Abstract
Stem cell therapy holds great promise for the replacement of damaged or dysfunctional myocardium. Nitric oxide (NO) has been shown to promote embryonic stem (ES) cell differentiation in other systems. We hypothesized that NO, through NO synthase gene transfer or exogenous NO exposure, would promote the differentiation of mouse ES cells into cardiomyocytes (CM). In our study, NO treatment increased both the number and the size of beating foci in embryoid body (EB) outgrowths. Within 2 weeks, 69% of the inducible NO synthase-transduced EB displayed spontaneously beating foci, as did 45% of the NO donor-treated EB, compared with only ≈15% in controls. Cardiac-specific genes and protein expression were significantly increased in NO-treated ES. Electron microscopy and immunocytochemistry revealed that these NO-induced contracting cells exhibited characteristics consistent with CM. At day 7 in culture, troponin T was expressed in 45.6 ± 20.6% of the NO-treated ES cells but in only 9.25 ± 1.77% of control cells. Interestingly, 50.4 ± 18.4% of NO-treated ES cells were troponin T-negative and annexin V-positive. This apoptotic phenotype was seen in <1% of the control ES cells. These data strongly support our hypothesis that mouse ES cells can be accelerated to differentiate into CM by NO treatment. NO may influence cardiac differentiation by both inducing a switch toward a cardiac phenotype and inducing apoptosis in cells not committed to cardiac differentiation.
Cell transplantation is one potential therapy to replace damaged or lost myocardial tissue to restore cardiac function (1). Approaches involving experimental cell transplantation to restore functional myocardium have been reported and include the use of skeletal myoblasts, fetal cardiomyocytes (CM), neonatal CM, adult CM, atrial tumor cell lines, cardiac stem cells, bone marrow stem cells, neuronal stem cells, endothelial stem cells, hepatocyte stem cells, and embryonic stem (ES) cells (reviewed in ref. 2). A promising candidate therapy makes use of CM derived from ES cells. ES cells are pluripotent and thus capable of differentiating into CM in vitro by appropriate cultivation (3). Furthermore, these cells retain the potential for unlimited proliferation, which is essential for large-scale production. Because the molecular basis of cardiac development is not fully understood, no method has been reported to induce cardiac specific differentiation from ES cells.
A potential differentiation signal is nitric oxide (NO). NO is synthesized in vivo by one of the three known NO synthases (NOSs) (4, 5) or through the conversion of nitrite to NO under acidic conditions, a process accelerated in the presence of deoxyhemoglobin (6). NO and NOS isoforms have been shown to induce or facilitate the differentiation of several cell types, including nerve cells (7), some tumor cell types (8), and the heart (9). Prominent expression of both inducible NOS (iNOS) and endothelial NOS (eNOS) proteins has been observed in the heart during embryonic development starting on day 9.5 (D9.5) (9). This prominent expression abates before birth, suggesting that a period of NO exposure is critical to normal development. Mice genetically deleted for the eNOS isoform exhibit several congenital heart malformations, such as bicuspid aortic valves and atrial and ventricular septal defects (10, 11). Work with mouse ES cells has shown that NOS inhibitors arrest differentiation toward a cardiac phenotype and that this effect can be rescued by NO donors (9).
Whether NO will induce ES cell differentiation to become functional CM is unknown. In the present study, we tested the hypothesis that NO promotes differentiation in part through its effects on cell survival, and that this leads to the differentiation of ES cells into CM. We demonstrate that optimized exposure of mouse ES cells to NO induces CM differentiation, as determined by flow cytometric (FCM) analysis of cardiac-specific protein expression, RT-PCR analysis of cardiac specific gene expression, and immunocytochemical imaging for the intracellular localization of cardiac contractile proteins. This differentiation was associated with higher apoptotic rates before a shift to the CM phenotype.
Materials and Methods
Cell Culture. Mouse ES cell line ES-D3 was obtained from American Type Culture Collection. ES cells were grown on a gelatin-coated tissue culture plastic substrate prepared by coating tissue culture flasks with a 0.1% gelatin solution. Cells were maintained in a standard medium (Iscove's Modified Dulbecco's Medium, Invitrogen) plus 15% newborn calf serum, nonessential amino acids, and nucleosides stock. Leukemia inhibitory factor (LIF) (Chemicon) at a final concentration of 1,000 units/ml and 10–4 M 2-mercaptoethanol were added to standard medium to prevent differentiation. For the induction of differentiation, ES cells were cultivated without LIF in hanging drops to form embryoid bodies (EBs) (≈400 cells per drop) for 2 days, then kept in suspension for 2 days, and finally plated on gelatin-coated single-culture dishes. Then they were exposed to NO by exogenous supplementation of S-nitroso-N-acetylpenicillamine (SNAP, 200 μM), 2-(N, N-diethylamino)-diazenolate-2-oxide (DEA/NO, 0.0.1 μM), or (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate (PAPA/NO, 1.5 mM) at D0. In some experiments, oxidized SNAP (200 μM) was used as indicated. By D3–D5 after plating, spontaneously contracting cell clusters could be observed within the EB outgrowths. In some experiments, EBs were transfected at a multiplicity of infection of 25 plaque-forming units of virus per cell by adenoviral vector containing the human iNOS transgene (AdiNOS; Programs of Excellence in Gene Therapy Vector Core Facility, University of Pittsburgh; transfected 1 day before plating (D–1). Culture media from both NO donor-treated and iNOS-transfected cells were changed every day after D3.
Flow Cytometry. EB outgrowths harvested by trypsinization were digested with collagenase II (0.5 mg/ml, Worthington) and pancreatin (0.6 mg/ml, Invitrogen), rinsed with PBS, fixed for 20 min at room temperature with 3.7% formaldehyde, and neutralized with 50 mM glycine. The cells were permeabilized by using 0.4% Triton X-100 in PBS and incubated with the primary antibody in a humidified chamber at 37°C for 2 h. After washing with 0.4% Triton X-100 and PBS, secondary antibody was added, and the specimens were incubated for 2 h at 37°C. Antibodies used in this study were as follows: anti-myosin light chain, anti-troponin T, and anti-troponin I antibodies (Abcam, Cambridge, U.K.), anti-β myosin heavy chain (MHC) antibody (Chemicon), and anti-α MHC antibody (American Type Culture Collection). Samples were then analyzed on a FACScan (Becton Dickinson). Data were analyzed on a Macintosh PC by using cell quest software (Becton Dickinson).
Western Blot Analysis. Cells were washed with PBS, collected in modified RIPA buffer (2 mM sodium orthovanadate/50 mM NaF/20 mM Hepes/150 mM NaCl/1.5 mM MgCl2/5 mM sodium pyrophosphate/10% glycerol/0.2% Triton X-100/5 mM EDTA/1 mM PMSF/10 μg/ml leupeptin/10 μg/ml aprotinin) and allowed to lyse for 30 min on ice. Samples were clarified by centrifugation for 15 min at 12,000 × g at 4°C. Western blotting was performed as described (12). Briefly, the samples containing equal amounts of protein were separated on SDS polyacrylamide gel under reducing conditions and transferred to polyvinylidene difluoride membranes. The blots were incubated for 1 h at room temperature with the indicated primary antibodies, followed by incubation with the SuperSignal chemiluminescence detection system (Pierce).
Immunostaining. For immunocytofluorescence studies, some EBs were cultured onto glass coverslips coated with 0.1% gelatin. For immunohistochemistry, heart grafts harvested at the indicated time point were frozen in liquid nitrogen, and 5-μm sections were cut for immunostaining. Slides were fixed with 3.7% formaldehyde and permeabilized with 0.1% Nonidet P-40 in PBS. Nonspecific binding sites were blocked with PBS containing 1% BSA and 10% skim milk. Cells were then incubated with monoclonal anti-troponin T or troponin I antibody (Abcam), polyclonal anti-GFP antibody (Santa Cruz Biotechnology), or rhodamine-conjugated phalloidin. Immunochemistry was carried out with the ABC staining system (Santa Cruz Biotechnology), according to the manufacturer's protocol.
RT-PCR Analysis. Total RNA from undifferentiated ES cells and contracting EB outgrowths was extracted at indicated time points by using Trizol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA by using SuperScript II reverse transcriptase (Life Technologies, Grand Island, NY). The PCR primers and reaction conditions were according to Hidaka et al. (13). The PCR products were size-fractionated by 2% agarose gel electrophoresis.
Transmission Electron Microscopy. Cells grown in culture dishes were fixed in 2.5% glutaraldehyde at 4°C, followed by postfixation in aqueous 1% osmium tetraoxide and 1% K3Fe(CN)6 for 1 h. Samples were dehydrated through a graded series of 30–100% ethanol, then infiltrated and embedded in Polybed 812 epoxy resin (Polysciences). Ultrathin (60-nm) sections were stained with 2% uranyl acetate, followed by 1% lead citrate. Sections were photographed by using a JEOL JEM 1210 transmission electron microscope.
ES Cell Transplantation. The experiments were performed in male Lewis rats with an initial body weight of ≈250 g. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996), and the protocol was approved by the University of Pittsburgh Institutional Animal Care Committee. Twelve Lewis rats were used as heart donors for cardiac transplantation. After induction of general anesthesia with inhaled isofluorane, a median sternotomy was performed under sterile conditions. All donor rats received heparin (1,000 units/kg) injected into the inferior vena cava (IVC) before heart harvest. After having been arrested with cold University of Wisconsin solution injected into the IVC, the donor heart was harvested, and myocardial infarction was induced by ligation of the left coronary artery by 8-0 silk.
Twelve other Lewis rats were used as heart recipients. After induction of general anesthesia with inhaled isofluorane, a laparotomy was performed under sterile conditions. The donor heart was heterotopically transplanted into the abdomen of recipient rats. The donor aorta was sutured to the abdominal aorta of the recipient, and the donor pulmonary artery was connected to the inferior vena cava of the recipient rat. Cell transplantation was performed within 30 min after developing myocardial infarction. In eight Lewis rat recipients, cell suspension (30 μl) was injected into three sites, one within the infarct area and two in the myocardium bordering the ischemic area. Each injection consisted of 10 μl of the medium, which contained beating ES cells (104 cells) for five of the rats or undifferentiated ES cells (104 cells) for three of the rats. The remaining four Lewis rats received the same operation but were injected only with the equivalent volume of the cell-free medium. After transplantation, recipient animals received a 7-day course of cyclosporine (Sandoz Pharmaceutical) at a dosage of 5 mg/kg per day i.m. Transplanted hearts were harvested for histological study at D14 after death of the animals by CO2 narcosis.
For further details, see Supporting Text and Figs. 9 and 10, which are published as supporting information on the PNAS web site.
Results
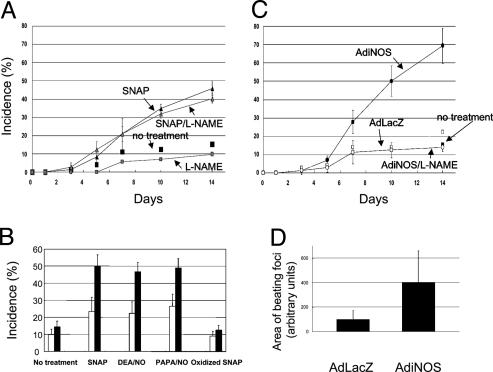
Spontaneous cardiac differentiation of mouse ES cells in vitro was assessed as the presence of rhythmically beating EB outgrowths. To determine the effects of NO on differentiation, some cultures were supplemented with 200 μM exogenous NO donor SNAP at plating on D0, and other cultures were transduced with AdiNOS at a multiplicity of infection of 25 1 day before plating (D-1). Transfection efficiency was determined to be 25–35% by immunocytochemistry using anti-iNOS antibody (data not shown). EB outgrowths were counted, whether or not they contained beating foci, by microscopy on D3, D5, D7, D10, and D14 after plating. As shown in Fig. 1A, SNAP exposure increased the percentage of beating EB outgrowths to 45% as compared with 15% in control cells at D14. Consistent with previous reports (9), the nonspecific NOS inhibitor l-nitroarginine methyl ester (l-NAME) decreased the incidence of beating foci compared with control cells (Fig. 1 A). To confirm that this increase in contracting EBs is NO-mediated, other NO donors, including 2-(N, N-diethylamino)-diazenolate-2-oxide/NO and (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)amino]-diazen-1-ium-1,2-diolate/NO, were also tested. As shown in Fig. 1B, all of the NO donors increased the incidence of beating foci compared with control cells. In addition, oxidized SNAP failed to increase in contracting EBs. AdiNOS transfection significantly increased the incidence of contracting EBs to 69%, and this was prevented by l-NAME (1 mM) (Fig. 1C). Overexpression of iNOS also increased the cluster size of beating cells in EB outgrowths 4-fold compared with cells transfected with a control adenovirus expressing β-galactosidase (AdLacZ) (Fig. 1D). All EB outgrowths analyzed showed a similar developmental pattern, with contracting cells first appearing at the periphery in the EB outgrowths.
Fig. 1.
Induction of cardiac differentiation of mouse ES cells by NO exposure through exogenous NO donor (A and B) or iNOS transduction (C and D). EBs were plated into four 24-well plates with one EB per well. (A and C) The numbers of wells that contain spontaneous contracting EB outgrowths were counted under a microscope at indicated time points. The results were plotted as percentages of the total counted wells (96 wells) and shown as mean ± SD of three separate sets of experiments. (B) To confirm that the increase in contracting EBs is NO-mediated, other NO donors, 2-(N,N-diethylamino)-diazenolate-2-oxide/NO (DEA/NO), (Z)-1-[N-(3-ammoniopropyl)-N-(n-propyl)amino]diazen-1-ium-1,2-diolate/NO (PAPA/NO), and oxidized SNAP were used. The open column is on D7 and the solid column on D14. The results were shown as mean ± SD of three separate sets of experiments. (D) The cluster sizes of beating cells in EB outgrowths were measured by using nih image software. Ninety-six EBs were photographed, and areas of beating foci in EB outgrowths were measured. The results were shown as mean ± SD of three separate sets of experiments. l-NAME, l-nitroarginine methyl ester.
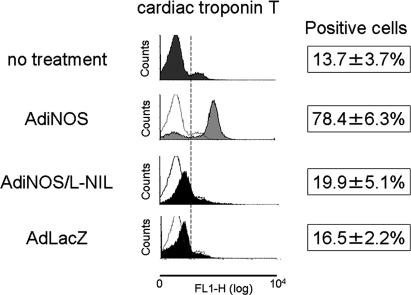
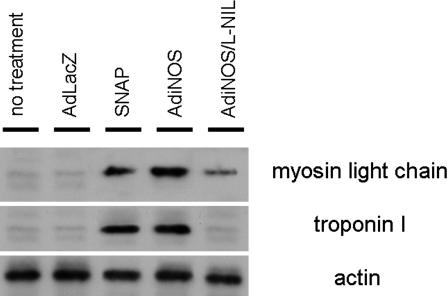
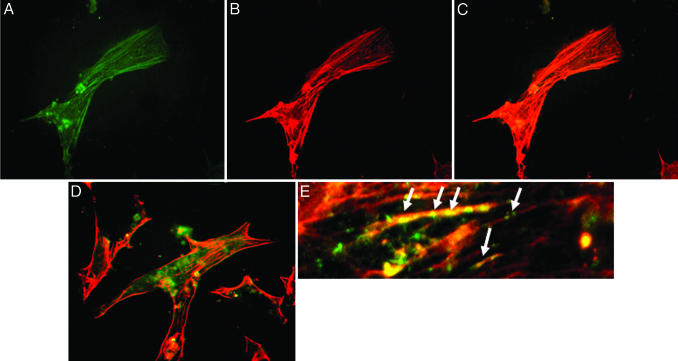
To establish the phenotypic characteristics of the EB outgrowths, the expression of cardiac-specific proteins was assessed at D14 by FCM analysis. EB outgrowths were dispersed by enzymatic digestion, fixed, and permeabilized for FCM analysis. Control vector AdLacZ-transfected cells showed no significant changes in the number of cardiac troponin T-positive cells (AdLacZ, 16.5 ± 2.2% vs. no treatment, 13.7 ± 3.7%) (Fig. 2). In contrast, AdiNOS-transfected cells showed a 6-fold increase in the number of troponin T-positive cells (78.4 ± 6.3%, P < 0.001) (Fig. 2). The iNOS-induced increase in troponin T was prevented by 20 μM the iNOS-selective inhibitor l-N-iminoethyllysine. Exposure to NO through the NO donor SNAP or via iNOS-transfection also significantly up-regulated the expression of myosin light chain and cardiac troponin I, compared with untreated cells and AdLacZ-transfected cells (Fig. 3). Immunocytochemistry revealed that AdiNOS-treated cells expressed cardiac troponin T and troponin I colocalizing with F-actin (Fig. 4). Taken together, these data indicate that NO-differentiated cells exhibit characteristics consistent with CM.
Fig. 2.
FCM analysis of single-cell preparation of EB outgrowths. EB outgrowths were enzymatically digested at D14. Single-cell suspension was stained with the antibody specific for cardiac troponin T and analyzed by flow cytometry. l-NIL, l-n-iminoethyllysine.
Fig. 3.
Western blot analysis of cardiac myosin light chain and cardiac troponin I. EBs were cultured with indicated treatment and harvested at D14, and immunoblot analysis was carried out with the indicated antibody. Actin staining of stripped blots was used as a control. l-NIL, l-n-iminoethyllysine.
Fig. 4.
Immunochemical characteristics of NO-exposed ES-derived CM. Immunostaining with antibody against cardiac troponin T (A) and actin (B). Shown is colocalization of troponin T and actin (C). Double staining of cardiac troponin I and actin (D and E at high magnification). Arrows indicate colocalization of actin and troponin I.
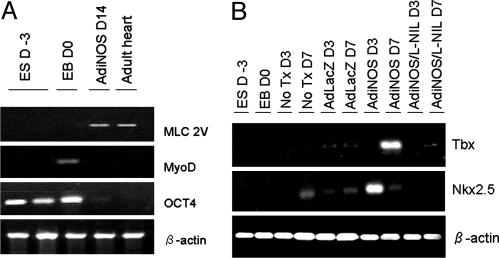
The differentiation status of the NO-exposed cells was further assessed by examining mRNA levels for cardiac-specific genes by RT-PCR (Fig. 5A). Undifferentiated ES cells were shown to express the pluripotency marker OCT4 at D-3. In contrast, AdiNOS-treated cells expressed much lower OCT4 mRNA levels. The cardiac-specific gene ventricular myosin light chain 2V was clearly detected at D14. EBs at D0 were also shown to express the skeletal muscle differentiation marker MyoD, which was not detected in either AdiNOS-treated cells or adult mouse heart samples. As shown in Fig. 5B, mRNA levels for the cardiac-specific transcription factor Nkx2.5 were up-regulated in AdiNOS-treated cells at D3. This declined to basal levels at D7. Tbx, another cardiac transcription factor, was clearly up-regulated at D7 in AdiNOS-treated cells.
Fig. 5.
NO exposure induced cardiac gene expression in ES cells. Cells were transfected with indicated adenovirus with or without iNOS inhibitor for indicated time periods. Semiquanititative RT-PCR was performed with the indicated specific primers. Figures are representative of three separate sets of experiments.
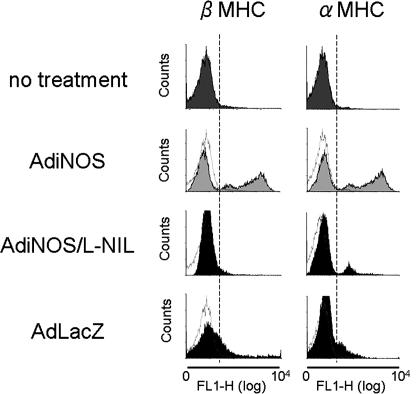
These data demonstrate that NO-induced contracting cells derived from ES cells possess characteristics of CM. To determine the developmental status of these contracting cells, FCM analysis was performed by using two different anti-MHC antibodies, including anti-α (adult type) MHC and anti-β (fetal type) MHC (Fig. 6). When treated with AdiNOS, cells exhibited 43.9% positive staining for β-MHC and 47.9% positive for α-MHC. In contrast, untreated cells exhibited only 4.8% positive staining for β-MHC and 3% positive staining for α-MHC. Thus, the D14 NO-differentiated cells represent a heterogeneous population of cells with both adult- and fetal-like characteristics.
Fig. 6.
FCM analysis of single cell preparation of EB outgrowths. EB outgrowths were enzymatically digested at D14. Single-cell suspension was stained with the antibodies specific for cardiac α- or β-MHC. l-NIL, l-n-iminoethyllysine; FL1-H, the levels of fluorescence.
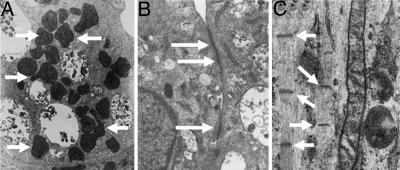
To determine the morphology of the contracting cells in the EB outgrowths, transmission electron microscopy was performed (Fig. 7). The NO-differentiated cells were shown to contain numerous mitochondria, and structures similar to adherens junctions were present between adjacent cells. The cells also displayed myosin filaments as characterized by Z-lines (Fig. 7C).
Fig. 7.
Ultrastructural analysis of NO-exposed ES-derived CM. (A) Transmission electron micrograph (TEM) showing the presence of numerous mitochondria (arrows). (B) Adherens junction-like structures observed between adjacent cells (arrows). (C) TEM demonstrating the presence of myosin filaments with Z-lines (arrows).
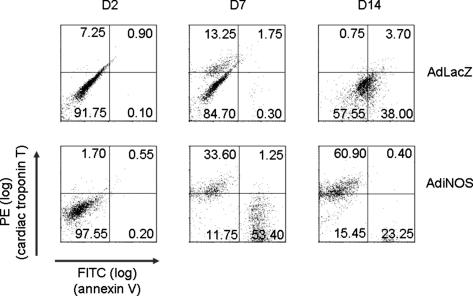
We hypothesized that NO would promote cardiac differentiation through a dual mechanism involving not only the phenotypic shift but also a selection pressure through the induction of apoptosis in undifferentiated cells. FCM analysis using phycoerythrin-labeled anti-cardiac troponin T was performed. Induction of apoptosis was concurrently measured by using FITC-labeled anti-annexin V. At D2, neither cardiac differentiation nor apoptosis was detected in iNOS-treated or control LacZ-treated ES cells. By D7, however, cardiac troponin T was expressed in 45.6 ± 20.6% (n = 3) of the NO-treated cells but only 9.25 ± 4.77% of control cells (Fig. 8). Interestingly, 50.4 ± 18.4% of NO-treated ES cells were troponin T-negative and annexin V-positive, compared with <1% of the control ES cells. At D14, the majority of the control cells were troponin T-negative with 38% annexin V-positive. The iNOS-treated cells were >60% troponin T-positive, with 23% annexin V-positive. Thus, NO not only induces differentiation but also leads to apoptosis in undifferentiated cells.
Fig. 8.
FCM analysis of single cell preparation of EB outgrowths. EB were transfected with AdiNOS or AdLacZ, then enzymatically digested at the indicated time points. Single-cell suspension was stained with antibodies specific for cardiac troponin T and annexin V. PE, phycoerythrin.
Discussion
Based on the published observation that NO participates in normal cardiac differentiation, we undertook studies to determine whether exposure to NO via donors or iNOS gene transfer would promote the differentiation of ES cells to CM. Our data indicate that exposure to NO at D0 promotes the conversion of ES cells to CM, as indicated by contractile function, expression of cardiac-specific genes, and cardiac morphology both in vitro and in vivo (see Supporting Text and Figs. 9 and 10).
Bloch et al. (9) demonstrated that expression of iNOS is transiently observed during early rodent embryonic heart development and that iNOS expression is strongly down-regulated by parturition. We have mimicked this transient expression of iNOS in cultured ES cells using adenoviral transfection of human iNOS transgene or a brief exposure to an NO donor. Transient NO exposure markedly increased the incidence of self-contracting foci in the EB outgrowths (Fig. 1), the numbers of cells that express cardiac troponin T (Fig. 2), and the expression of cardiac-specific markers. It is known that the cardiac transcription factors Nkx2.5 and Tbx are expressed in the early stages of cardiac development and before the expression of contractile proteins (14). Indeed, Tbx and Nkx2.5 were detected earlier than myosin light chain 2V in our system (Fig. 5). Furthermore, FCM analysis using anti-α- and -β-MHC antibodies revealed that CM derived from NO-exposed ES cells were heterogeneous at D14, containing cells expressing either α- or β-MHC (Fig. 6). This heterogeneity is consistent with the known MHC transition from β to α isoform during cardiac development in vivo (15) and in vitro (16). Thus, NO exposure appears to induce a recapitulation of the normal sequence seen in development.
Methods of specific induction of CM from ES cells have not been previously developed, and molecular pathways leading to commitment to the cardiac lineage are not well understood. Antioxidants have been reported to inhibit cardiac differentiation, whereas reactive oxygen species (ROS) or prooxidants induce CM differentiation from ES cells (17). These data as well as ours indicate that cardiomyogenesis in ES cells might be regulated by the intracellular redox state. Recently Takahashi et al. (18) showed ascorbic acid also enhances cardiac differentiation. Interestingly, these investigators reported that ascorbic acid has no significant effect on cardiac differentiation when cardiac commitment has already been taken place, and that the effects may be independent from the antioxidant properties of ascorbic acid. In contrast, our group, along with Sauer et al. (17), used an EB-forming system to develop CM differentiation. ROS and NO clearly work in this system, even if cardiac commitment, as well as commitment to other cell lines, has occurred. In our case, the selection process appears to involve not only a phenotypic shift but also the variable susceptibilities of differentiated and undifferentiated cells to apoptosis by NO (Fig. 8). Non-CM-committed cells seem to be more sensitive to NO-induced apoptosis, whereas CM-committed cells are resistant to NO-induced apoptosis. Thus, NO not only up-regulates cardiac-specific genes but also may inhibit programmed cell death by down-regulating apoptotic signaling in CM-committed cells, as has been shown in other cell types (19, 20).
Conclusion
Our data demonstrate that NO markedly increases cardiac differentiation in mouse ES cells through positive and negative selection mechanisms. The exposure of ES cells to NO may be a useful method to efficiently produce CM from ES cells. These findings will hopefully lay the foundation for future application of these laboratory observations to bedside regenerative medicine for treating patients with ischemic heart disease.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants HL 066949 and GM 044100.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ES, embryonic stem; NOS, NO synthase; CM, cardiomyocytes; iNOS, inducible NOS; AdiNOS, adenovirus encoding human iNOS gene; AdLacZ, adenovirus encoding β-galactosidase gene; SNAP, S-nitroso-N-acetylpenicillamine; EB, embryoid body; FCM, flow cytometric; MHC, myosin heavy chain; Dn, day n.
References
- 1.Hassink, R. J., Brutel de la Riviere, A., Mummery, C. L. & Doevendans, P. A. (2003) J. Am. Coll. Cardiol. 41, 711–717. [DOI] [PubMed] [Google Scholar]
- 2.Dowell, J. D., Rubart, M., Pasumarthi, K. B., Soonpaa, M. H. & Field, L. J. (2003) Cardiovasc. Res. 58, 336–350. [DOI] [PubMed] [Google Scholar]
- 3.Boheler, K. R., Czyz, J., Tweedie, D., Yang, H. T., Anisimov, S. V. & Wobus, A. M. (2002) Circ. Res. 91, 189–201. [DOI] [PubMed] [Google Scholar]
- 4.Moncada, S. & Higgs A. (1993) N. Engl. J. Med. 329, 2002–2012. [DOI] [PubMed] [Google Scholar]
- 5.Moncada, S., Palmer, R. M. & Higgs, E. A. (1991) Pharmacol. Rev. 43, 109–142. [PubMed] [Google Scholar]
- 6.Cosby, K., Partovi, K. S., Crawford, J. H., Patel, R. P., Reiter, C. D., Martyr, S., Yang, B. K., Waclawiw, M. A., Zalos, G., Xu, X., et al. (2003) Nat. Med. 12, 1498–1505. [DOI] [PubMed] [Google Scholar]
- 7.Peunova, N. & Enikolopov, G. (1995) Nature 375, 68–73. [DOI] [PubMed] [Google Scholar]
- 8.Magrinat, G., Mason, S. N., Shami, P. J. & Weinberg, J. B. (1992) Blood 80, 1880–1884. [PubMed] [Google Scholar]
- 9.Bloch, W., Fleischmann, B. K., Lorke, D. E., Andressen, C., Hops, B., Hescheler, J. & Addicks, K. (1999) Cardiovasc. Res. 43, 675–684. [DOI] [PubMed] [Google Scholar]
- 10.Feng, Q., Song, W., Lu, X., Hamilton J. A., Lei, M., Peng, T. & Yee, S. P. (2002) Circulation 106, 873–879. [DOI] [PubMed] [Google Scholar]
- 11.Lee, T. C., Zhao, Y. D., Courtman, D. W. & Stewart, D. J. (2000) Circulation 101, 2345–2348. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaka, C., Tanaka, K., Abe, M. & Sato, Y. (1996) J. Cell. Physiol. 169, 522–531. [DOI] [PubMed] [Google Scholar]
- 13.Hidaka, K., Lee, J. K., Kim, H. S., Ihm, C. H., Iio, A., Ogawa, M., Nishikawa, S., Kodama, I. & Morisaki, T. (2003) FASEB J. 17, 740–742. [DOI] [PubMed] [Google Scholar]
- 14.Akazawa, H. & Komuro, I. (2003) Circ. Res. 92, 1079–1088. [DOI] [PubMed] [Google Scholar]
- 15.Morkin, E. (2000) Microsc. Res. Technol. 50, 522–531. [DOI] [PubMed] [Google Scholar]
- 16.Metzger, J. M., Lin, W. I., Johnston, R. A., Westfall, M. V. & Samuelson, L. C. (1995) Circ. Res. 76, 710–719. [DOI] [PubMed] [Google Scholar]
- 17.Sauer, H., Rahimi, G., Hescheler, J. & Wartenberg, M. (2000) FEBS Lett. 476, 218–223. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi, T., Lord, B., Schulze, P. C., Fryer, R. M., Sarang, S. S., Gullans, S. R. & Lee, R. T. (2003) Circulation 107, 1912–1916. [DOI] [PubMed] [Google Scholar]
- 19.Kim, Y. M., Talanian, R. V. & Billiar, T. R. (1997) J. Biol. Chem. 272, 31138–31148. [DOI] [PubMed] [Google Scholar]
- 20.Kim, Y. M., Kim, T. H., Seol, D. W., Talanian, R. V. & Billiar, T. R. (1998) J. Biol. Chem. 273, 31437–31441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.