Abstract
Objectives. The IL-1 family member IL-37 was recently characterized as a fundamental inhibitor of innate inflammation. We investigated the effects of recombinant IL-37 in joint inflammation and joint pathology in a mouse model of arthritis. In addition, we explored the potential for therapeutic use in human joint inflammation.
Methods. Wild-type mice were treated systemically with a recombinant form of the naturally occurring human IL-37, and then the knee joints were injected with streptococcal cell wall fragments; joint inflammation, synovial cytokine concentrations and histology were evaluated after 24 h. Mice deficient in the IL-1 family decoy receptor IL-1R8 were treated in a similar manner. The effects of IL-37 treatment were also assessed in a model of streptococcal cell wall-induced systemic inflammation. Changes in IL37 and IL1R8 gene expression were evaluated in the synovia of patients with rheumatoid arthritis.
Results. In wild-type mice, low doses (40 µg/kg) of IL-37 suppressed joint inflammation by 51.7% (P < 0.001) and significantly decreased synovial IL-1β by 84%, IL-6 by 73%, TNF-α by 33%, chemokine (C-X-C motif) ligand 1 by 58%, Chemokine (C-C motif) ligand 3 or macrophage inflammatory protein 1-alpha by 64%, IL-1α by 40% and MPO by 60%. These reductions were associated with a lower recruitment of neutrophils into the joint. The anti-inflammatory properties of IL-37 were dependent on the presence of IL-1R8, also in streptococcal cell wall-induced peritonitis. We found that gene expression of IL1R8, but not IL37, is markedly increased in the synovia of patients with rheumatoid arthritis.
Conclusion. IL-37 emerges as a key suppressor of joint and systemic inflammation. These findings indicate a rationale for using recombinant IL-37 in the treatment of arthritis.
Keywords: cytokine, IL-1β, TNF-α, neutrophils, rheumatoid arthritis, chemokines, immunotherapy
Rheumatology key messages
Recombinant IL-37 suppressed joint inflammation, reduced cell influx and lowered histological scores in experimental arthritis.
IL-1R8 expression, but not IL-37, is increased in the synovia of patients with rheumatoid arthritis.
This study shows there is a rationale for using recombinant IL-37 in the treatment of joint inflammation.
Introduction
RA is a systemic autoimmune disease characterized by joint inflammation, leading to progressive destruction of articular cartilage and bone erosion. Treatment strategies for patients with RA refractory to presently available therapies remain an unmet clinical need. Therapeutic use of naturally occurring regulatory cytokines that reduce tissue inflammation would allow the re-establishment of homeostasis [1]. The spectrum of anti-inflammatory cytokines has recently been expanded to include the IL-1 family member IL-37, a natural suppressor of innate inflammation and acquired immunity [2]. IL-37 is transcribed as five different splice variants (IL-37a–e), with IL-37b being the largest isoform [3–5]. The anti-inflammatory effects of IL-37 have emerged in a rapidly expanding number of studies demonstrating suppression of the pro-inflammatory cytokines IL-1α, IL-1β, IL-6, IL-12 and TNF-α, whereas silencing of IL-37 enhanced cytokine production in response to pro-inflammatory stimuli [2].
Studies with IL-37 transgenic (IL-37tg) mice revealed that human IL-37 is functional in the mouse and provided insights on the ability of IL-37 to affect inflammatory and immune responses. For example, IL-37tg mice are protected from lipopolysaccharide-induced shock, experimental colitis, hepatitis, cardiac ischaemia, metabolic syndrome and hypersensitivity dermatitis [2, 6–10]. Protective effects are paralleled by a decrease in the concentrations of circulating and tissue cytokines [2]. Expression of endogenous IL-37 is likely to act as a protective mechanism to curb excessive inflammation and to prevent tissue damage. In the present study, we investigated the effects of treatment with recombinant human IL-37 on joint and systemic inflammation in a model of experimental arthritis characterized by rampant activation of innate immunity. In addition, we explored a potential rationale for the use of exogenous IL-37 in the treatment of human joint inflammation.
Methods
Animals
Male C57BL/6J mice, 8–10 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). For all experimental animals, the weight range was between 22 and 25 g. Age- and sex-matched IL-1R8-deficient mice used in the experiments were generated on a C57Bl/6J background as previously reported [2, 11]. Animal procedures were conducted under protocols approved by the Institutional Animal Care and Use Committees of the University of Colorado Denver, Aurora, CO, USA.
Recombinant human IL-37
There is no signal peptide to indicate the N-terminus of IL-37. Instead, all IL-1 family members share a consensus sequence (alanine-X-aspartic acid); nine amino acids towards the N-terminal methionine, and the N-terminus has the optimal β-sheet folding. However, in the case of IL-37 there is more than one consensus sequence, and the optimal N-terminus for binding to the IL-18Rα is unknown. We used R&D Systems recombinant IL-37 with an N-terminus at valine 46 and C-terminus at 218, which was expressed in Escherichia coli, extracted, refolded and purified (BioTechne, Minneapolis, MN, USA; R&D Systems catalogue number: 7585-IL-025).
The N-terminus of valine 46 was first reported by Edman degradation of IL-37 isoform b expressed in mammalian cells [12]. Prior to induction of arthritis or peritonitis, mice received three intraperitoneal doses of IL-37 (1 µg per mouse, ∼40 µg/kg) in 200 µl, or 1 µg of human serum albumin as a vehicle control.
Streptococcal cell wall-induced arthritis and peritonitis
Cell wall fragments from Streptococcus pyogenes (SCW) were prepared as previously described [13]. Mice were anaesthetized with isofluorane. Arthritis was induced in each knee joint with a single intra-articular injection of 25 μg SCW, dissolved in 6 μl PBS. Joint swelling was graded macroscopically on a scale ranging from 0 to 3 points (0 points: normal joint; 1 point: slight swelling and/or erythema; 2 points: pronounced oedematous swelling; 3 points: severe swelling and redness), as previously described [14]. Peritonitis was induced with a single intraperitoneal injection of 25 µg SCW dissolved in 200 µl PBS.
Histological analyses
Mice were anaesthetized with isofluorane and killed. Knee joints were removed and fixed in 4% formaldehyde for 5 days followed by 5% formic acid for decalcification. Joints were processed for paraffin embedment. Haematoxylin and eosin staining of tissue sections (7 μm thick) was used to study cell influx and tissue destruction; Safranin O was used to evaluate cartilage damage, proteoglycan depletion and bone erosion. Each parameter was scored on a scale of 0–3 by two blinded observers.
Sample processing and cytokine studies
Blood was collected in EDTA via orbital bleeding. Bone marrow cells were obtained from the femurs by flushing with ice-cold Roswell Park Memorial Institute medium (RPMI). Total and differential white blood cell counts were determined using an automated HemaTrue Hematology Analyzer (Heska, Loveland, CO, USA). Plasma was obtained by centrifugation at 1000g. Synovial tissue was removed and placed in lysis buffer containing PBS with 1% Triton X for 2 h and then frozen at −20°C. Peritoneal fluid was collected by instillation of 10 ml ice-cold PBS into the peritoneal cavity, followed by gentle massage; the peritoneal lavage fluid was then collected, cells were counted, and the fluid was centrifuged at 1000g for 10 min. Peritoneal fluid supernatants were concentrated in Spectra/Por dialysis membranes (11.5 mm diameter; molecular cut-off of 3.5 kDa), which were boiled and rinsed with sterile water. The fluid was added, and the tube was closed and placed in thick slurry of polyethylene glycol (molecular weight 6000–8000; Sigma-Aldrich, St Louis, MO, USA). The peritoneal fluid was reduced to between 10 and 15% of the starting volume. The protein concentration was assessed by means of the Bio-Rad Protein Assay (Bio-Rad Laboratories Inc., Hercules, CA, USA). Cytokine concentrations were determined using specific ELISAs (BioTechne). For peritoneal fluid, the protein content was used to normalize the values.
RNA isolation and gene expression studies
Studies on human samples were conducted in accordance with the Declaration of Helsinki and were approved by the Human Studies Ethical Committee of the Radboud University Medical Center, Nijmegen, The Netherlands. Informed consent was obtained from all patients. Synovial tissue was obtained from RA patients who underwent total joint replacement surgery or synovectomy at the Radboud University Medical Center. RNA was isolated from the synovial tissue using TRI-reagent (Sigma) and treated with DNAse to destroy genomic DNA. The RNA was subsequently reverse transcribed with oligo(dT) primers in a reverse transcriptase procedure. Quantitative real-time PCR was performed using the Step-One Plus (Applied Biosystems, Carlsbad, CA, USA), with specific primer pairs and SYBR Green PCR Master Mix. Primer sequences were as follows: Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (housekeeping gene), 5′-ATC-TTC-TTT-TGC-GTC-GCC-AG-3′ (forward) and 5′-TTC-CCC-ATG-GTG-TCT-GAG-C-3′ (reverse); and IL37, 5′-CCA-AGC-CTC-CCC-ACC-ATG-AA-3′ (forward) and 5′-TCC-AGG-ACC-AGT-ACT-TTG-TGA-TC-3′ (reverse). The change in cycle threshold (ΔCt) method was used to normalize IL37 mRNA expression to GAPDH.
Microarray
Raw microarray data of GSE55235 and GSE12021 were obtained from the National Center for Biotechnology Information Gene Expression Omnibus data set. Gene expressions were normalized using the Robust Microarray Average method and extracted using Affymetrix Power Tools. Genes with multiple probesets were collapsed by using the maximal value of the probeset representing the gene. Statistical differences were assessed by analysis of variance .
Statistical analysis
Group measures are expressed as the mean (s.e.m.). Statistical significance was assessed using the Mann–Whitney U-test and Student’s unpaired t-test (GraphPad Prism 6.0, La Jolla, CA, USA).
Results
Administration of recombinant human IL-37 ameliorates SCW-induced arthritis
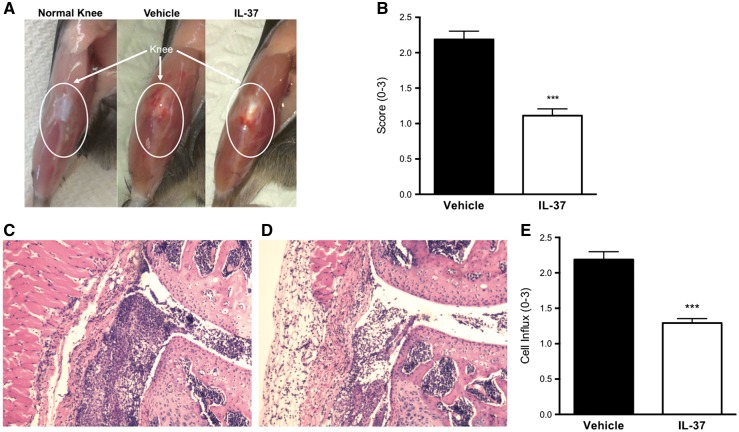
To evaluate the role of IL-37 in joint inflammation, wild-type mice received 1 µg of recombinant human IL-37 or vehicle 24, 12 and 2 h before the induction of SCW-induced arthritis. In SCW-induced arthritis, the intra-articular delivery of bacterial cell wall fragments activates Toll-like receptor 2 and triggers the alternative complement activation cascade to attract neutrophils to the joint [15, 16]. IL-1 and TNF are central to the inflammation that ensues, which leads to severe joint swelling and cartilage destruction [14, 17]. We used this model of innate inflammation because previous studies reported a reduction in joint pathology upon administration of the anti-inflammatory cytokines IL-10 and IL-1Ra [18, 19]. Joint inflammation was assessed 24 h after intra-articular challenge. As shown in Fig 1A and B, IL-37-treated mice exhibited a 51.7% reduction in the severity of arthritis compared with vehicle-treated mice (P < 0.001). Histological changes were compared between IL-37-treated and vehicle-treated mice. IL-37-treated mice displayed a significant reduction in synovial inflammation and cell influx into the joint space, consistent with macroscopic findings of reduced joint swelling (Fig. 1–E).
Fig. 1.
Effects of recombinant IL-37 treatment on streptococcal cell wall-induced arthritis
(A) Representative photographs of joints of a normal knee and from vehicle- and IL-37-treated mice (see Methods section). (B) Mean (s.e.m.) of joint scores. (C and D) Microphotographs of representative knee joint sections from vehicle- (C) and IL-37-treated mice (D); original magnification 100×. (E) Mean (s.e.m.) of synovial cell influx. Student’s unpaired t-test was used for statistical analyses; data are expressed as the mean (s.e.m.) of 10 knee joints per group; ***P < 0.001.
IL-37 treatment reduces synovial pro-inflammatory mediators
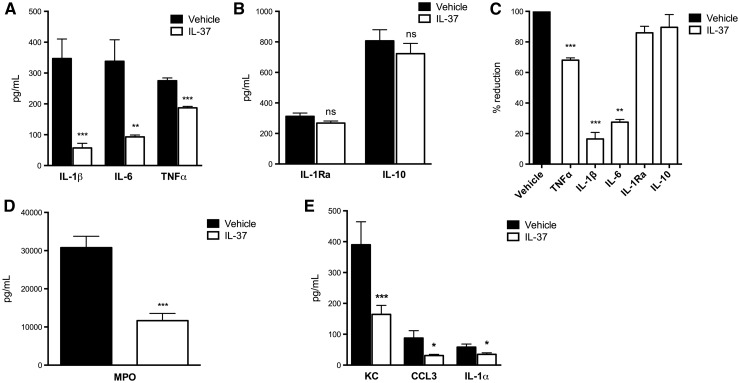
The synovium was removed from the left knee joint of each mouse 24 h after induction of SCW arthritis. Synovial tissue extracts were analysed for the levels of pro-inflammatory cytokines and chemokines. As shown in Fig. 2A, in the synovial extracts of IL-37-treated mice IL-1β was reduced by 84% (P < 0.001), IL-6 by 73% (P = 0.002) and TNF-α by 33% (P < 0.001). In contrast, levels of the anti-inflammatory cytokines IL-1Ra and IL-10 were not affected (Fig. 2B). The percentage changes in the synovial levels of these cytokines are shown in Fig. 2C. The reduction in cytokines paralleled the reduction in the levels of the neutrophil enzyme MPO, which was decreased by 60% (P < 0.001; Fig. 2D). Given that IL-37 treatment reduced the neutrophil influx into the joint space, the synovial levels of neutrophil-attractant chemokines were also evaluated. As shown in Fig. 2E, in the synovia of IL-37-treated mice chemokine (C-X-C motif) ligand 1 (KC) was reduced by 58% (P = 0.005), Chemokine (C-C motif) ligand 3 or macrophage inflammatory protein 1-alpha (CCL3/MIP1α) by 64% (P = 0.03) and IL-1α by 40% (P = 0.04).
Fig. 2.
Cytokine changes in the synovia of mice subjected to streptococcal cell wall-induced arthritis
Mice were treated as described in Fig. 1. Levels of synovial cytokines were assessed 24 h after the induction of streptococcal cell wall-induced arthritis. (A) Mean (s.e.m.) of IL-1β, TNF-α and IL-6. (B) IL-1Ra and IL-10. (C) Mean percentage reduction of cytokine levels relative to values shown in A and B. (D) MPO. (E) KC, CCL3/MIP1α and IL-1α. Groups were compared using Student’s unpaired t-test; data are derived from 10 mice per group; *P < 0.05, **P < 0.005 and ***P < 0.001. KC: CXCL1 or (C-X-C motif) ligand 1; NS: non-significant.
Effects of IL-37 treatment on systemic indicators
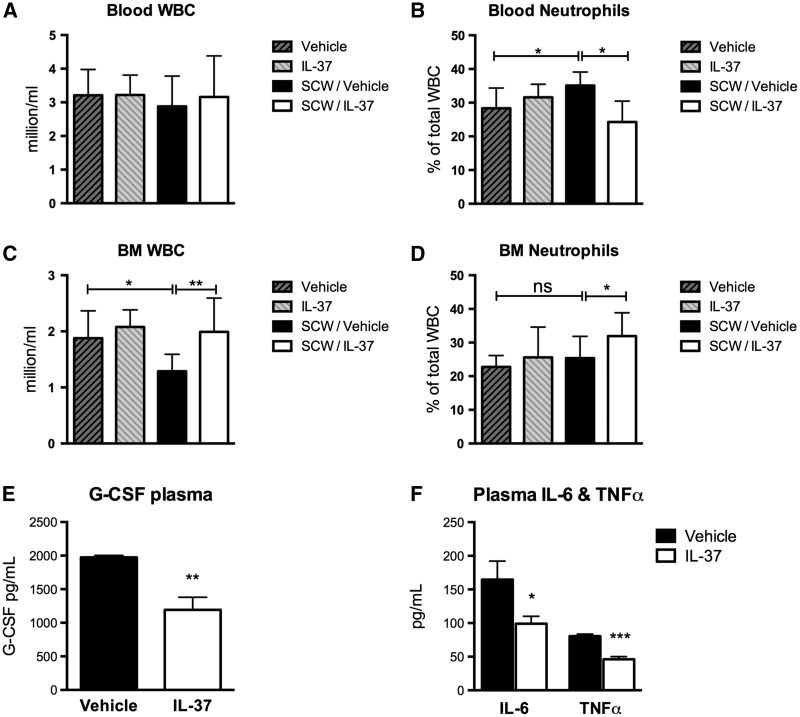
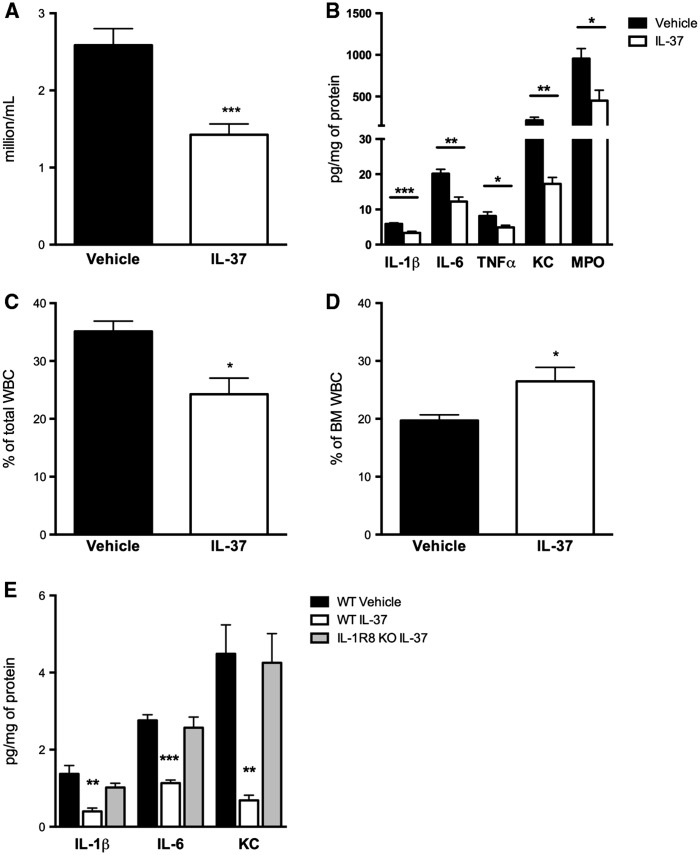
Joint inflammation in SCW arthritis is primarily neutrophil driven [15]. We thus evaluated whether changes in circulating and bone marrow neutrophils were affected by the reduced recruitment into the joints of IL-37-treated mice. Blood was collected from IL-37- and vehicle-treated mice 24 h after induction of joint inflammation, and complete blood counts were performed. Although there were no differences in the number of total leucocytes between the groups (Fig. 3A), mice subjected to SCW-induced arthritis and treated with IL-37 exhibited a 31% reduction in the percentage of circulating neutrophils (P = 0.01; Fig. 3B). Bone marrow cells were harvested and counted. In comparison with vehicle-treated mice subjected to SCW, treatment with IL-37 was associated with a 47% increase in the total number of leucocytes (P = 0.008) as well as a 27% increase in the percentage of neutrophils (P = 0.02) in the bone marrow (Fig. 3C and D). These changes upon IL-37 treatment are consistent with decreased synovial levels of the neutrophil chemo-attractants KC, MIP1α and IL-1α and with decreased neutrophil influx into the joint, as also confirmed by the reduction of the neutrophil activation marker MPO (see Figs 1 and 2). Plasma concentrations of G-CSF, which mobilizes neutrophils from the bone marrow [20], were evaluated. IL-37-treated mice exhibited a 40% decrease in the concentrations of G-CSF (P = 0.003; Fig. 3E). In addition, plasma concentrations of IL-6 and TNF-α in IL-37-treated mice were decreased by 40% (P = 0.04) and 46% (P = 0.0001), respectively (Fig. 3F).
Fig. 3.
Cell counts and systemic cytokines in mice subjected to streptococcal cell wall-induced arthritis
(A) Mean (s.e.m.) circulating leucocytes in vehicle- and IL-37-treated mice 24 h after induction of SWC-induced arthritis. (B) Percentage of circulating neutrophils relative to values shown in A. (C) Mean (s.e.m.) bone marrow leucocytes in vehicle- and IL-37-treated mice. (D) Percentage of bone marrow neutrophils relative to values shown in C. (E) Mean (s.e.m.) plasma concentrations of G-CSF. (F) Plasma concentrations of IL-6 and TNF-α. Groups were compared using Student’s unpaired t-test. Data are derived from 10 mice per group; *P < 0.05 and **P < 0.005. BM: bone marrow; NS: non-significant; SWC: streptococcal cell wall; WBC: white blood cells.
IL-37 requires the anti-inflammatory receptor IL-1R8
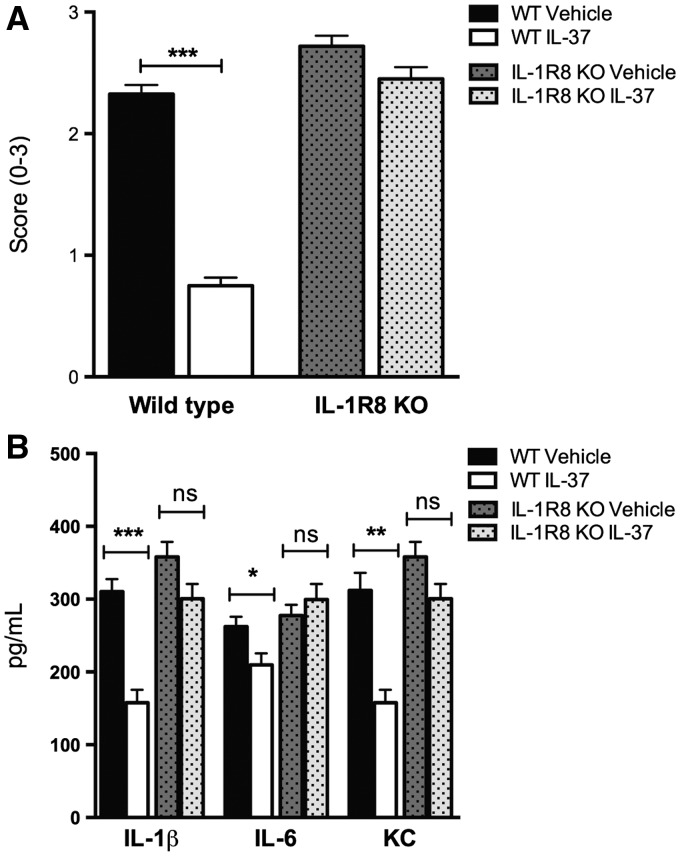
In several models of acquired and innate immunity, IL-1R8 deficiency is associated with a hyper-inflammatory phenotype [11]. For example, IL-1R8-deficient mice exhibit an overwhelming inflammatory response to Mycobacterium tuberculosis and Aspergillus fumigatus infection, endotoxaemia and Dextran sulfate sodium-induced colitis [11, 21–23]. We reported that IL-1R8 forms a surface tripartite complex with IL-37 and the α-chain of the IL-18 receptor [24]. Given that IL-1R8 is required for the anti-inflammatory properties of recombinant IL-37, SCW-induced arthritis was induced in mice deficient in IL-1R8. As shown in Fig. 4A, the anti-inflammatory effects of IL-37 in IL-1R8-deficient mice were nearly absent, and treatment with IL-37 failed to reduce synovial levels of IL-1, IL-6 and KC (Fig. 4B).
Fig. 4.
Effects of recombinant IL-37 treatment in IL-1R8-deficient mice
Mice received 1 µg of recombinant IL-37 at 24, 12 and 2 h before the induction of SCW-induced arthritis. Vehicle- and IL-37-treated mice were killed 24 h after the induction of arthritis. (A) Mean (s.e.m.) of joint scores in WT and IL-1R8-deficient mice. (B) Mean (s.e.m.) of IL-1β, IL-6 and KC concentrations in the synovia of WT and IL-1R8-deficient mice. Groups were compared using Student’s unpaired t-test. Data are expressed as the mean (s.e.m.) of 10 mice per group; *P < 0.05, **P < 0.005 and ***P < 0.001. NS: non-significant; SWC: streptococcal cell wall; WT: wild-type.
Effects of IL-37 treatment on SCW-induced peritonitis
We next studied the anti-inflammatory properties of IL-37 in a model of SCW-induced peritonitis. Wild-type mice received IL-37 or vehicle at 24, 12 and 2 h before the intraperitoneal administration of SCW; peritoneal inflammation was assessed after 24 h. Mice treated with IL-37 exhibited a 42% decrease in the number of leucocytes infiltrating the peritoneal cavity (P = 0.0005; Fig. 5A) and a reduction in the concentrations of pro-inflammatory mediators in the peritoneal fluid (Fig. 5B and E). Specifically, IL-1β was reduced by 42% (P = 0.0001), IL-6 by 40% (P = 0.002), TNF-α by 41% (P = 0.02) and KC by 91% (P = 0.001); concentrations of the neutrophil enzyme MPO were decreased by 53% (P = 0.01). As observed in mice subjected to SCW-induced arthritis, treatment with IL-37 was associated with a 31% decrease in circulating neutrophils (P = 0.01), paralleled by a 34% increase in bone marrow neutrophils (P = 0.03; Fig. 5C and D). These observations are consistent with decreased peritoneal concentrations of KC and MPO and with reduced transmigration into the peritoneal cavity. Again, in mice deficient for IL-1R8 the protective effects of IL-37 on SCW-induced peritonitis were nearly absent, as there were no significant changes in the concentrations of peritoneal cytokines IL-1, IL-6 and KC (Fig. 5E).
Fig. 5.
Effects of recombinant IL-37 treatment in streptococcal cell wall-induced peritonitis
WT mice received 1 µg of recombinant IL-37 at 24, 12 and 2 h before the induction of SCW-induced peritonitis. Vehicle- and IL-37-treated mice were killed 24 h after the induction of peritonitis. (A) Mean numbers of leucocytes infiltrating the peritoneal cavity. (B) Concentrations of IL-1β, IL-6, TNF-α, KC and MPO in the peritoneal fluid of mice treated with IL-37. (C and D) Mean (s.e.m.) percentage of circulating (C) and bone marrow (D) neutrophils in vehicle- and IL-37-treated mice 24 h after induction of SCW-induced peritonitis. (E) Concentrations of IL-1β, IL-6 and KC in the peritoneal fluid of WT or IL-1R8-deficient mice treated with IL-37. For cytokine determinations in peritoneal fluid, cytokine concentrations were normalized to the protein content after concentration and expressed as picograms or milligrams of protein. *P < 0.05, **P < 0.005 and ***P < 0.001. KC: CXCL1 or (C-X-C motif) ligand 1; SWC: streptococcal cell wall; WT: wild-type.
Expression of IL-37 and IL-1R8 in the synovia of patients with RA
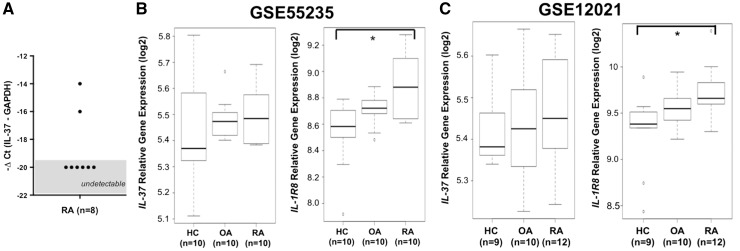
We next evaluated IL-37 gene expression in synovial tissues from eight patients with active RA. No relevant expression of IL-37 was detected by quantitative real-time PCR in the synovia of these patients (Fig. 6A). In order to confirm these findings, we analysed two different microarray data sets: GSE12021 (synovial samples collected from synovectomy surgery in 12 patients with RA, from joint replacement surgery in 10 with OA and post mortem from 9 healthy controls) and GSE55235 (10 patients with RA, 10 with OA and 10 healthy controls). As shown in Fig. 6B and C, synovial expression of IL-37 was low regardless of the clinical condition. In contrast, RA patients exhibited a markedly increased synovial expression of IL-1R8, the receptor required for the anti-inflammatory effects of IL-37 (P < 0.05). IL-1R8 expression was higher in the synovia of RA than OA patients, but this did not reach statistical significance.
Fig. 6.
Gene expression of IL-37 and its receptor IL-1R8 in human rheumatoid synovia
(A) IL37 gene expression in the synovia of RA patients. Synovial tissue was obtained from eight RA patients who underwent total joint replacement surgery or synovectomy. Quantitative real-time PCR was used to assess IL37 gene expression. Values on the y-axis are calculated with the ΔCt method to normalize IL37 mRNA expression to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B and C) Mean (s.e.m.) levels of IL-37 and IL-1R8 gene expression in the synovia of HCs, OA and RA patients from two different microarray data sets: GSE55235 (B) and GSE12021 (C). GSE55235 included 10 patients with RA, 10 with OA and 10 HCs; GSE12021 included 12 patients with RA, 10 with OA and 9 HCs. Analysis of variance was used to evaluate differences between groups; *P < 0.05. ΔCt: change in cycle threshold; HCs: healthy controls.
Discussion
In the present study, we investigated the therapeutic potential of the novel anti-inflammatory cytokine IL-37 in the treatment of joint inflammation. Short-term, low-dose treatment with recombinant IL-37 suppressed joint inflammation, reduced cell influx and lowered histological scores by > 50%. In mice treated with recombinant IL-37, synovial IL-1β was reduced by 84%, IL-6 by 73%, TNF-α by 33%, KC by 58%, CCL3/MIP1α by 64%, IL-1α by 40% and MPO by 60%. In contrast, the anti-inflammatory cytokines IL-1Ra and IL-10 were unaffected. We expanded these findings in a model of systemic inflammation, that is, SCW-induced peritonitis. Again, reduced recruitment of inflammatory cells and suppression of inflammatory cytokines and chemokines revealed the protective effects of IL-37. The total amount of IL-37 administered was 3 µg per mouse (0.12 mg/kg), divided into three doses. This low dosing of IL-37 in vivo is consistent with in vitro observations that the anti-inflammatory effects of IL-37 are most evident at concentrations between 10 and 100 picomolar [25]. Also, in a model of acute inflammatory lung disease, IL-37 reduced neutrophil infiltration at a dose of 100 ng per mouse [22].
We showed that the decoy receptor IL-1R8 is required for IL-37 to suppress joint inflammation. Unlike other members of the IL-1 receptor family, IL-1R8 has a non-canonical function, in that it reduces IL-1R- and Toll-like receptor-dependent inflammation [24]. Several mechanisms have been reported for the anti-inflammatory properties of IL-1R8, including inhibition of nuclear factor-κB and c-Jun N-terminal kinases [24, 26]. Consistently, IL-1R8 deficiency is associated with a hyper-inflammatory phenotype [11, 24, 25]. In our studies, the anti-inflammatory effects of IL-37 on SCW-induced arthritis were abrogated in mice deficient for IL-1R8.
We used a recombinant form of IL-37 with the naturally occurring N-terminus at valine 46 [5]. Once administered, mice exhibited a marked decrease in joint swelling, paralleled by a decline in the levels of synovial IL-1β, TNFα, IL-6 and systemic IL-6 and TNFα. Histological analysis of affected joints revealed a decrease in tissue inflammation and cell influx, thus confirming the protective effects of IL-37. The suppression of articular inflammation was not attributable to increased IL-10 or IL-1Ra, consistent with previous studies in which antibody blockade of the IL-10 receptor did not affect IL-37-mediated protection [6]. The SCW-induced arthritis model is too short lived to evaluate the effects of IL-37 treatment on Th17 differentiation and function. However, recent evidence suggests that IL-37 suppresses IL-17 production and limits Th17 polarization specifically in RA, and that overexpression of IL-37 in a model of collagen-induced arthritis can alleviate the severity of joint inflammation through inhibition of IL-17 and suppression of Th17 cell proliferation [27].
We used intra-articular instillation of SCW fragments, which is ideally suited for the therapeutic use of recombinant IL-37. Given that this model of arthritis is characterized by a rapid increase and similar rapid reduction in inflammation, the optimal timing for administration of any treatment (including CSs, anti-TNF-α agents and different anti-cytokine therapies) is shortly before the induction of joint inflammation [18, 28]. Inhibition of innate inflammation is the primary effect of IL-37 [2]. We thus investigated the therapeutic potential of this anti-inflammatory cytokine in a model of arthritis characterized by rampant production of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, activation of complement, and neutrophil infiltration. These cytokines and neutrophils are found in the synovial fluid from the early stages of RA [29]. Here, neutrophil-derived immune mediators initiate acute inflammation and recruit other cell types. For instance, neutrophils produce abundant pro-inflammatory cytokines, including TNF-α, T cell-attracting chemokines and B-lymphocyte stimulator [30–32]. Infiltrating neutrophils express the peptidyl-arginine deiminase 4 enzyme and promote the accumulation of citrullinated proteins within the joint, a crucial early event in RA pathogenesis [33]. In RA, neutrophils also exhibit defective apoptosis, thus augmenting the release of reactive oxygen species and proteases, which activate cytokine precursors and perpetuate inflammation and tissue damage (reviewed by Cascão et al. [29]). In addition to the suppression of local inflammation, reduced recruitment of neutrophils to the joint was deemed a likely mechanism for the protective effects of IL-37. Supporting this hypothesis, elevated levels of the neutrophil activation marker MPO characterized synovial inflammation in SCW-induced arthritis and were markedly reduced upon IL-37 treatment. Consistently, IL-37 treatment was associated with a decrease in circulating neutrophils. A corresponding increase in bone marrow neutrophils indicates that reduced recruitment and mobilization, rather than reduced proliferation, accompany the clinical response to IL-37 treatment. Mechanistic confirmation for this hypothesis comes from synovial levels of the main neutrophil chemo-attractants KC, CCL3/MIP1α and IL-1α, all of which were markedly reduced in mice treated with IL-37.
It is not unexpected that the anti-inflammatory effects of recombinant IL-37 would result in a decrease in neutrophil populations in peripheral inflammatory sites. In humans, IL-1 injected at doses as low as 3 ng/kg induces neutrophil mobilization from the bone marrow and neutrophilia, whereas a reduction in circulating neutrophils often heralds a clinical response to IL-1-blocking therapies (reviewed by Cavalli and Dinarello [34]). It is reasonable that the observed increase in bone marrow neutrophils in our model reflects reduced recruitment and mobilization, rather than a primary increase in proliferation. Indeed, besides suppression of inflammatory cytokines and of neutrophil chemo-attractants upon IL-37 treatment, we also observed a decrease in circulating G-CSF. G-CSF induces neutrophil proliferation, as well as activation and mobilization from the bone marrow, and delays neutrophil apoptosis [29]. Of note, serum and synovial levels of G-CSF are directly correlated with RA severity, and exogenous administration of G-CSF exacerbates arthritis (reviewed by Cornish et al. [20]).
Delivery of regulatory cytokines remains an attractive perspective for arthritis treatment [35–38]. The broad-spectrum anti-inflammatory cytokine IL-37 dampens the production of pro-inflammatory cytokines and protects against inflammation-mediated tissue damage [3]. The present study demonstrates that by curbing excessive inflammation IL-37 exerts a protective role against arthritis. We also demonstrated that IL-37 is scarcely expressed in the diseased synovia of patients with RA, but expression of IL-1R8, the receptor transducing the anti-inflammatory effects of IL-37, is elevated. Given this clinical reality, there is a therapeutic potential for administration of IL-37 in humans; although levels of endogenous IL-37 are too low to contain the overwhelming inflammatory reaction in the synovia of RA patients, exogenous administration might suppress the pathological process. Altogether, the present findings provide rationale for using recombinant IL-37 in the treatment of joint inflammation.
Acknowledgements
The authors thank T. Azam, S. Li, B. Swartzwelter, I. Tengesdal and M. M. A. Helsen. These studies were supported by National Institutes of Health Grant AI 15614 (to C.A.D.) and by The Interleukin Foundation (to G.C.).
Funding: These studies were supported by National Institutes of Health Grant AI 15614.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 2012;13:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nold MF, Nold-Petry CA, Zepp JA. et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 2010;11:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol 2013;25:466–8. [DOI] [PubMed] [Google Scholar]
- 4.Bulau AM, Nold MF, Li S. et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc Natl Acad Sci USA 2014;111:2650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Hanning CR, Brigham-Burke MR. et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 2002;18:61–71. [DOI] [PubMed] [Google Scholar]
- 6.McNamee EN, Masterson JC, Jedlicka P. et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA 2011;108:16711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai X, Luo Y, Nold-Petry C. et al. IL-37 suppresses contact hypersensitivity by inhibiting maturation of dendritic cells. J Invest Dermatol 2011;131:S102. [Google Scholar]
- 8.Luo Y, Cai X, Liu S. et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA 2014;111:15178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulau AM, Fink M, Maucksch C. et al. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. ScientificWorldJournal 2011;11:2480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballak DB, van Diepen JA, Moschen AR. et al. IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun 2014;5:4711. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda C, Riva F, Polentarutti N. et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA 2004;101:3522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan G, Risser P, Mao W. et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine 2001;13:7. [DOI] [PubMed] [Google Scholar]
- 13.Cromartie WJ, Craddock JG, Schwab JH, Anderle SK, Yang CH. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med 1977;146:1585–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinhuis B, Koenders MI, van de Loo FA. et al. IL-32γ and Streptococcus pyogenes cell wall fragments synergise for IL-1-dependent destructive arthritis via upregulation of TLR-2 and NOD2. Ann Rheum Dis 2010;69:1866–72. [DOI] [PubMed] [Google Scholar]
- 15.van den Broek MF, van den Berg WB, van de Putte LB, Severijnen AJ. Streptococcal cell wall-induced arthritis and flare-up reaction in mice induced by homologous or heterologous cell walls. Am J Pathol 1988;133:139–49. [PMC free article] [PubMed] [Google Scholar]
- 16.Joosten LA, Koenders MI, Smeets RL. et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol 2003;171:6145–53. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper S, Joosten LAB, Bendele AM. et al. Different roles of tumour necrosis factor α and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine 1998;10:690–702. [DOI] [PubMed] [Google Scholar]
- 18.Joosten LA, Helsen MM, van Den Berg WB. Blockade of endogenous interleukin 12 results in suppression of murine streptococcal cell wall arthritis by enhancement of interleukin 10 and interleukin 1Ra. Ann Rheum Dis 2000;59:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubberts E, Joosten LA, Helsen MM, van den Berg WB. Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine 1998;10:361–9. [DOI] [PubMed] [Google Scholar]
- 20.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol 2009;5:554–9. [DOI] [PubMed] [Google Scholar]
- 21.Garlanda C, Di Liberto D, Vecchi A. et al. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol 2007;179:3119–25. [DOI] [PubMed] [Google Scholar]
- 22.Moretti S, Bozza S, Oikonomou V. et al. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS Path 2014;10:e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald D, Qin J, Zhao Z. et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 2003;4:920–7. [DOI] [PubMed] [Google Scholar]
- 24.Nold-Petry CA, Lo CY, Rudloff I. et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol 2015;16:354–65. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Neff CP, Barber K. et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA 2015;112:2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garlanda C, Riva F, Bonavita E, Gentile S, Mantovani A. Decoys and regulatory “receptors” of the IL-1/Toll-like receptor superfamily. Front Immunol 2013;4:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye L, Jiang B, Deng J. et al. IL-37 alleviates rheumatoid arthritis by suppressing IL-17 and IL-17-triggering cytokine production and limiting Th17 cell proliferation. J Immunol 2015;194:5110–9. [DOI] [PubMed] [Google Scholar]
- 28.Plater-Zyberk C, Joosten LA, Helsen MM. et al. GM-CSF neutralisation suppresses inflammation and protects cartilage in acute streptococcal cell wall arthritis of mice. Ann Rheum Dis 2007;66:452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 2010;9:531–5. [DOI] [PubMed] [Google Scholar]
- 30.Scapini P, Carletto A, Nardelli B. et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood 2005;105:830–7. [DOI] [PubMed] [Google Scholar]
- 31.Auer J, Bläss M, Schulze-Koops H. et al. Expression and regulation of CCL18 in synovial fluid neutrophils of patients with rheumatoid arthritis. Arthritis Res Ther 2007;9:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assi LK, Wong SH, Ludwig A. et al. Tumor necrosis factor α activates release of B lymphocyte stimulator by neutrophils infiltrating the rheumatoid joint. Arthritis Rheum 2007;56:1776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vossenaar ER, Nijenhuis S, Helsen MM. et al. Citrullination of synovial proteins in murine models of rheumatoid arthritis. Arthritis Rheum 2003;48:2489–500. [DOI] [PubMed] [Google Scholar]
- 34.Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology 2015;54:2134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeij EA, Broeren MG, Bennink MB. et al. Disease-regulated local IL-10 gene therapy diminishes synovitis and cartilage proteoglycan depletion in experimental arthritis. Ann Rheum Dis 2015;74:2084–91. [DOI] [PubMed] [Google Scholar]
- 36.van Roon J, Wijngaarden S, Lafeber FP. et al. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol 2003;30:648–51. [PubMed] [Google Scholar]
- 37.Schwager K, Kaspar M, Bootz F. et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther 2009;11:R142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadoun D, Rosenzwajg M, Joly F. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. New Engl J Med 2011;365:2067–77. [DOI] [PubMed] [Google Scholar]