Abstract
Objectives. To identify and quantify resource required and associated costs for implementing TNF-α inhibitor (TNFi) drug level and anti-drug antibody (ADAb) tests in UK rheumatology practice.
Methods. A microcosting study, assuming the UK National Health Service perspective, identified the direct medical costs associated with providing TNFi drug level and ADAb testing in clinical practice. Resource use and costs per patient were identified via four stages: identification of a patient pathway with resource implications; estimation of the resources required; identification of the cost per unit of resource (2015 prices); and calculation of the total costs per patient. Univariate and multiway sensitivity analyses were performed using the variation in resource use and unit costs.
Results. Total costs for TNFi drug level and concurrent ADAb testing, assessed using ELISAs on trough serum levels, were £152.52/patient (range: £147.68–159.24) if 40 patient samples were tested simultaneously. For the base–case analysis, the pre-testing phase incurred the highest costs, which included booking an additional appointment to acquire trough blood samples. The additional appointment was the key driver of costs per patient (67% of the total cost), and labour accounted for 10% and consumables 23% of the total costs. Performing ELISAs once per patient (rather than in duplicate) reduced the total costs to £133.78/patient.
Conclusion. This microcosting study is the first assessing the cost of TNFi drug level and ADAb testing. The results could be used in subsequent cost-effectiveness analyses of TNFi pharmacological tests to target treatments and inform future policy recommendations.
Keywords: microcosting, immunogenicity, TNFi drug levels, opportunity costs, health economics
Rheumatology key messages
Microcosting analysis enabled quantification of resource use and costs required to implement TNF inhibitor pharmacological monitoring in practice.
The cost of £152.52/patient for TNF inhibitor pharmacological monitoring (base case analysis) was comparable to other novel diagnostics.
The additional appointment for trough level TNF inhibitor pharmacological monitoring was the key driver of costs per patient.
Introduction
TNF-α inhibitors (TNFi) have transformed the treatment of several chronic inflammatory diseases. Given their effectiveness in the most severely affected patients, the use of biologics in rheumatology continues to increase, but is associated with significant expenditure (£10 000/year/patient). TNFi agents such as adalimumab, etanercept and infliximab are currently represented within the top five highest medicinal expenditures in England [1], with an estimated cost to the National Health Service (NHS) of ∼£160 million annually for RA [2]. A targeted approach using robust predictive biomarkers of response in TNFi-treated patients may add value to the clinical decision-making process by potentially informing the selection of which TNFi drug to use first in specific patients, the appropriate biologic sequence and whether to continue the drug in patients established on therapy. However, there remain considerable gaps in the evidence base supporting the introduction of a targeted approach into clinics [3]. In the era of finite budgets, robust economic evidence is required in order to ensure that the alternative uses for funds are considered in any decision, and decision-making groups must be aware of other funding pressures and service developments that will otherwise be forgone (opportunity costs) [4].
An important mechanism for treatment failure of certain TNFi agents is immunogenicity involving the formation of anti-drug antibodies (ADAb) and low drug levels [5, 6]. While the presence of ADAbs and low TNFi drug levels, detected soon after treatment initiation, have been shown to predict subsequent treatment response [7], tests quantifying levels are not currently available in rheumatology clinical practice in the UK NHS. Such testing needs to be both effective in improving outcomes and a cost-effective use of the healthcare budget before it can be recommended for implementation into the clinic. To date, a description of the types and quantity of resources needed to provide the test is not available in the published literature. Identifying the resources required will facilitate the calculation of the costs of implementing these tests in a UK clinical setting if the introduction of such testing is shown to be clinically useful.
Microcosting is a method that allows robust assessment of the types and quantities of resources and associated costs of health interventions consumed [8]. It is particularly useful for estimating the costs of new interventions and for interventions with large variability across providers, thereby potentially providing a key input for undertaking subsequent economic evaluations. The aim of this study was to identify and quantify the resource use and associated costs required for introducing drug level and ADAb testing to assess response to TNFi drugs in routine practice in the UK setting.
Methods
A microcosting study assumed the NHS (service provider) perspective for identifying the resource use and cost per patient of providing TNFi drug level and ADAb testing (the test). Costs of providing the test were determined from the point of a patient established on treatment (for ⩾3 months) presenting to clinic, to the results being fed back to the clinician to inform a treatment decision. Direct medical costs associated with providing the test were identified; indirect non-medical costs (such as absence from work) were not consistent with the study perspective and beyond the scope of the paper. Ethical approval was not required. This study was essentially an audit of practice in North West England. (Regional guidelines for biologics in RA [9] allow use of these tests in rheumatology practice if clinicians have access.) The four study stages are now described.
Stage 1: identifying the testing pathway
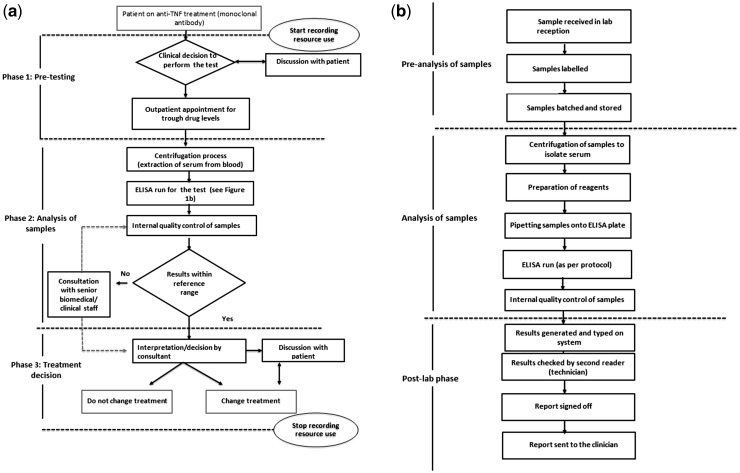
The test is not routinely available in UK rheumatology practice, and it was necessary to define an explicit pathway for a patient being offered testing with input from six experts from North West England (four rheumatology consultants and two clinical/laboratory staff) (Fig. 1A and B). The pathway eventually encompassed three phases: pre-testing, analysis of samples, and treatment decision (Fig. 1A). This study assumed that the test is reliant on identifying a pre-defined drug trough level, requiring an additional outpatient appointment, rather than using random sampling, which mirrors the current availability of the technology available in the UK (bridging ELISAs) to measure ADAbs.
Fig. 1.
Pathway for immunogenicity and drug level testing (the test)
(A) Overview of the pathway from the clinical decision to perform the test. (B) Summary of laboratory processes.
Stage 2: use of resources
The use of direct medical resources for the pathway was estimated using structured face-to-face interviews and elicitation with six experts. The expert elicitation process is described in more detail in supplementary Table S1, available at Rheumatology Online. Direct non-participant observation of staff was undertaken in a hospital setting to generate an estimate of the time taken for selected procedures. The Central Manchester Foundation NHS Trust Immunology Department was asked to name resources required relating to laboratory staff time. The level of each resource use was estimated per patient for each phase and per batch of 40 samples for the laboratory processes (Fig. 1A, phase 2).
Stage 3: identifying unit costs
It was assumed that most hospital laboratories would have the necessary room requirements and stock standard equipment required to perform ELISAs, and the following items of resource use were therefore excluded: equipment costs of centrifuge systems; ELISA readers; pipettes; personal protective equipment; phlebotomy equipment costs; overhead; and capital costs.
A variety of approaches were taken to identify unit costs (price year 2015) for each type of resource use (Table 1). The unit cost of a rheumatology blood-monitoring appointment was obtained from operational managers for rheumatology directorates of two hospitals (Central Manchester University Hospitals and Salford Royal NHS Foundation Trust). Published estimates of unit costs for labour time were not available for all types of staff by the Personal Social Services Research Unit [10]. Therefore hospital-based health care staff time was valued using relevant labour unit costs from the national pay system for the NHS (Agenda for change—pay rates 2015–16) [11] and the British Medical Association pay scale for medical staff in England (2015–16) [12]. Salary scales per annum were converted to a per-minute rate by dividing the number of workable minutes per year, as described previously [13] (see supplementary Table S2, available at Rheumatology Online).
Table 1.
Resource use and costs of implementing drug level and immunogenicity testing per patient in a hospital setting
| Type of resource use | Staff member | Mean volume of resource use, minutes | Range of resource use, minutes | Source to obtain resource use | Unit costs for base-case analysis 2015, £a | Range of unit costs 2015, £ | Source to obtain unit costs [reference] | Total costs (£) (minimum to maximum range) |
|---|---|---|---|---|---|---|---|---|
| Phase 1: pre-testing | ||||||||
| Outpatient appointment for discussion about need for test | Consultant rheumatologist | 3.6 min | 2–5 min | Expert estimation | £39.24/h | £33.42–45.06/h | BMA pay scales [12] | £2.35 (£1.11–3.76) |
| Clerical staff (to book the appointment and send out a letter to patient) | Clerical assistant | 8.2 min | 6–11 min | Direct observation | £8.43/h | £7.72–9.12/h | NHS pay scale, band 5 [11] | £1.15 (£0.77–1.67) |
| Appointment for trough blood levels | Phlebotomist/ clinical support worker | Resource use incorporated in unit cost | NA | NA | £102 per appointment | NA | DOH national tariff [14] | £102 (NA) |
| Phase 2: analysis of samples | ||||||||
| Receipt and labelling of samples—central specimen receptionb | Medical lab assistant | 15 min | NA | Expert estimation | £8.86/h | £7.74–9.98/h | NHS pay scale band 5 [11] | £2.22 (£1.94–2.50) |
| Data entry of patient information to lab systemb | Medical lab assistant | 15 min | NA | £2.22 (£1.94–2.50) | ||||
| Sample preparation—extraction of serum from bloodb | Medical lab assistant | 15 min | NA | £2.22 (£1.94–2.50) | ||||
| Transport, receipt and storage of sample—immunology labb | Medical lab assistant | 15 min | NA | £2.22 (£1.94– 2.50) | ||||
| Preparation of reagents (wash solution, setting up assay, conjugate)b | Biomedical scientist | 15 min | NA | Expert estimation | £12.79/h | £11.12–14.45/h | NHS pay scale band 5 [11] | £3.20 (£2.78–3.61) |
| ELISA kit for ADAbs or drug levelsb | NA | NA | NA | NA | £700.00 per ELISA | NA | UK commercial price from Grifolsc | £700.00 |
| Pipette tips for ELISAsb | NA | NA | NA | NA | £6.00 per ELISA | NA | University of Manchester Centre for Musculoskeletal Research laboratory | £6.00 |
| Semi-deep well plates for ELISAsb | NA | NA | NA | NA | £2.20 per ELISA | NA | Laboratory costs | £2.20 |
| Troughs for ELISAsb | NA | NA | NA | NA | £1 per ELISA | NA | Laboratory costs | £1.00 |
| Retrieval of patient/IQC samples from storageb | Biomedical scientist | 10 min | NA | Expert estimation | £12.79/h | £11.12–14.45/h | NHS pay scale- band 5 [11] | £2.13 (£1.85 –2.40) |
| Checking and sorting samples to match worklistb | Biomedical scientist | 10 min | NA | £2.13 (£1.85–2.40) | ||||
| Pipetting samples onto ELISA plateb | Biomedical scientist | 20 min | NA | £4.26 (£3.71– 4.81) | ||||
| Pipetting calibrators, IQC samples and incubation of samplesb | Biomedical scientist | 10 min | NA | £2.13 (£1.85 –2.40) | ||||
| Washing ELISA plate and addition of conjugateb | Biomedical scientist | 10 min | NA | £2.13 (£1.85 –2.40) | ||||
| Washing ELISA plate and addition of substrateb | Biomedical scientist | 10 min | NA | £2.13 (£1.85–2.40) | ||||
| Addition of stop solutionb | Biomedical scientist | 5 min | NA | £1.06 (£0.93 –1.20) | ||||
| ELISA plate reading and printing of resultsb | Biomedical scientist | 10 min | NA | £2.13 (£1.85–2.40) | ||||
| Technical validation involving review of Internal quality controlb | Biomedical scientist | 5 min | NA | £1.06 (£0.93–1.20) | ||||
| Results transcribed to worksheetb | Biomedical scientist | 5 min | NA | £1.06 (£0.93–1.20) | ||||
| Data entry of results to patient record in lab systemb | Biomedical scientist | 10 min | NA | £2.13 (£1.85 – 2.40) | ||||
| Transcribed results/data entry reviewed by a second independent biomedical scientistb | Biomedical scientist | 5 min | NA | £1.06 (£0.93 – 1.20) | ||||
| Clinical authorisation using reference range/delta check failure resultsb | Senior Biomedical scientist or Consultant immunologist | 5 min | NA | Expert estimation | £30.48/h | £15.9–45.06/h | NHS pay scale band 7 assumed [11]; BMA pay scale [12] | £2.54 (£1.33 –3.76) |
| Hardcopy report sent to clinicianb | Clerical assistant | 15 min | NA | Expert estimation | £8.43/h | £7.74 –9.12/h | NHS pay scale [11] | £2.11 (£1.93 –2.28) |
| Phase 3: Treatment decision | ||||||||
| Interpretation of results by rheumatologist | Consultant Rheumatologist | 6 min | 4–10 | Expert estimation | £39.24/h | £33.42–45.06/h | NHS pay scale [11] | £3.92 (£2.23 –7.51) |
| Discussion with patient (phone call) | 5.3 min | 5–6 | £3.47 (£2.79–3.92) | |||||
| Letter with results and decision | 3.3 min | 3–4 | £2.16 (£1.61–2.62) | |||||
| Total costs (best case to worst case scenario)d | £152.52 (£147.68–159.24) | |||||||
Mid-point of salary grade used to calculate base–case sample.
Resource use estimated per batch (40 samples).
Previously known as Progenika Biopharma.
Base–case (multiway sensitivity analyses were conducted by varying the following parameters using pre-defined lower and upper ranges of estimated resource use: lowest time taken to perform tasks using the lowest pay grade (best case scenario) and highest amount of time taken to perform procedures using the highest pay grade (worst case scenario). BMA: British Medical Association;
DOH: department of health; ELISA: enzyme-linked immunosorbent assay; IQC: internal quality control; NA: not applicable; NHS: National Health Service.
Stage 4: data analysis
The base–case analysis calculated the total cost of the test by multiplying unit costs with the identified items and quantities of resource for each phase of the pathway (see Fig. 1A). Multiway sensitivity analyses were conducted by varying the following parameters using lower and upper ranges of estimated resource use: lowest time taken to perform tasks using the lowest pay grade (best case scenario) and highest amount of time taken to perform procedures using the highest pay grade (worst case scenario). Three one-way sensitivity analyses and one two-way sensitivity analysis were used to understand the impact of varying pre-defined assumptions made when calculating the cost of the test.
Results
Table 1 summarizes the items and quantity of resource use and unit costs for each of the three phases of the pathway (Fig. 1A).
Base–case analysis
The total cost for performing the test was £152.52/patient for the base–case analysis. The most expensive element of the pathway was the cost of the additional appointment to conduct blood sampling for drug trough levels. Therefore the pre-testing phase incurred the highest costs due to the additional appointment to perform trough blood sampling (total costs: £105.50/patient). The total cost for processing 40 samples during laboratory phase (phase 2, analysis of samples) was £749.34 [£18.73 (cost in phase 2 divided by 40) × 2 (for both tests) = £37.47/patient to simultaneously perform the test]. The final treatment decision cost was £9.55/patient. The additional trough level appointment accounted for 67% of the total cost, and labour and consumables accounted for 10% and 23% of the total costs, respectively.
Sensitivity analyses
The multiway sensitivity analysis varied the estimated and directly observed time and pay grade for each phase (see Table 1). Using the lowest values, the estimated best-case scenario was £147.68/patient/test. Using the highest values, the worst-case scenario estimated a cost of £159.24.
Three one-way sensitivity analyses were performed (see Sensitivity analysis in the supplementary data, available at Rheumatology Online). Performing the tests singly and not in duplicate may reduce test accuracy, but lowered the total cost to £133.78/patient. If the patient was due to take their TNFi on the day following their rheumatology appointment, an additional trough level appointment was not required, lowering the test cost to £50.52. If there were 50 samples to be processed by the laboratory, a new batch would need to be started, increasing the resource use in phase 2 and the total cost to £173.79/patient.
One two-way sensitivity analysis examined the impact of using various pay grades. For costs attributed to consultant time (base–case), varying the pay scale to the lower grade using the mean volume of resource use (Table 1) changed the total costs to £145.26/patient. The variation in grade included a specialty trainee in rheumatology at £38 588.50/annum (mid-point of paygrade, supplementary Table S2, available at Rheumatology Online), a consultant rheumatologist (Table 1, phases 1 and 3) and a senior clinical biochemist (mid-point of paygrade £35 891/annum, supplementary Table S2, available at Rheumatology Online) instead of a consultant immunologist (Table 1, phase 2).
Discussion
This microcosting study has identified the potential direct medical costs associated with TNFi pharmacological testing from a service provider’s perspective in the UK. Since these tests for TNFi-treated patients are not routinely performed in UK clinical practice, a testing pathway was developed to allow a detailed estimation of the quantities of resources required in order to calculate a total cost. The developed pathway provides a framework for reporting resource use, presenting unit costs and allowing decision-makers from various jurisdictions to use their country-specific data if required.
There is accumulating evidence that monoclonal TNFi drug levels and ADAb levels correlate with future response to the drugs [7, 15]. If the testing strategy is to translate to clinical practice, a number of points will need to be addressed. First, the test must be shown to be useful in changing clinical decision-making; second, robust evidence must confirm that the change in practice will result in better outcomes for patients; finally the test intervention should be a cost-effective use of health care budgets. The current work is the first step in informing the last requirement. To date, the costs associated with providing TNFi drug level/ADAb testing are not known because no national tariff exists for diagnostic tests. Emerging numbers of microcosting studies in other areas have enabled rigorous comparison of health interventions in order to inform efficient resource allocation [16]. A recent NICE diagnostic assessment committee evaluating test performance of ELISA kits for ADAbs and TNFi levels in Crohn’s disease was not able to draw definitive conclusions about the relative cost-effectiveness of the test compared with current practice because of insufficient evidence to inform the analysis. Early analysis suggested that the test may save the NHS money, but would also result in some loss of health in the population tested. The high degree of uncertainty in the economic analysis, particularly around the impact of the test on quality-adjusted life-years meant that the committee concluded that further research was required before the test could be recommended for use in clinical practice [17].
Our microcosting analysis identified a unit cost of £152.52/patient, making this biomarker test for guiding decisions regarding future treatment with TNFi comparable with that of other novel diagnostics and theranostics [18]. A robust economic evaluation that identifies the incremental costs and health benefits (quality-adjusted life-years) of using the test for targeting TNFi treatments compared with current prescribing practice in RA is required to determine whether this targeted approach is a cost-effective use of health care budgets.
The overall cost of testing per patient in the UK was influenced most by the cost of an additional appointment for obtaining trough levels. When the cost of trough levels was excluded, the cost per patient reduced to £50.52. To deal with batching and capacity, the base–case analysis assumed batching of samples from 40 patients/ELISA. However, uneven sample numbers would require a new batch with changes in marginal costs (cost of doing one more test) impacting on consumables, staff resources and time. If results are to be fed back in sufficient time for referring clinicians to make treatment decisions, it is unlikely that samples from 40 patients would be available for testing unless test sites were restricted to regional or national laboratories. When processing 50 rather than 40 samples, the cost per sample rose to £173.79 because each ELISA kit only allows for 40 samples to be analysed at a time.
This analysis made several assumptions in order to estimate the total cost. The base–case assumed a tertiary level setting in the north-west of England; however, it is acknowledged that the cost of a trough level appointment may vary elsewhere in the UK, thus influencing the total costs/sample. We assumed a concurrent testing strategy for all samples, in which tests for TNFi drug levels and ADAbs were performed at the same time, rather than reflex testing, which may be an alternative to reduce costs. Reflex testing would involve testing the TNFi drug levels first and only testing for ADAbs if the drug was undetectable. Direct non-medical costs such as patient out-of pocket expenses for trough level testing were not included, which may have wider societal implications [19]. Capital/overhead costs were not included in the analysis. While some hospital laboratories have automated ELISA systems or multiplex platforms, this was not assumed and was deemed unlikely to significantly lower resource estimates, following consultation with the hospital laboratory team. Furthermore, while numerous ELISA kits are commercially available for ADAb and TNFi drug level testing, unit costs were based on those ELISA kits frequently used in the literature [20].
In conclusion, using a microcosting approach, we have explicitly identified and quantified the types and quantities of resources required in order to provide TNFi drug and ADAbs level testing in an NHS clinical setting and found that the costs were comparable with those of other tests already available. The identified cost of the test will be of use for future cost-effectiveness analysis of TNFi pharmacological testing. The results of the study will also help inform potential resource implications per patient for hospital trusts considering incorporating pharmacological monitoring into clinical practice.
Supplementary Material
Acknowledgements
M.J. is currently supported by a National Institute for Health Research (NIHR) clinical lectureship and was a Medical Research Council (MRC) Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the MRC (grant number G1000417/94909), ICON, GlaxoSmithKline, AstraZeneca and the Medical Evaluation Unit. We thank Arthritis Research UK for their support (grant reference 20385 and 20380) and the NIHR Manchester Musculoskeletal Biomedical Research Unit. This report includes independent research supported by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. W.G.D. was supported by an MRC Clinician Scientist Fellowship (G092272). We also acknowledge support from MATURA, a stratified medicines initiative jointly funded by MRC and Arthritis Research UK (MR/K015346/1) and Innovate UK.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: A.B. has received honoraria or grants from Pfizer and Eli-Lilly in the past 2 years. H.C. has received honoraria, lecture fees and/or research grants from Abbvie, Janssen, Pfizer, UCB, Roche, Celgene, outside the submitted work. M.J. has received honoraria/travel support from Pfizer, Abbvie and UCB. S.G. is supported by a National Institute for Health Research Manchester Musculoskeletal Biomedical Research Unit PhD studentship. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Prescribing & Medicines Team, Health and Social Care Information Centre. Prescribing Costs in Hospitals and the Community, England 2014–15. November 2015.
- 2.National Audit Office. Services for People with Rheumatoid Arthritis. July 2009. https://www.nao.org.uk/wp-content/uploads/2009/07/0809823.pdf (28 June 2016, date last accessed).
- 3.Gavan S, Harrison M, Iglesias C. et al. Economics of stratified medicine in rheumatoid arthritis. Curr Rheumatol Rep 2014;16:468.. [DOI] [PubMed] [Google Scholar]
- 4.Faculty of Public Health. Priority Setting in the NHS. Position Statement by Faculty of Public Health, 2014. http://www.fph.org.uk/uploads/Policy_reports (28 June 2016, date last accessed).
- 5.Garcês S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis 2013;72:1947–55. [DOI] [PubMed] [Google Scholar]
- 6.Jani M, Isaacs JD, Morgan AW. et al. High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis 2016. May 31. pii: annrheumdis-2015-208849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jani M, Chinoy H, Warren RB. et al. Clinical utility of random anti–tumor necrosis factor drug–level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheumatol 2015;67:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Grossetta Nardini HK, Ruger JP. Micro-costing studies in the health and medical literature: protocol for a systematic review. Syst Rev 2014;3:47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jani M, Reid V, Parker B, et al. Harmonised Biologics Pathway for Rheumatoid Arthritis. Greater Manchester Medicines Management/MAHSC Guidelines. http://gmmmg.nhs.uk/docs/guidance/GMMMG RA Pathway 22 april_2015.pdf (28 June 2016, date last accessed).
- 10.Personal Social Services User Research Unit (PSSURU). Unit Costs of Health and Social Care 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/ (28 June 2016, date last accessed).
- 11.Health Careers, NHS. NHS Careers, Agenda for Change – Pay Rates from 1 April 2015. http://www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates/ (1 September 2015, date last accessed).
- 12.British Medical Association. Pay Scales for 2015–2016. 2015. http://bma.org.uk/practical-support-at-work/pay-fees-allowances/pay-scales (28 June 2016, date last accessed).
- 13.Hanly P, Céilleachair AÓ, Skally M. et al. Direct costs of radiotherapy for rectal cancer: a microcosting study. BMC Health Serv Res 2015;15:184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GOV.UK. NHS National Tariff Payment System 2015/16: draft price-setting models - Publications - GOV.UK (July 2014). https://www.gov.uk/government/publications/nhs-national-tariff-payment-system-201516-draft-price-setting-models (31 August 2015, date last accessed).
- 15.Bartelds G, Krieckaert C. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460–8. [DOI] [PubMed] [Google Scholar]
- 16.Tan SS, Oppe M, Zoet-Nugteren SK. et al. A microcosting study of diagnostic tests for the detection of coronary artery disease in the Netherlands. Eur J Radiol 2009;72:98–103. [DOI] [PubMed] [Google Scholar]
- 17.NICE diagnostic guidance [DG22]. Therapeutic Monitoring of TNF-alpha Inhibitors in Crohn’s Disease (LISA-TRACKER ELISA kits, IDKmonitor ELISA kits, and Promonitor ELISA kits). London: NICE, 2016. https://www.nice.org.uk/guidance/dg22 (28 June 2016, date last accessed). [Google Scholar]
- 18.Parker D, Belaud-Rotureau M-A. Micro-cost analysis of ALK rearrangement testing by FISH to determine eligibility for crizotinib therapy in NSCLC: implications for cost effectiveness of testing and treatment. Clin Med Insights Oncol 2014;8:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson S, Roberts T, Barton P. et al. Healthcare and patient costs of a proactive chlamydia screening programme: the Chlamydia Screening Studies project. Sex Transm Infect 2007;83:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D-Y, Chen Y-M, Tsai W-C. et al. Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis. Ann Rheum Dis 2015;74:e16.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.