Abstract
Objective. To determine if ischaemia is a causal factor in the development of calcinosis in SSc.
Methods. Patients with SSc were assessed yearly. Physicians reported the presence of calcinosis, digital ischaemia (digital ulcers, digital necrosis/gangrene, loss of digital pulp on any digits and/or auto- or surgical digital amputation) and nailfold capillary dropout assessed using a dermatoscope. The number of digits with digital ischaemia was used as an assessment of the severity of digital ischaemia. SSc specific antibodies were detected with a line immunoassay. Multiple logistic regression and Cox proportional hazards models were generated to determine associations between calcinosis, digital ischaemia and capillary dropout.
Results. One thousand three hundred and five patients were included in this study, of whom 300 (23.0%) had calcinosis at study entry. In a cross-sectional multivariate analysis, at baseline, calcinosis was associated with digital ischaemia (odds ratio (OR) = 2.37, 95% CI: 1.66, 3.39), severity of ischaemia (OR = 1.12, 95% CI: 1.06, 1.18), capillary dropout (OR = 1.41, 95% CI: 1.05, 1.89), ACAs (OR = 1.68, 95% CI: 1.17, 2.43) and anti-RNA polymerase III antibodies (OR = 1.77, 95% CI: 1.08, 2.89). Current use of calcium channel blockers was inversely associated with the presence of calcinosis (OR = 0.70, 95% CI: 0.52, 0.96). Of the 805 patients with no calcinosis at study entry and at least one follow-up visit, 215 (26.7%) developed calcinosis during follow-up. Significant baseline predictors of the development of calcinosis in follow-up were digital ischaemia (hazard ratio (HR) = 1.82, 95% CI: 1.30, 2.54), capillary dropout (HR = 1.46, 95% CI: 1.08, 1.99), dcSSc (HR = 1.57, 95% CI: 1.11, 2.21), ACA (HR = 2.18, 95% CI: 1.50, 3.17) and anti-RNA polymerase III antibodies (HR = 2.58, 95% CI: 1.65, 4.04).
Conclusion. Ischaemia may play a role in the development of calcinosis in SSc.
Keywords: scleroderma, systemic, ischaemia, calcinosis, capillaroscopy, autoantibodies, multivariate analysis, calcium channel blockers, smoking
Rheumatology key messages
Indices of tissue ischaemia are cross-sectionally associated with the presence of calcinosis in SSc.
In SSc patients without calcinosis, indices of tissue ischaemia predict the future development of calcinosis.
Ischaemia may play a role in the development of calcinosis in SSc.
Introduction
Calcinosis has long been appreciated as a common and often disabling feature of SSc [1–3]. The aetiology of calcinosis in SSc is, however, not well understood. One hypothesis, that tissue ischaemia may contribute to the development of calcinosis, is supported by some recent studies reporting an association between calcinosis and digital ulcers (DUs) [4–7], acro-osteolysis or digital amputation [6, 7]. These studies are not without limitations, including small, cross-sectional designs and use of only radiography to define the presence of calcinosis. In the current study, we sought to confirm these observations in a much larger SSc cohort and to investigate the temporal associations between calcinosis and ischaemia.
Methods
Study subjects
The study sample consisted of patients enrolled in the Canadian Scleroderma Research Group registry with baseline visits between August 2004 and February 2015. Patients in the registry are recruited from 15 centres across Canada and Mexico. They must have a diagnosis of SSc made by the referring rheumatologist, be over 18 years of age and be fluent in English, French or Spanish. Patients in the registry undergo a yearly standardized evaluation including a detailed medical history, physical evaluation and laboratory investigations. In particular, demographic and disease-related information is recorded, including age, sex and disease duration since the onset of the first non-Raynaud’s symptoms. Physicians were expected to enrol all willing subjects with SSc that were seen in their practices. Ethics committee approval for this study was obtained at each site and each patient provided written informed consent to participate in this study.
Study measures
At each visit, a physician performed a complete physical exam and recorded the presence of visible or palpable calcinosis anywhere on the body. X-rays were not routinely required but if they had been performed and calcinosis was noted then the presence of calcinosis was recorded. There was no attempt to quantify the extent of calcinosis. The physician simply indicated the presence or absence of calcinosis at each visit. Similarly, at each visit, a physician recorded the presence of DUs defined as ulcers on the volar aspect of the digits distal to the PIP joints thought to be caused by ischaemia and specifically excluding ulcers over calcium deposits [8–13]. In addition, a physician reported a history of DUs, as well as the presence of digital necrosis/gangrene, loss of digital pulp on any digits or auto- or surgical amputation of digits. As a surrogate for tissue ischaemia, we used the presence or a history of DUs, digital necrosis/gangrene, loss of digital pulp on any digits or auto- or surgical amputation of digits. We refer to this as clinical digital ischaemia. The severity of ischaemia was estimated by the summed number of digits with ulcers, digital necrosis or surgical amputation. Finally, nailfold capillaroscopy was performed with a DermLite dermatoscope (3Gen, San Juan Capistrano, CA, USA) and the physician recorded the presence of drop-out areas [14].
Covariates
Disease duration was computed as the time from the onset of the first non-Raynaud’s sign or symptom until baseline registry visit. Serum was collected from each patient at baseline and shipped on ice packs to the University of Calgary where aliquots were stored at −70 °C until used. Anti-centromere (centromere protein (CENP) A and CENP B), anti-topo I and anti-RNA polymerase III (RP11 and RP155) antibodies were detected by the EurolineSSc line immunoassay (Euroimmun, Luebeck, Germany) according to manufacturer’s instructions (http://www.euroimmun.com). The modified Rodnan skin score was used to assess the extent of skin involvement. Subjects were further grouped into lcSSc and dcSSc subsets [15]. Subjects without skin involvement were included with lcSSc. Calcium channel blocker use was assessed at baseline. The subtype of calcium channel blocker was not identified. Other vasodilator medications included prostaglandins, endothelin blockers or PDE5 inhibitors.
Missing data
Subjects with complete data for antibodies and calcinosis at baseline were included in the study (n = 1305). Only those without calcinosis at baseline and with at least one follow-up visit, were included for the longitudinal study (n = 805). No imputations were performed for missing data.
Statistical analysis
Descriptive statistics were used to summarize and compare the baseline characteristics of the patients with and without calcinosis at baseline. Chi-squared tests and Mann–Whitney U tests were used as appropriate. Multiple logistic regression was performed to assess the cross-sectional relationship between the presence of calcinosis and clinical digital ischaemia, controlling for possible confounders. A Cox proportional hazards model was generated to determine the association between the development of calcinosis over time and the presence of tissue ischaemia at baseline. In sensitivity analyses, we repeated the multivariate analyses with the presence of dropout areas on capillaroscopy as the measure of ischaemia. All analysis were performed with SAS software version 9.4 (SAS Institute, Cary, NC, USA) and P < 0.05 was considered significant.
Results
A total of 1305 patients were included in this study, of whom 300 (23.0%) had calcinosis at the baseline visit and 1005 had no calcinosis. Of those 1005, 200 cases had not yet had a follow-up at the time of the study. Of the 805 patients with a follow-up visit and no baseline calcinosis, 215 (26.7%) developed calcinosis during follow-up. The duration of follow-up ranged from 0.7 to 10.1 years with a mean of 3.8 years. Table 1 shows the demographic and clinical characteristics of the study subjects at baseline.
Table 1.
Baseline characteristics of the study cohort
| Entire cohort (n = 1305) | Calcinosis present (n = 300) | Calcinosis absent (n = 1005) | P-value | |
|---|---|---|---|---|
| Age, mean (s.d.), years | 55.3. (12.2) | 55.7 (12.2) | 55.2 (12.2) | 0.559 |
| Female, n (%) | 1128 (85.9) | 261 (87.0) | 867 (86.3) | 0.745 |
| Disease duration, mean (S.D.), years | 10.5 (9.5) | 14.5 (10.2) | 9.2 (8.9) | <0.001 |
| dcSSc, n (%) | 469 (36.0) | 121 (40.3) | 348 (34.7) | 0.073 |
| ACA, n (%) | 510 (39.1) | 128 (42.7) | 382 (38.0) | 0.147 |
| Anti-topo, n (%) | 208 (15.9) | 45 (15.0) | 163 (16.2) | 0.613 |
| Anti-RNA polymerase III, n (%) | 191 (14.6) | 47 (15.7) | 144 (14.3) | 0.565 |
| Current smoker, n (%) | 173 (14.1) | 44 (15.5) | 129 (13.7) | 0.438 |
| Current use of calcium channel blockers, n (%) | 508 (38.9) | 113 (37.7) | 395 (39.3) | 0.610 |
| Current other vasodilators, n (%) | 59 (4.5) | 12 (4.0) | 47 (4.7) | 0.621 |
| Clinical digital ischaemia, n (%)a | 679 (55.7) | 222 (77.6) | 457 (48.9) | <0.001 |
| Severity of digital ischaemia, mean (s.d.)b | 1.2 (2.8) | 2.5 (4.0) | 0.9 (2.3) | <0.001 |
| Nailfold capillary dropout, n (%) | 687 (54.9) | 173 (60.1) | 514 (53.3) | 0.043 |
See Methods section.
Number of digits with clinical digital ischaemia.
Cross-sectional data
Clinical digital ischaemia was present in 679 (55.7%) patients and dropout areas on capillaroscopy in 687 (54.9%). At baseline, clinical digital ischaemia, the severity of digital ischaemia and capillary dropout were all significantly associated with the presence of calcinosis in univariate analyses (P < 0.001, P < 0.001 and P = 0.043, respectively; Table 1). Significant associations were also found for disease duration (P < 0.001). DcSSc tended to be more frequent in those with calcinosis (P = 0.073).
A multivariate logistic regression model with calcinosis at baseline as the dependent variable was generated using clinical digital ischaemia and severity of digital ischaemia as indices of tissue ischaemia, adjusting for age, gender and smoking status (Table 2). In this model, calcinosis was highly associated with clinical digital ischaemia (odds ratio (OR) = 2.37, 95% CI: 1.66, 3.39) and severity of ischaemia (OR = 1.12, 95% CI: 1.06, 1.18). In a sensitivity analysis, when we replaced clinical digital ischaemia with the presence of capillary dropout, ischaemia again had a significant association with calcinosis (OR = 1.41, 95% CI: 1.05, 1.89). Other variables associated independently with calcinosis at baseline included disease duration (OR = 1.05, 95% CI: 1.03, 1.06), ACAs (OR = 1.68, 95% CI: 1.17, 2.43) and anti-RNA polymerase III antibodies (OR = 1.77, 95% CI: 1.08, 2.89). There was a protective effect of current use of calcium channel blockers on calcinosis (OR = 0.70, 95% CI: 0.52, 0.96) but not of other vasodilators.
Table 2.
Multivariate logistic regression model with calcinosis at baseline as the dependent variable
| β | OR (95% CI) | P-value | |
|---|---|---|---|
| Clinical digital ischaemiaa | 0.86 | 2.37 (1.66, 3.39) | <0.001 |
| Severity of digital ischaemiab | 0.11 | 1.12 (1.06, 1.18) | <0.001 |
| Current calcium channel blocker use | –0.35 | 0.70 (0.52, 0.96) | 0.026 |
| Current other vasodilators | –0.22 | 0.80 (0.39, 1.63) | 0.537 |
| Age | 0.01 | 1.01 (1.00, 1.02) | 0.234 |
| Female vs male | 0.13 | 1.14 (0.74, 1.76) | 0.560 |
| Disease duration | 0.05 | 1.05 (1.03, 1.06) | <0.001 |
| Current vs not current smoker | 0.17 | 1.19 (0.79, 1.80) | 0.414 |
| Diffuse vs limited SSc | 0.31 | 1.36 (0.98, 1.89) | 0.064 |
| ACA vs all other antibodies | 0.52 | 1.68 (1.17, 2.43) | 0.006 |
| Anti-topo antibody vs all other antibodies | 0.13 | 1.16 (0.70, 1.94) | 0.566 |
| Anti-RNA polymerase III antibody vs all other antibodies | 0.57 | 1.77 (1.08, 2.89) | 0.024 |
See Methods section.
Number of digits with clinical digital ischaemia.
Longitudinal data
Table 3 shows the characteristics of the patients without calcinosis at baseline according to whether calcinosis did or did not develop during follow-up. Clinical digital ischaemia, severity of digital ischaemia and capillary dropout were all significant univariate predictors of the development of calcinosis. Subjects who developed calcinosis were more likely to have dcSSc than lcSSc (P = 0.024). Anti-RNA polymerase III antibody was also more common in those who developed calcinosis (P < 0.001).
Table 3.
Univariate associations with the development of calcinosis during follow-up
| Calcinosis not present during follow-up (n = 590) | Calcinosis present during follow-up (n = 215) | P-valuea | |||
|---|---|---|---|---|---|
| Missing | Missing | ||||
| Age, mean (s.d.), years | 55.3 (12.2) | 0 | 54.0 (11.1) | 0 | 0.239 |
| Female, n (%) | 506 (85.8) | 0 | 192 (89.3) | 0 | 0.191 |
| Disease duration, mean (s.d.), years | 9.3 (9.0) | 0 | 9.9 (8.5) | 0 | 0.140 |
| Diffuse cutaneous disease, n (%) | 196 (33.3) | 1 | 90 (41.9) | 0 | 0.024 |
| ACA, n (%) | 206 (34.9) | 0 | 88 (40.9) | 0 | 0.117 |
| Anti-topo, n (%) | 99 (16.8) | 0 | 36 (16.7) | 0 | 0.991 |
| Anti-RNA polymerase III, n (%) | 73 (12.4) | 0 | 49 (22.8) | 0 | <0.001 |
| Current smoker, n (%) | 80 (14.0) | 18 | 23 (11.1) | 8 | 0.295 |
| Current use of calcium channel blockers, n (%) | 230 (39.0) | 0 | 95 (44.2) | 0 | 0.183 |
| Current other vasodilators, n (%) | 23 (3.9) | 0 | 6 (2.8) | 0 | 0.456 |
| Clinical digital ischaemia, n (%)b | 254 (47.0) | 49 | 131 (64.5) | 12 | <0.001 |
| Severity of digital ischaemia, mean (s.d.)c | 0.8 (2.3) | 0 | 1.2 (2.2) | 0 | <0.001 |
| Capillary dropout, n (%) | 294 (52.3) | 28 | 125 (59.8) | 6 | 0.063 |
| Time to development of calcinosis, mean (s.d.) | — | — | 2.7 (1.7) | 0 | — |
Chi-squared tests and Mann–Whitney U tests were used as appropriate.
See Methods section.
Number of digits with clinical digital ischaemia.
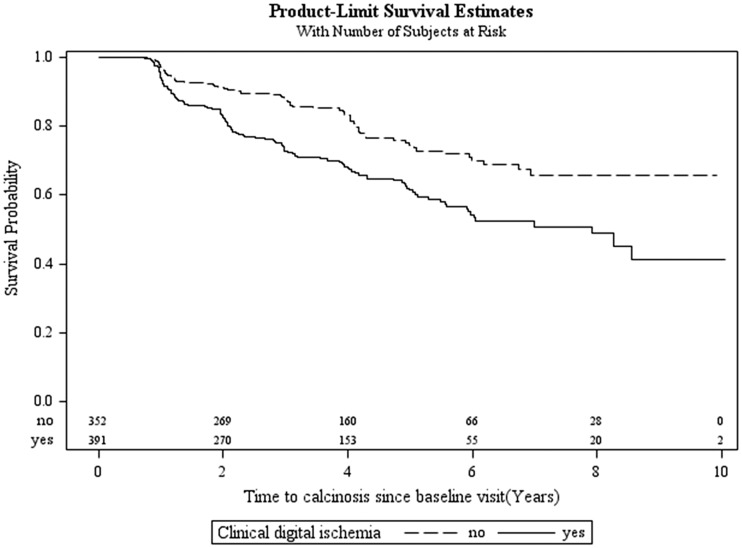
Figure 1 shows the time to onset of calcinosis since the baseline visit according to the presence or absence of clinical digital ischaemia at baseline. Calcinosis developed more rapidly in those with baseline evidence of ischaemia (log-rank P < 0.0001). The mean time to development of calcinosis was 2.6 years in those with digital ischaemia and 2.9 years in those without.
Fig. 1.
Probability of remaining calcinosis-free during follow-up in SSc subjects
Kaplan–Meier curve demonstrating that subjects with clinical digital ischaemia at baseline develop calcinosis earlier in follow-up than those with no ischaemia (log rank P < 0.0001).
Table 4 shows the results of a multivariate Cox proportional hazards model that was adjusted for age, sex, disease duration and smoking status. Only baseline variables were used in this analysis. Clinical digital ischaemia at baseline was a significant predictor of the development of calcinosis (hazard ratio (HR) = 1.82, 95% CI: 1.30, 2.54). Other significant predictors were dcSSc (HR = 1.57, 95% CI: 1.11, 2.21), ACAs (HR = 2.18, 95% CI: 1.50, 3.17) and anti-RNA polymerase III antibodies (HR = 2.58, 95% CI: 1.65, 4.04). Unlike the cross-sectional baseline analysis, neither severity of digital ischaemia nor the use of calcium channel blockers was a significant predictor of the development of future calcinosis. Nor was the use of other vasodilators. In a sensitivity analysis, capillary dropout on capillaroscopy at baseline (instead of clinical digital ischaemia) was also associated with the development of calcinosis (HR = 1.46, 95% CI: 1.08, 1.99).
Table 4.
Multivariate Cox proportional hazards model fitted to the development of calcinosis during follow-up
| β | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|
| Clinical digital ischaemiaa | 0.60 | 1.82 (1.30, 2.54) | 0.001 |
| Severity of digital ischaemiab | 0.02 | 1.02 (0.96, 1.08) | 0.541 |
| Calcium channel blocker current yes vs no | −0.07 | 0.93 (0.69, 1.26) | 0.637 |
| Current other vasodilators | −0.65 | 0.52 (0.19, 1.41) | 0.200 |
| Age | −0.01 | 0.99 (0.98, 1.01) | 0.253 |
| Female vs male | 0.16 | 1.17 (0.73, 1.88) | 0.520 |
| Disease duration | 0.02 | 1.02 (1.00, 1.04) | 0.072 |
| Current vs not current smoker | −0.11 | 0.90 (0.55, 1.46) | 0.661 |
| Diffuse vs limited SSc | 0.45 | 1.57 (1.11, 2.21) | 0.011 |
| ACA vs all other antibodies | 0.78 | 2.18 (1.50, 3.17) | <0.001 |
| Anti-topo antibody vs all other antibodies | 0.29 | 1.33 (0.81, 2.19) | 0.255 |
| Anti-RNA polymerase III antibody vs all other antibodies | 0.95 | 2.58 (1.65, 4.04) | <0.001 |
All independent variable values refer to baseline.
See Methods section.
Number of digits with clinical digital ischaemia.
Discussion
Several findings in this study support the association between calcinosis and tissue ischaemia in SSc. In cross-sectional analyses, calcinosis was strongly associated with signs of digital ischaemia. In addition, the severity of digital ischaemia, estimated by the number of digits involved, was also associated with calcinosis independent of the presence of digital ischaemia. Finally, the presence of drop-out areas on nailfold capillaroscopy was also associated with calcinosis. In longitudinal analyses, we found that the presence of digital ischaemia and capillary drop-out at baseline independently predicted the development of calcinosis during follow-up. Although cross-sectional associations do not necessarily imply causality, the ability of baseline variables to predict the development of calcinosis in follow-up supports the hypothesis that ischaemia may be a contributor to the development of calcinosis in SSc.
Our findings support and extend previous observations in smaller cohorts. In a radiological study of hand features in 120 SSc subjects, Avouac et al. [5] found calcinosis in 23% of hand radiographs. Acro-osteolysis, which presumably represents ischaemia of bone, and DUs were associated with calcinosis, but the frequency of calcinosis was similar in patients with lcSSc and dcSSc and was not associated with the presence of ACAs detected by IIF. In a subsequent report [4], multivariate analysis identified male gender and DUs, but not disease sub-type, as independent predictors of worsening calcinosis over time [4]. The current study extends these observations in that we assessed the development of newly observed calcinosis rather than worsening of existing calcinosis. We have also studied a much larger population of patients and could not support the findings of sex as a predictor. In addition we found that the dcSSc subtype predicted development of calcinosis suggesting that perhaps the previous study was underpowered to find that. We also found that anti-topo and anti-RNA polymerase III antibodies were predictive of the development of calcinosis. Avouac et al. [4] did not assess for anti-RNA polymerase III antibodies and did not find an association between ACA and anti-topoisomerase and the development of calcinosis, also possibly due to the small sample size.
In a study of 101 hand radiographs, Johnstone et al. [6] found no association between acro-osteolysis and calcinosis after adjusting for confounders. Finally, in another cross-sectional study of 167 subjects, Koutaissoff et al. [7] reported calcinosis was associated with longer disease duration, higher modified Rodnan skin score, more current DUs and pitting scars, and a more frequent history of digital gangrene, but not disease subtype or auto-antibody status, in univariate analyses. Multivariate analyses were not reported, nor the details of the assays used to detect auto-antibodies.
There is no single validated measure of tissue ischaemia in SSc. We therefore used either clinical signs of digital ischaemia or the presence of capillary dropout on nailfold capillaroscopy as surrogates. In the cross-sectional analysis, we found that the number of digits affected by clinical digital ischaemia was a predictor of calcinosis independent of the presence of clinical digital ischaemia, a categorical variable. The fact that the degree of that ischaemia adds more predictive power to the cross-sectional baseline model is an important finding in that it suggests a dose–response relationship, and by the same token provides further support for the relationship between ischaemia and calcinosis.
There is some evidence to suggest that soft tissue ischaemia contributes to calcinosis in other circumstances. Perhaps one of the best examples occurs in medullary bone infarction where calcification is often seen on radiography. Calcifications also occur in sickle cell disease where ischaemia is important. Rotator cuff calcification often occurs at a region of the tendon that is thought to be a vascular watershed [16, 17]. One hypothesis is that tendon damage may be caused by relative ischaemia and subsequent to that calcification occurs [18]. In calciphylaxis (calcific uraemic arteriolopathy), soft tissue calcification occurs in addition to calcification within the vessel walls [19]. Although there is often soft tissue necrosis and frequent cutaneous ulcers, presumably caused by the underlying ischaemia, the exact reason for the calcification is not known and may indeed be more related to a deficiency of matrix Gla protein, an inhibitor of calcifications [20]. It is of interest to note that aberrations of this protein in SSc skin have been linked to calcinosis [21].
In this study, we also noted some other interesting findings. dcSSc was significantly associated with the development of future calcinosis, and showed a trend towards an association with current calcinosis. This novel finding is in contradistinction to the commonly held belief that calcinosis is more common in limited disease in which it is part of the CREST syndrome [22]. This relationship to dcSSc persisted even though ACAs were also associated with calcinosis. On the other hand, anti-topo antibodies were not associated with calcinosis. These observations suggest that, in addition to its association with the vasculopathy of SSc, calcinosis is also linked to the two other main pathophysiological abnormalities that characterize SSc, an autoimmune diathesis and fibrosis.
An unexpected finding was the relationship between anti-RNA polymerase III antibodies and calcinosis. We are not aware that this has been previously reported. This antibody is associated with very rapid onset of disease and skin thickening progression [23], development of malignancy and contractures [24, 25], renal crises [26] and gastric antral vascular ectasia [27]. The association with these latter two phenomena suggests some underlying predisposition to vascular complications that might be the link to calcinosis.
We also noted that in the cross-sectional study, the use of calcium channel blockers was associated with less calcinosis. Although it is true that calcium channel blockers are often used to treat Raynaud’s and one could postulate that improving Raynaud’s and associated ischaemia might lead to less calcinosis, there are other possible explanations for this finding. Calcium channel blockers have been suggested as treatment for calcinosis, although efficacy has never been proven [28, 29], and it is possible that some patients with calcinosis had been treated for that with calcium channel blockers but the drugs were discontinued if there was no response. This would lead to a negative association with calcinosis. In the longitudinal follow-up, we could not find the same association with the use of calcium channel blockers. But we must point out that since radiographs was not routinely performed we may have missed some calcinosis that developed during follow-up that may have biased us against finding any association. Another possible explanation for our findings may be that if there is a role for calcium channel blockers it may be only in very early disease.
This study is not without limitations. First, the physicians who collected the data were asked to indicate if any calcinosis was present, either by clinical or radiological assessment. Radiographs were not required and we do not have information on how many subjects had radiographs. Therefore, we are unable to discern if the calcinosis was only clinical, only radiological, or both. We may therefore have missed calcinosis present only radiographically. We did not attempt to quantify the calcinosis and thus could not assess changes in the size of deposits over time and thus could not assess the relationship between digital ischaemia and changes in the size of the deposits. In contrast, in previous studies reporting an association between calcinosis and ischaemia, only hand radiographs were used to identify calcinosis and calcinosis that may have been found on clinical exam but may not have been X-rayed was ignored. Second, we used surrogate measures of tissue ischaemia, although these have not been validated. On the other hand, there is currently no validated measure of ischaemia in SSc. Third, it is possible that some of the DUs recorded were actually overlying areas of calcinosis, despite instructions to exclude those ulcers. Fourth, we have previously reported that detection of capillary dropout with the dermatoscope is less reliable than detection of dilated vessels [30]. However, the fact that we found the hypothesized relationship between calcinosis and capillary dropout despite this limitation, which should have worked against finding a significant association, strengthens our findings. Finally, it is possible that some areas of dystrophic calcification could be due to one mechanism and other areas to another. For example, distal finger calcification may be due to ischaemia whereas more proximal deposits, such as in the forearms, may be related to the skin changes. Further studies will be required to investigate this hypothesis.
In summary, we found a relationship both cross-sectionally and longitudinally between calcinosis and digital ischaemia in SSc. We also identified other independent associations with calcinosis, including dcSSc, ACAs and anti-RNA polymerase III antibody. Calcium channel blocker use at baseline was associated with fewer patients having calcinosis at that time, although it did not predict the future development of calcinosis.
We feel that a further study with a more detailed assessment of the exact location of calcium deposits, including both clinical assessment and routine hand radiographs, is warranted. This may permit a better assessment of the relationship of different factors to the development of dystrophic calcification in different sites. In addition, the use of calcium channel blockers, perhaps early in the disease, to prevent the development of calcinosis should be revisited.
Funding: This work was supported by the Canadian Institutes of Health Research (grant #FRN 83518).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Abrahams I. CRST syndrome (calcinosis, Raynaud's disease, sclerodactyly, and telangiectasias). Arch Dermatol 1965;92:209–10. [DOI] [PubMed] [Google Scholar]
- 2.Dellipiani A, George M. Syndrome of sclerodactyly, calcinosis, Raynaud's phenomenon and telangiectasis. Br Med J 1967;4:334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schimke R, Kirkpatrick C, Delp M. Calcinosis, Raynaud's phenomenon, sclerodactyly, and telangiectasis. The CRST syndrome. Arch Intern Med 1967;119:365–70. [PubMed] [Google Scholar]
- 4.Avouac J, Mogavero G, Guerini H. et al. Predictive factors of hand radiographic lesions in systemic sclerosis: a prospective study. Ann Rheum Dis 2011;70:630–3. [DOI] [PubMed] [Google Scholar]
- 5.Avouac J, Guerini H, Wipff J. et al. Radiological hand involvement in systemic sclerosis. Ann Rheum Dis 2006;65:1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone EM, Hutchinson CE, Vail A, Chevance A, Herrick AL. Acro-osteolysis in systemic sclerosis is associated with digital ischaemia and severe calcinosis. Rheumatology 2012;51:2234–8. [DOI] [PubMed] [Google Scholar]
- 7.Koutaissoff S, Vanthuyne M, Smith V. et al. Hand radiological damage in systemic sclerosis: comparison with a control group and clinical and functional correlations. Semin Arthritis Rheum 2011;40:455–60. [DOI] [PubMed] [Google Scholar]
- 8.Clements PJ, Wong WK, Hurwitz EL. et al. The Disability Index of the Health Assessment Questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum 2001;44:653–61. [DOI] [PubMed] [Google Scholar]
- 9.Chung L, Fiorentino D. A pilot trial of treprostinil for the treatment and prevention of digital ulcers in patients with systemic sclerosis. J Am Acad Dermatol 2006;54:880–2. [DOI] [PubMed] [Google Scholar]
- 10.Garcia de la Pena-Lefebvre P, Rodriguez Rubio S, Valero Exposito M. et al. Long-term experience of bosentan for treating ulcers and healed ulcers in systemic sclerosis patients. Rheumatology 2008;47:464–6. [DOI] [PubMed] [Google Scholar]
- 11.Korn JH, Mayes M, Matucci Cerinic M. et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum 2004;50:3985–93. [DOI] [PubMed] [Google Scholar]
- 12.Matucci-Cerinic M, Denton CP, Furst DE. et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2011;70:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seibold J, Wigley FM, Schiopu E. et al. Digital ischemic ulcers in scleroderma treated with oral treprostinil diethanolamine: a randomized, double-blind, placebo-controlled, multicenter study. [abstract]. Arthritis Rheum 2011;63:2483. [Google Scholar]
- 14.Baron M, Bell M, Bookman A. et al. Office capillaroscopy in systemic sclerosis. Clin Rheumatol 2007;26:1268–74. [DOI] [PubMed] [Google Scholar]
- 15.LeRoy EC, Medsger TA. Criteria for the classification of early Systemic Sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 16.Brooks CH, Revell WJ, Heatley FW. A quantitative histological study of the vascularity of the rotator cuff tendon. J Bone Joint Surg Br 1992;74:151–3. [DOI] [PubMed] [Google Scholar]
- 17.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clin Orthop Relat Res 1990;35–8. [PubMed] [Google Scholar]
- 18.Uhthoff HK, Loehr JW. Calcific tendinopathy of the rotator cuff: pathogenesis, diagnosis, and management. J Am Acad Orthop Surg 1997;5:183–91. [DOI] [PubMed] [Google Scholar]
- 19.Yerram P, Chaudhary K. Calcific uremic arteriolopathy in end stage renal disease: pathophysiology and management. Ochsner J 2014;14:380–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Luo G, Ducy P, McKee MD. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- 21.Davies CA, Jeziorska M, Freemont AJ, Herrick AL. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology 2006;45:1349–55. [DOI] [PubMed] [Google Scholar]
- 22.Velayos E, Masi A, Stevens M, Shulman L. The ‘CREST' syndrome. Comparison with systemic sclerosis (scleroderma). Arch Intern Med 1979;139:1240–4. [DOI] [PubMed] [Google Scholar]
- 23.Cavazzana I, Ceribelli A, Airo P. et al. Anti-RNA polymerase III antibodies: a marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev 2009;8:580–4. [DOI] [PubMed] [Google Scholar]
- 24.Nikpour M, Hissaria P, Byron J. et al. Prevalence, correlates and clinical usefulness of antibodies to RNA polymerase III in systemic sclerosis: a cross-sectional analysis of data from an Australian cohort. Arthritis Res Ther 2011;13:R211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moinzadeh P, Fonseca C, Hellmich M. et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res Ther 2014;16:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domsic RT. Scleroderma: the role of serum autoantibodies in defining specific clinical phenotypes and organ system involvement. Curr Opin Rheumatol 2014;26:646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceribelli A, Cavazzana I, Airo P, Franceschini F. Anti-RNA polymerase III antibodies as a risk marker for early gastric antral vascular ectasia (GAVE) in systemic sclerosis. J Rheumatol 2010;37:1544. [DOI] [PubMed] [Google Scholar]
- 28.Dolan AL, Kassimos D, Gibson T, Kingsley GH. Diltiazem induces remission of calcinosis in scleroderma. Br J Rheumatol 1995;34:576–8. [DOI] [PubMed] [Google Scholar]
- 29.Vayssairat M, Hidouche D, Abdoucheli-Baudot N, Gaitz JP. Clinical significance of subcutaneous calcinosis in patients with systemic sclerosis. Does diltiazem induce its regression? Ann Rheum Dis 1998;57:252–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron M, Bell M, Bookman A. et al. Office capillaroscopy in systemic sclerosis. Clin Rheumatol 2007;26:1268–74. [DOI] [PubMed] [Google Scholar]