 |
a,bValues in row with no common superscripts letter are significantly different (P < 0.05).
1Data are least squares means of 5 observations per treatment.
2Pooled error of mean.
3BMC = bone mineral contents.
4BMD = bone mineral density.
CONCLUSION
These data indicate that increasing the stock density from 5 to 10 birds/m2 of floor space negatively influenced laying performance, leukocytes concentration, serum corticosterone, litter moisture, gas emission, and bone mineral density, but blood biochemistry was not significantly altered.
Acknowledgments
This work supported by the “Cooperative Research Program for Agriculture Science & Technology Development (Project title: The study of management and disease for animal welfare of laying hens, Project No.PJ010952).” Rural Development Administration, Republic of Korea.
REFERENCES
- Adams A. W., Craig J. V. Effect of crowding and cage shape on productivity and profitability of caged layers: a survey. Poult. Sci. 1985;64:238–242. [Google Scholar]
- Alzawqari M., Moghaddam H. N., Kermanshahi H., Ragi A. R. The effect of desiccated ox bile supplementation on performance, fat digestibility, gut morphology and blood chemistry of broiler chickens fed tallow diets. J. Appl. Anim. Res. 2011;39:169–174. [Google Scholar]
- Anderson K. E., Adams A. W., Craig J. V. Behavioral adaptation of floor-reared white leghorn pullets to different café densities and cage shapes during the initial settling in period. Poult. Sci. 1989;68:70–78. [Google Scholar]
- Anderson K. E., Davis G. S., Jenkins P. K., Carroll A. S. Effect of bird age, density and molt on behavioral profiles of two commercial layer strains in cages. Poult. Sci. 2004;83:15–23. doi: 10.1093/ps/83.1.15. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) Official Methods of Analysis. 15th ed. Assoc. Off. Anal. Chem.; Arlington, VA: 1990. [Google Scholar]
- Appleby M. C. 1993. Should cages for laying hens be banned or modified? Anim. Welfare. 2 67–80. [Google Scholar]
- Appleby M. C., Smith S. F., Hughes B. O. Nesting, dust bathing and perching by laying hens in cages: Effects of design on behavior and welfare. Br. Poult. Sci. 1993;34:835–847. doi: 10.1080/00071669308417644. [DOI] [PubMed] [Google Scholar]
- APQA . Livestock farm animal welfare certification standards (regulation No. 2012-68) Animal and Plant Quarantine Agency; Republic of Korea: 2012. [Google Scholar]
- Baxter M. R. The welfare problems of laying hens in battery cages. Vet. Rec. 1994;134:614–619. doi: 10.1136/vr.134.24.614. [DOI] [PubMed] [Google Scholar]
- Bell D. Cage selection and management. Feedstuffs. 1981;53:20–24. [Google Scholar]
- Carey J. B. Effect of pullet-stocking density on performance of laying hens. Poult. Sci. 1987;66:1283–1287. doi: 10.3382/ps.0661283. [DOI] [PubMed] [Google Scholar]
- Carr L. E., Wheaton F. W., Douglass L. W. Empirical models to determine ammonia concentrations from broiler chicken litter. Trans. ASAE. 1990;33:1337–1342. [Google Scholar]
- Cheng H. W., Muri W. M. Chronic social stress differentially regulates neuroendocrine response in laying hens: II. Genetic basis of adrenal responses under three different social conditions. Psychoneuroendocrinology. 2004;29:961–971. doi: 10.1016/j.psyneuen.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Craig J. V., Craig J. A., Vargas J. Corticosteroids and other indicators of hens’ well-being in four laying house environments. Poult. Sci. 1986;65:856–863. doi: 10.3382/ps.0650856. [DOI] [PubMed] [Google Scholar]
- Craig J. V., Milliken G. A. Further studies of space and group size effect in caged hens of stock differing in fearful behavior. Productivity and behavior. Poult. Sci. 1989;68:9–16. [Google Scholar]
- Craig J. V., Swanson J. C. Review: Welfare perspectives on hens kept for egg production. Poult. Sci. 1994;74:921–938. doi: 10.3382/ps.0730921. [DOI] [PubMed] [Google Scholar]
- Eisen E. J., Bohren B. B., McKean H. E. Haugh unit as a measure of egg albumen quality. Poult. Sci. 1962;41:1361–1368. [Google Scholar]
- Ekstrand C., Algers B., Svedberg J. Rearing conditions and foot-pad dermatitis in Swedish broiler chickens. Prevent. Vet. Med. 1997;31:167–174. [PubMed] [Google Scholar]
- El-Lethey H., Aerni V., Jungi T. W., Wechsler B. Stress and feather pecking in laying hens in relation to housing condition. Br. Poult. Sci. 2000;41:22–28. doi: 10.1080/00071660086358. [DOI] [PubMed] [Google Scholar]
- European Communities . Council Directive 1999/74/EC. Official Journal of the European Communities; 1999. L203/53. [Google Scholar]
- Feddes J. J., Emmanuel E. J., Zuidhoft M. J. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 2002;81:774–779. doi: 10.1093/ps/81.6.774. [DOI] [PubMed] [Google Scholar]
- Ganong W. F. Review of medicinal physiology. 19th ed. Stanford, Connecticut: Appleton and Lange; 1999. p. 353. [Google Scholar]
- Gunnarsson S., Keeling L. J., Svedberg J. Effect of rearing factors on the prevalence of floor eggs, cloacal cannibalism and feather pecking in commercial flocks of loose housed laying hens. Br. Poult. Sci. 1999;40:12–18. doi: 10.1080/00071669987773. [DOI] [PubMed] [Google Scholar]
- Hayat Z., Cherian G., Pasha T. N., Khattak F. M., Jabber M. A. Effect of feeding flax and two types of antioxidants on egg production, egg quality, and lipid composition eggs. J. Appl. Poult. Res. 2009;18:541–551. [Google Scholar]
- Hester P. Y., Wilson E. K. Performance of White Leghorn hens in response to cage density and the introduction of cage mates. Poult. Sci. 1986;65:2029–2033. doi: 10.3382/ps.0652029. [DOI] [PubMed] [Google Scholar]
- Hocking P. M., Maxwell M. H., Robertson G. W., Mitchell M. A. Welfare assessment of modified rearing programmes for broiler breeders. Br. Poult. Sci. 2001;42:424–432. doi: 10.1080/00071660120070677. [DOI] [PubMed] [Google Scholar]
- Hughes B. O., Appleby M. C. Increase in bone strength of spent laying hens housed in modified cages with perches. Vet. Rec. 1989;124:483–484. doi: 10.1136/vr.124.18.483. [DOI] [PubMed] [Google Scholar]
- Jalal M. A., Scheidelser S. E., Marx D. Effect of bird cage space and dietary metabolizable energy level on production parameters in laying hens. Poult. Sci. 2006;85:306–311. doi: 10.1093/ps/85.2.306. [DOI] [PubMed] [Google Scholar]
- Johnson T. S., Zuk M. Parasites, morphology, and blood characters in male red jungle fowl during development. Condor. 1998;100:749–752. [Google Scholar]
- Knowles T. G., Broom D. M. Limb bone strength and movement in laying hens from different housing systems. Vet. Rec. 1990;126:354–356. doi: 10.1136/vr.126.15.354. [DOI] [PubMed] [Google Scholar]
- Lee K., Moss C. V. Effect of population density on layer performance. Poult. Sci. 1995;74:1754–1760. doi: 10.3382/ps.0741754. [DOI] [PubMed] [Google Scholar]
- Lucas A. M., Jamroz C. Atlas of Avian Hematology. USDA Monograph 25. USDA; Washington DC: 1961. [Google Scholar]
- Martrenchar A., Morisse J. P., Huonnic D., Cotte J. P. Influence of stock density on some behavioural, physiological, and productivity traits of broilers. Vet. Res. 1997;28:473–480. [PubMed] [Google Scholar]
- Mashaly M. M., Webbs L., Youtz S. L., Roush W. B., Graves H. B. Changes in serum corticosterone concentration of laying hens as a response to increased population density. Poult. Sci. 1984;63:2271–2274. doi: 10.3382/ps.0632271. [DOI] [PubMed] [Google Scholar]
- Masoudi A., Chagi M., Bojaropur M., Mirzadeh K. Effects of different levels of date pits on performance, carcass characteristics and blood parameters of broiler chickens. J. Appl. Anim. Res. 2011;39:399–405. [Google Scholar]
- McFarlane J. M., Curtis S. E. Multiple concurrent stressors in chicks. 3. Effects on plasma corticosterone and the heterophil:lymphocyte ratio. Poult. Sci. 1989;68:522–527. doi: 10.3382/ps.0680522. [DOI] [PubMed] [Google Scholar]
- Newman S., Leeson S. Effect of housing birds in cages or an aviary system on bone characteristics. Poult. Sci. 1998;77:1492–1496. doi: 10.1093/ps/77.10.1492. [DOI] [PubMed] [Google Scholar]
- Nicol C. J. Behavioural responses of laying hens following a period of spatial restriction. Anim. Behav. 1987;35:1709–1719. [Google Scholar]
- Nicol C. J., Brown S. N., Glen E., Pope S. J., Short F. J., Waross P. D., Zimmerman P. H., Wilkins L. J. Effects of stocking density flock size and management on the welfare of laying hens in single-tier aviaries. Br. Poult. Sci. 2006;47:135–146. doi: 10.1080/00071660600610609. [DOI] [PubMed] [Google Scholar]
- NRC . Nutrient Requirements of Poultry. 9th ed. National Academy Press; Wahington, DC: 1994. [Google Scholar]
- Onbasilar E. E., Aksoy F. T. Stress parameters and immune response of layers under different cage floor and density conditions. Livest. Prod. Sci. 2005;95:255–263. [Google Scholar]
- Pesti G. M., Howarth B. Effects of population density on the growth, organ weights, and plasma corticosterone of young broiler chicks. Poult. Sci. 1983;62:1080–1083. doi: 10.3382/ps.0621080. [DOI] [PubMed] [Google Scholar]
- Post J., Rebel J. M., Terhurne A. H. M. Automated blood cell count: A sentitive and reliable method to study corticosterone related stress in broilers. Poult. Sci. 2003;82:591–595. doi: 10.1093/ps/82.4.591. [DOI] [PubMed] [Google Scholar]
- Roush W. B., Mashaly M. M., Graves H. B. Effect of increased bird production in a fixed cage area on production and economic response of Single Comb White Leghorn laying hens. Poult. Sci. 1984;63:45–48. doi: 10.3382/ps.0630045. [DOI] [PubMed] [Google Scholar]
- Sandoval M., Miles R. D., Jacobs R. D. Cage space and house temperature gradient effects on performance of White Leghorn hens [Abst.] Poult. Sci. 1991;70:103. [Google Scholar]
- Schreiweis M. A., Orban J. I., Ledur M. C., Hester P. Y. The use of densitometry to detect differences in bone mineral density and content of live White Leghorns fed varying levels of dietary calcium. Poult. Sci. 2003;82:1292–1301. doi: 10.1093/ps/82.8.1292. [DOI] [PubMed] [Google Scholar]
- Shanawanny M. M. Broiler performance under high stocking densities. Br. Poult. Sci. 1988;29:43–52. doi: 10.1080/00071668808417025. [DOI] [PubMed] [Google Scholar]
- Sorensen P., Su G., Kestin S. C. Effects of age and stocking density on leg weakness in broiler chickens. Poult. Sci. 2000;79:864–870. doi: 10.1093/ps/79.6.864. [DOI] [PubMed] [Google Scholar]
- Suto Z., Horn P., Ujvari J. The effect of different housing systems on production and egg quality traits of brown and Leghorn type layers. Acta Agr. Kaposv. 1997;1:29–35. [Google Scholar]
- Tactacan G. B., Guenter W., Lewis N. J., Rodriguez-Lecompte J. C., House J. D. Performance and welfare of laying hens in conventional and enriched cages. Poult. Sci. 2009;88:698–707. doi: 10.3382/ps.2008-00369. [DOI] [PubMed] [Google Scholar]
- Webster A. B. Welfare implications of avian osteoporosis. Poult. Sci. 2004;83:184–192. doi: 10.1093/ps/83.2.184. [DOI] [PubMed] [Google Scholar]
- Wells R. G. The effect of varying stocking density on the development and subsequent laying performance of floor reared pullets. Br. Poult. Sci. 1972;13:13–25. [Google Scholar]
- Yannakopulos A. L., Tserveni-Gousi A. S. Quality characteristics of quail eggs. Br. Poult. Sci. 1986;27:171–176. doi: 10.1080/00071668608416863. [DOI] [PubMed] [Google Scholar]
- Zulkifli I., Liew P. K., Israf D. A., Omar A. R., Hair-Bejo M. Effect of early age feed restriction and heat conditioning on heterophil/lymphocyte ratios, heat shock protein 70 expression and body temperature of heat-stressed broiler chickens. J. Therm. Biol. 2003;28:217–222. [Google Scholar]


