Fatigue during whole body exercise influences the efficacy of the corticospinal pathway. Specifically, the augmented motoneuronal excitability typically observed with a given increase in muscle activation during cycling in the absence of fatigue is abolished during the same exercise performed in the presence of fatigue. This suggests that the previously observed unaltered corticospinal excitability from start of intense leg cycling to exhaustion is partly determined by motoneuronal inhibition/disfacilitation obscuring the facilitatory effects resulting from muscle activation.
Keywords: corticospinal pathway, electromyography, motor cortex, motor neuron
Abstract
Exercise-induced fatigue influences the excitability of the motor pathway during single-joint isometric contractions. This study sought to investigate the influence of fatigue on corticospinal excitability during cycling exercise. Eight men performed fatiguing constant-load (80% Wpeak; 241 ± 13 W) cycling to exhaustion during which the percent increase in quadriceps electromyography (ΔEMG; vastus lateralis and rectus femoris) was quantified. During a separate trial, subjects performed two brief (∼45 s) nonfatiguing cycling bouts (244 ± 15 and 331 ± 23W) individually chosen to match the ΔEMG across bouts to that observed during fatiguing cycling. Corticospinal excitability during exercise was quantified by transcranial magnetic, electric transmastoid, and femoral nerve stimulation to elicit motor-evoked potentials (MEP), cervicomedullary evoked potentials (CMEP), and M waves in the quadriceps. Peripheral and central fatigue were expressed as pre- to postexercise reductions in quadriceps twitch force (ΔQtw) and voluntary quadriceps activation (ΔVA). Whereas nonfatiguing cycling caused no measureable fatigue, fatiguing cycling resulted in significant peripheral (ΔQtw: 42 ± 6%) and central (ΔVA: 4 ± 1%) fatigue. During nonfatiguing cycling, the area of MEPs and CMEPs, normalized to M waves, similarly increased in the quadriceps (∼40%; P < 0.05). In contrast, there was no change in normalized MEPs or CMEPs during fatiguing cycling. As a consequence, the ratio of MEP to CMEP was unchanged during both trials (P > 0.5). Therefore, although increases in muscle activation promote corticospinal excitability via motoneuronal facilitation during nonfatiguing cycling, this effect is abolished during fatigue. We conclude that the unaltered excitability of the corticospinal pathway from start of intense cycling exercise to exhaustion is, in part, determined by inhibitory influences on spinal motoneurons obscuring the facilitating effects of muscle activation.
NEW & NOTEWORTHY
Fatigue during whole body exercise influences the efficacy of the corticospinal pathway. Specifically, the augmented motoneuronal excitability typically observed with a given increase in muscle activation during cycling in the absence of fatigue is abolished during the same exercise performed in the presence of fatigue. This suggests that the previously observed unaltered corticospinal excitability from start of intense leg cycling to exhaustion is partly determined by motoneuronal inhibition/disfacilitation obscuring the facilitatory effects resulting from muscle activation.
the transmission of descending drive from higher brain areas to evoke skeletal muscle contractions occurs predominately through the corticospinal motor pathway, including the motor cortex and spinal motoneurons. Maintaining or enhancing the functional integrity of these neuronal structures during physical activity is an important prerequisite for the central nervous system (CNS) to optimally activate the muscles required for a given task. Alterations in the efficacy of the corticospinal pathway to relay neural signals (i.e., changes in corticospinal excitability) have implications for the development of central fatigue (CNS-mediated reduction in neural drive to the muscle; Taylor et al. 2016) and can impact the control of voluntary movements (Klass et al. 2008; Martin et al. 2006b; Petersen et al. 2003). For example, a decrease in corticospinal excitability during exercise requires an increased input from higher brain areas to maintain a given power output (Di Lazzaro et al. 1998; Martin et al. 2006b; Mazzocchio et al. 1994). It is therefore important to identify factors that influence the integrity of this major motor pathway.
During nonfatiguing single-joint exercise, increases in muscle activation, estimated via electromyography (EMG; Bigland-Ritchie et al. 1983; Lepers et al. 2002), facilitate the excitability of both the motor cortex and motoneurons (Sidhu et al. 2009; Taylor et al. 1997; Todd et al. 2003; Weavil et al. 2015). This facilitatory effect on the motor pathway remains in the presence of peripheral fatigue (force loss accounted for by mechanisms distal to neuromuscular junction) (Levenez et al. 2008; McNeil et al. 2011a; Todd et al. 2003) as cortical and motoneuronal excitability progressively rises during fatiguing submaximal single-joint exercise characterized by progressive increases in EMG (Smith et al. 2007; Sogaard et al. 2006). However, when EMG is held constant during a low-intensity voluntary contraction, fatigue has been documented to compromise corticospinal excitability by suppressing the motoneuron pool (Martin et al. 2006b; McNeil et al. 2011a), an effect presumably mediated by an increase in the motoneuronal afterhyperpolarization period (Matthews 1999). Therefore, the facilitatory effect associated with an increase in muscle activation outweighs the disfacilitation, or inhibition, of motoneurons induced by fatigue and results in an overall increase in corticospinal excitability during fatiguing single-joint exercise (McNeil et al. 2011a).
Increases in muscle activation also have been documented to enhance the excitability of the corticospinal pathway during nonfatiguing whole body (cycling) exercise (Weavil et al. 2015). However, in contrast to the rise observed during fatiguing single-joint exercise, the excitability of the motor pathway remains unaltered during fatiguing cycling exercise to exhaustion (Sidhu et al. 2012a). Although the consequence of fatigue, per se (i.e., independent of the facilitating influence of muscle activation), on cortical and motoneuronal excitability during cycling exercise is largely unknown (Sidhu et al. 2014), the unaltered corticospinal excitability during fatiguing cycling exercise alludes to a disfacilitating, or inhibitory influence of fatigue. Specifically, a fatigue-related depression of cortical and/or motoneuronal excitability may counteract the previously documented facilitation associated with the increase in muscle activation (Martin et al. 2006b; Martin et al. 2008b) with the net effect of an overall unchanged corticospinal excitability during strenuous cycling exercise to exhaustion.
The purpose of this study was to investigate the excitability of the corticospinal pathway during fatiguing and nonfatiguing cycling exercise while considering the associated facilitatory influence of muscle activation (estimated via EMG). To quantify alterations in the overall excitability of the corticospinal pathway (including motor cortex and spinal motoneurons) during exercise, we evaluated changes in quadriceps motor-evoked potentials (MEP) triggered via transcranial magnetic stimulation (TMS) of the motor cortex (Goodall et al. 2009; Rothwell et al. 1991; Taylor et al. 1996; Todd et al. 2003). Electrical stimulation of the cervicomedullary junction (CMS) was utilized to directly activate corticospinal axons eliciting short-latency cervicomedullary evoked potentials (CMEP), which were monitored to evaluate the influence of exercise and fatigue on the excitability of spinal motoneurons (Taylor 2006; Taylor and Gandevia 2004). Alterations in the excitability of motor cortical output cells were quantified by tracking changes in the ratio of MEP to CMEP (Martin et al. 2008b). We hypothesized that for a given increase in muscle activation, corticospinal excitability would be diminished during fatiguing cycling compared with nonfatiguing cycling.
METHODS
Participants
Eight healthy, recreationally active men [age: 26 ± 2 yr, height: 182 ± 2 cm, weight: 77 ± 4 kg, maximal oxygen consumption (V̇o2max): 47 ± 5 ml·kg·min−1] participated in this study. Written informed consent was obtained from all participants before their inclusion in this study. The Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center approved this protocol.
Protocol
Participants completed 7 exercise sessions separated by at least 24 h. Each participant's seat height was kept consistent throughout the study, and their feet were securely fastened to the pedals. To minimize head movement, participants were centered in relation to a fixed mouthpiece while their arms rested on a custom-made frame during exercise. At the beginning of each exercise session, participants performed a standardized 5-min warm-up (1 W/kg body wt). The first visit (session 1) consisted of a maximal incremental exercise test (20 W + 25 W/min; Amann et al. 2006) on a cycle ergometer (VeloTron, Elite model; RacerMate, Seattle, WA) to determine peak power output (Wpeak: 301 ± 17 W) and V̇o2max. Wpeak was recorded as the workload during the last completed stage; V̇o2max was defined as the highest oxygen uptake during the maximal incremental exercise test.
During session 2, participants practiced fatiguing constant-load cycling exercise (fatiguing cycling; 80% Wpeak: 241 ± 13 W, cycling cadence: 80 rpm) to task failure (10% drop in cadence for >5 s). Vastus lateralis (VL) and rectus femoris (RF) EMG were measured throughout the exercise. MEP, CMEP, and maximal M waves (Mmax) were evoked 30 s into exercise and again at task failure. The percent increase in EMG (ΔEMG) was quantified using the difference in EMG obtained during the first and the last minute of exercise, and exercise-induced fatigue was quantified by neuromuscular assessments of fatigue. During session 3, participants repeated the same task and the same measurements were performed. This fatiguing cycling session was considered the experimental trial.
Sessions 4 and 5 were designed to verify that a given change in EMG could be consistently measured across days. On each of these 2 days, 8 participants performed cycling exercise at 50, 100, 150, and 200 W, while 5 participants also exercised at 300 and 400 W. Each of these 1-min stages was performed at a cadence of 80 rpm, and EMG in the VL and RF was assessed during the final 10 s. The placement of EMG electrodes was marked with indelible ink to ensure identical placement across days.
In session 6, brief (∼45 s) nonfatiguing cycling bouts were performed with the aim of matching the change in EMG (i.e., ΔEMG) observed during fatiguing cycling. The initial power output was determined by rounding to the nearest 50 W relative to each individuals' power output used in fatiguing cycling. On the basis of preliminary work and results from the initial visits, power output was then increased in 50-W increments until a ΔEMG similar to that in fatiguing cycling was identified. For the seventh session, participants performed the two nonfatiguing cycling bouts identified in session 6 (∼45 s, 244 ± 15 and 331 ± 23 W), and corticospinal excitability was measured (via M waves, MEPs, and CMEPs) during exercise. These bouts were randomized and performed in duplicate (reporting the mean motor cortical and motoneuronal excitability between trials). To prevent any carryover effect and the development of fatigue, a 5-min rest was provided between cycling bouts. Neuromuscular function was assessed after nonfatiguing cycling to determine if exercise-induced fatigue occurred.
To quantify corticospinal alterations during experimental sessions 3 and 7, participants received a stimulation set consisting of three TMS, one CMS, and one peripheral nerve stimulation (PNS). The order of stimulation type was randomized and separated by at least eight pedal revolutions. Crank angle was monitored continuously, and all stimulations were elicited, without interrupting cycling, at 45°Clockwise (a knee angle of ∼100°) relative to top dead center to evoke a response during the peak cycling EMG burst of the VL (Sidhu et al. 2012b). To quantify fatigue, maximum voluntary contractions (MCV) and evoked twitches were performed before and after sessions 3 and 7.
Instrumentation
EMG.
The EMG signals were recorded by surface electrodes (Ag-AgCl, 10-mm diameter, 5-cm interelectrode distance) placed on the muscle belly of the RF and VL in a bipolar configuration. Before electrode placement, the skin was lightly abraded with fine sandpaper and cleaned with an alcohol swab. EMG signals were amplified 1,000 times (NeuroLog Systems; Digitimer, Welwyn Garden City, UK), bandpass filtered (20–1,000 Hz; NL-844, Digitimer), and analog-to-digitally converted at a sampling rate of 2,000 Hz using a 16-bit Micro1401 mkII data acquisition unit and Spike2 data collection software (Cambridge Electronic Design, Cambridge, UK) running custom-written scripts.
PNS.
The peripheral motor nerve was stimulated with the anode placed between the greater trochanter and the iliac crest and with the cathode placed over the femoral nerve in the femoral triangle. Optimal position (i.e., greatest twitch force) for the stimulating electrode was determined by delivering low-intensity single-pulse stimuli (200-μs pulse width; 100–150 mA) via a movable cathode probe and a constant-voltage stimulator (model DS7AH; Digitimer). Once the optimal position was located, the cathode electrode was fixed and remained in this position until all experiments were completed. Thereafter, stimulation intensity was increased by 20-mA increments until the size of the evoked twitch and compound muscle action potential (M wave) demonstrated no further increase (i.e., maximal M wave, Mmax) at rest and during 50% MVC. Stimulation intensity was set at 130% of Mmax intensity (group mean intensity: 342 ± 21 mA) and kept constant throughout the testing session. Mmax intensity was confirmed during warm-up cycling and adjusted, if necessary, to ensure a supramaximal stimulation (353 ± 22 mA).
CMS.
The corticospinal pathway was electrically activated (100-μs pulse width; D185-Mark IIA MultiPulse stimulator; Digitimer) at the cervicomedullary junction to evoke CMEPs. Self-adhesive electrodes were placed in the grooves behind the mastoid processes with the cathode placed on the left side to stimulate the contralateral leg (Ugawa et al. 1991). For dynamic cycling, simulator intensity (326 ± 24 V) was set to achieve a CMEP of ∼20% Mmax during a 20% MVC quadriceps contraction to provide room for either an increase or decline in corticospinal responses during cycling.
TMS.
Magnetic brain stimulation was delivered over the vertex of the motor cortex (left hemisphere, ∼2–3 cm lateral of the vertex) with the use of a concave double-cone coil (Magstim 200; Magstim, Whitland, UK) to elicit MEPs in the quadriceps. Optimal positioning of the TMS coil was determined before the experiment and marked on the scalp for accurate placement throughout the study. The stimulator intensity (39 ± 4%) was set to evoke MEPs of similar size to the CMEP during a 20% MVC quadriceps contraction to test a similar portion of the motoneuronal pool. This intensity was used to measure changes in corticospinal excitability during all cycling exercise.
Neuromuscular quadriceps function and force acquisition.
To examine exercise-induced fatigue, neuromuscular quadriceps function of the dominant leg was assessed before and immediately (36 ± 2 s) after exercise. Participants were seated upright in a custom-built chair with their hip and knee flexed at 120° and 90°, respectively. A noncompliant cuff was attached to a calibrated load cell (MLP 300; Transducer Techniques, Temecula, CA) 2–3 cm superior to the right lateral malleolus. Cuff position was marked on the ankle to maintain placement within a session. The assessment of quadriceps function included three MVCs, over which a superimposed twitch (SIT) was elicited by PNS, followed by another PNS at rest to evoke a resting potentiated quadriceps twitch force (Qtw). The average SIT and Qtw were used for the calculation of voluntary quadriceps activation (VA) as previously described (Merton 1954): VA = [1 − (SIT/Qtw)] × 100. Peripheral and central fatigue were quantified as the percent reduction in Qtw and VA, respectively, from before to within 30 s after exercise.
Data Analysis
All data were stored and analyzed offline using Spike2 data acquisition software. The averaged peak-to-peak amplitude and area of evoked responses (MEP, CMEP, and Mmax) were measured. Because the results for both area and peak-to-peak amplitude of MEP, CMEP, and Mmax were similar, the presentation of the results is limited to area (Martin et al. 2008b). To account for potential changes within the muscle, MEPs and CMEPs were normalized to Mmax. To isolate alterations in the excitability of the motor cortex, we normalized MEP to CMEP (Martin et al. 2008b). For background cycling EMG analysis, 10 s of rectified EMG waveforms, collected before any stimulations, were overlaid and then averaged around the same centering point (i.e., 45° clockwise from top dead center) used for eliciting stimulations. EMG was averaged over a 100-ms period, i.e., 50 ms before and 50 ms after the centering point (Weavil et al. 2015) to provide the average cycling EMG occurring at the stimulation. Simple linear regression analyses were conducted to explain the relationship between EMG (VL and RF) and workload (sessions 4 and 5, nonfatiguing trials) and exercise time (session 3, fatiguing trial). All data are means ± SE.
Statistical Analysis
A one-way ANOVA with a priori planned comparisons was performed to determine if there was a significant effect of fatiguing and nonfatiguing cycling on the neuromuscular variables (i.e., Mmax, MEP, CMEP, MEP/CMEP). A priori planned comparisons were conducted across 1) the start of fatigue cycling and task failure and 2) the lower and higher workload during nonfatiguing cycling. In addition, a Student's paired t-test was performed to compare pre- and postexercise Qtw and VA, and a Pearson product-moment correlation coefficient was used to assess the mean ΔEMG between nonfatiguing cycling and fatiguing cycling. To examine the consistency of rectified mean EMG measurements across days, a two-way repeated-measures ANOVA (trial day × workload) was performed with a priori planned comparisons conducted within workloads. Alpha was set at 0.05. All statistical analyses were performed using SPSS version 17.0 for Windows (IBM, Chicago, IL).
RESULTS
Reproducibility of Electromyography and Relationship with Workload
VL EMG obtained during the brief cycling bouts was similar between days (Table 1). Furthermore, the percent increase in VL EMG from one workload to the next higher workload was similar between days (trial × workload main effect, P = 0.93): 50–100 W (100 ± 19% vs. 96 ± 17%), 100–150 W (32 ± 5% vs. 31 ± 5%), 150–200 W (18 ± 4% vs. 17 ± 5%), and 300–400 W (40 ± 10% vs. 37 ± 11%) between days 1 and 2, respectively. The relationship between workload and EMG was linear on both days (R2 > 0.96, P < 0.001). The calculated coefficients of variation reflect acceptable between-day variability of VL EMG: 10.9%, 7.0%, 3.7%, 5.8%, 4.2%, and 6.5% for 50, 100, 150, 200, 300, and 400 W, respectively.
Table 1.
Reproducibility of electromyography
| Electromyography, mV |
||||||
|---|---|---|---|---|---|---|
| Cycling Workload, W | n | Day 1 | Day 2 | P Value | CV | |
| Vastus lateralis | 50 | 8 | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.65 | 10.9 |
| 100 | 8 | 0.21 ± 0.03 | 0.22 ± 0.03 | 0.79 | 7.0 | |
| 150 | 8 | 0.27 ± 0.03 | 0.28 ± 0.03 | 0.72 | 3.7 | |
| 200 | 8 | 0.31 ± 0.05 | 0.33 ± 0.05 | 0.31 | 5.8 | |
| 300 | 5 | 0.59 ± 0.06 | 0.58 ± 0.07 | 0.27 | 4.2 | |
| 400 | 5 | 0.84 ± 0.10 | 0.81 ± 0.12 | 0.73 | 6.5 | |
| Rectus femoris | 50 | 8 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.98 | 7.4 |
| 100 | 8 | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.98 | 7.2 | |
| 150 | 8 | 0.17 ± 0.04 | 0.17 ± 0.03 | 0.79 | 5.5 | |
| 200 | 8 | 0.20 ± 0.06 | 0.22 ± 0.06 | 0.76 | 8.4 | |
| 300 | 5 | 0.39 ± 0.06 | 0.34 ± 0.04 | 0.27 | 9.5 | |
| 400 | 5 | 0.54 ± 0.09 | 0.53 ± 0.09 | 0.59 | 6.3 | |
Data are means ± SE.
CV, coefficient of variation between days.
Similarly, RF EMG obtained during each of the brief bouts was similar between days (Table 1). Furthermore, the percent increase in RF EMG from one workload to the next higher workload was similar between days (trial by workload main effect, P = 0.46): 50–100 W (91 ± 16% vs. 95 ± 14), 100–150 W (41 ± 4% vs. 36 ± 5%), 150–200 W (21 ± 5% vs. 28 ± 6%), and 300–400 W (44 ± 16% vs. 48 ± 17%) between days 1 and 2, respectively. The relationship between workload and EMG (VL and RF) was linear on both days (R2 > 0.94, P < 0.001).The calculated coefficients of variation reflect acceptable between-day variability of RF EMG: 7.4%, 7.2%, 5.5%, 8.4%, 9.5%, and 6.3% for 50, 100, 150, 200, 300, and 400 W, respectively.
Electromyography and Fatigue
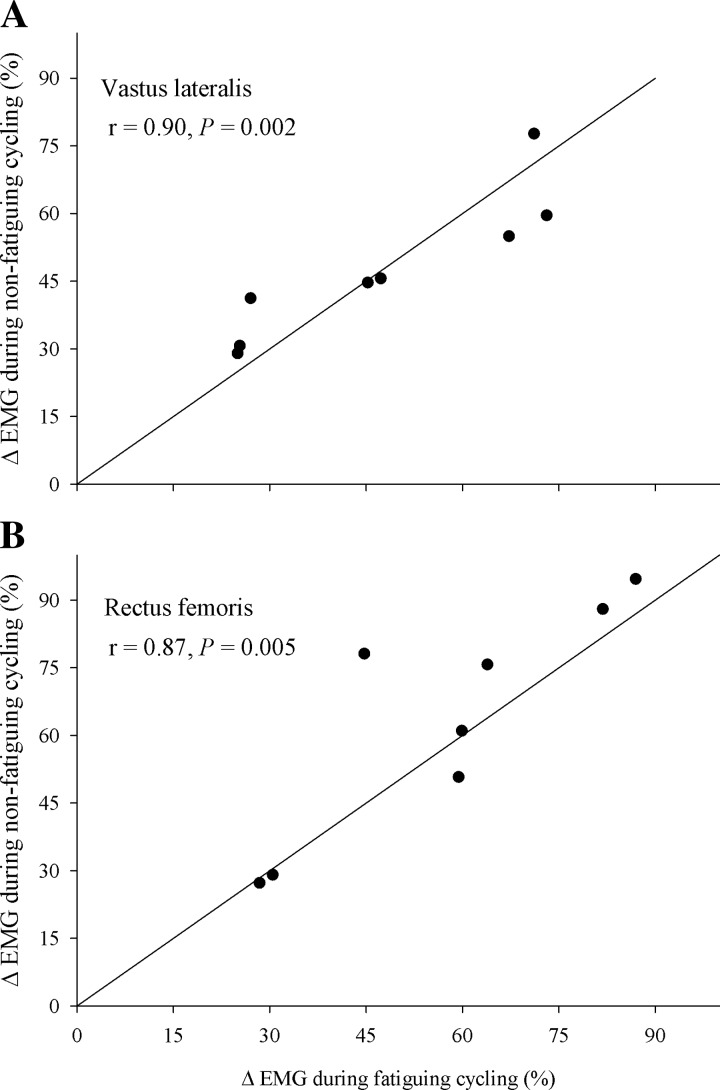
During nonfatiguing cycling, EMG increased from the low workload to the high workload in VL (48 ± 6%; P < 0.001) and RF (63 ± 9%; P < 0.001). During fatiguing cycling (time to task failure: 7.7 ± 0.5 min.), EMG increased from the start of exercise to exhaustion in both the VL (48 ± 7%; P < 0.01) and RF (57 ± 8%; P < 0.05). The relationship between endurance time and EMG (VL and RF) was linear (R2 > 0.98, P < 0.001). By design, the ΔEMG in VL obtained from nonfatiguing cycling and fatigue cycling was not different (P = 0.97) and positively correlated (r = 0.90, P < 0.01). Furthermore, the ΔEMG in RF obtained from nonfatiguing cycling and fatigue cycling was also not different (P = 0.61) and positively correlated (r = 0.87, P < 0.01; Fig. 1). Additionally, similar findings were revealed when EMG was normalized to Mmax obtained during exercise.
Fig. 1.
Identity plots showing the percent change in EMG (ΔEMG) from the start of fatiguing constant-load cycling exercise to exhaustion (241 ± 13 W; time: 7.7 ± 0.5 min) plotted against the change in vastus lateralis (A) and rectus femoris (B) EMG obtained by switching from a short-duration (∼45 s) nonfatiguing low workload (244 ± 15 W) to a nonfatiguing high workload (331 ± 23 W).
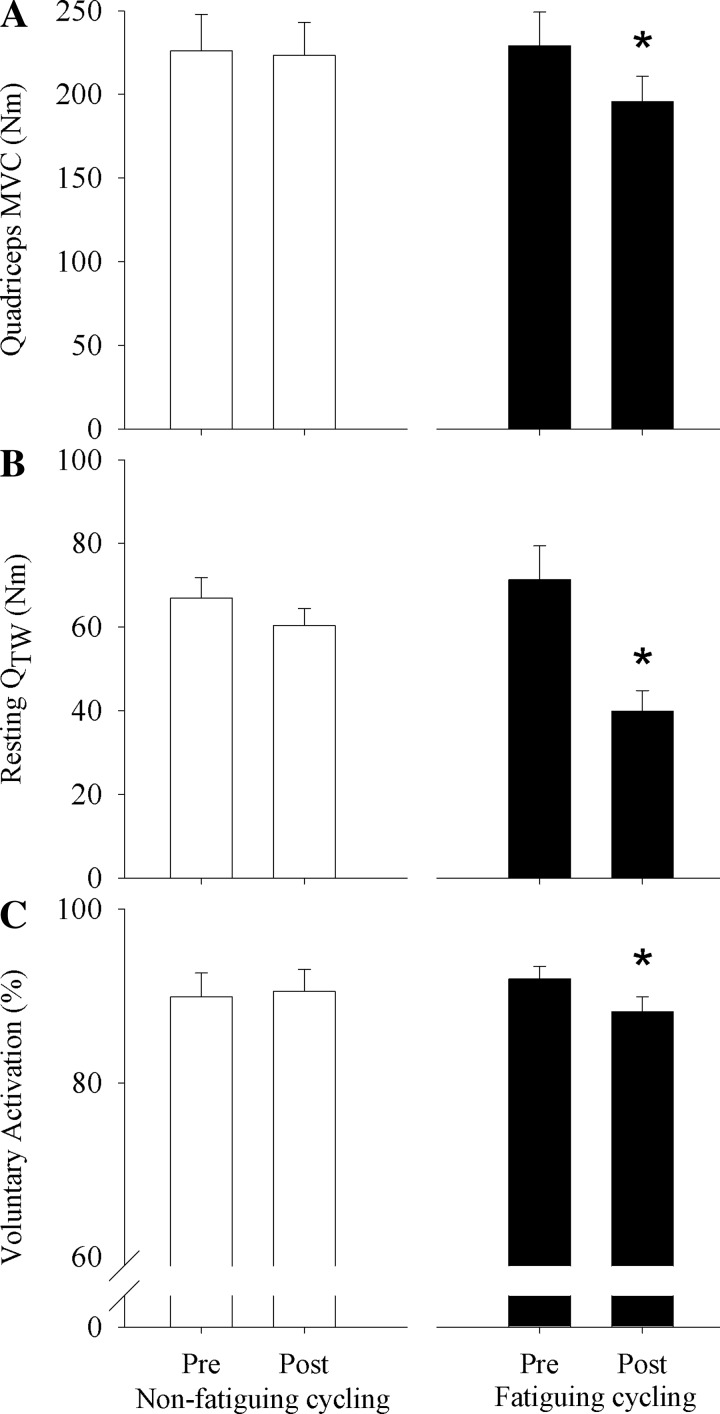
Nonfatiguing cycling did not result in a significant pre- to postexercise decrease in MVC (−2.6 ± 2.8 Nm), Qtw (−6.5 ± 2 Nm), or VA (−0.6 ± 0.5%). In contrast, fatiguing cycling caused a pre- to postexercise decrease (P < 0.05) in MVC (−13.7 ± 3.4%), Qtw (−42.6 ± 6.2%), and VA (−4.1 ± 1.2%; Fig. 2).
Fig. 2.
Central and peripheral fatigue induced by nonfatiguing (left) and fatiguing (right) cycling exercise. A–C show pre- and postexercise values (Pre and Post) of quadriceps MVC (A), potentiated quadriceps twitch force (QTW; B), and voluntary quadriceps activation (C). * P < 0.05, significant difference from Pre.
Mmax, MEP, and CMEP During Nonfatiguing Cycling and Fatiguing Cycling
Nonfatiguing cycling.
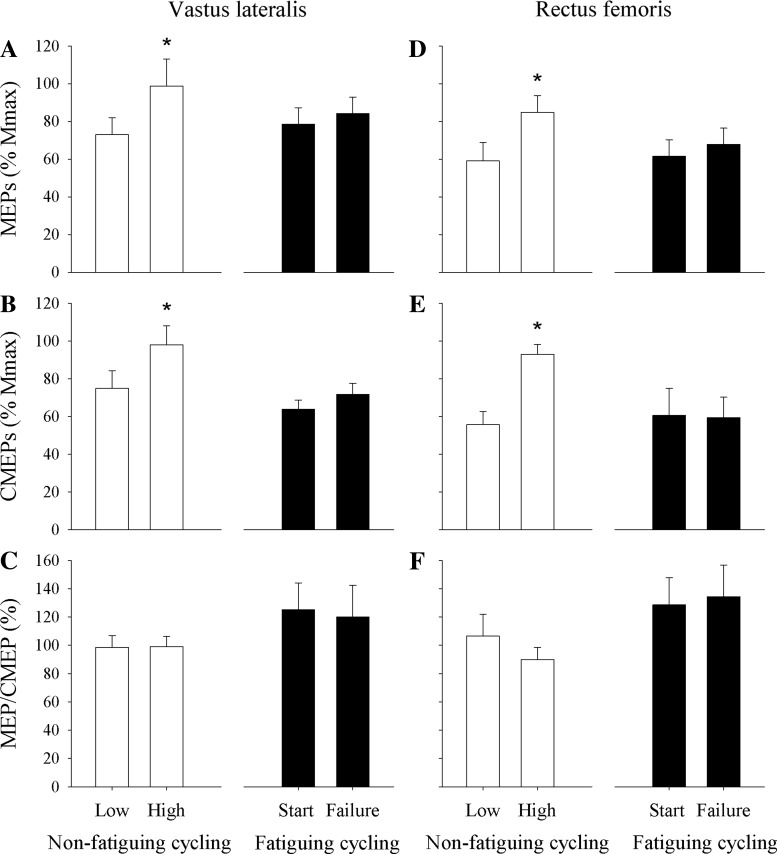
Mmax during the low-workload nonfatiguing cycling bout was similar to that during the high-workload nonfatiguing cycling bout in VL (40 ± 5 vs. 36 ± 4 μV·s, respectively; P = 0.15) and RF (50 ± 8 vs. 50 ± 7 μV·s, respectively; P = 0.92). Increasing EMG by ∼48% from the low to the high workload caused an increase in VL MEP (normalized to Mmax; P < 0.05) and VL CMEP (normalized to Mmax; P < 0.01). The MEP/CMEP ratio in VL did not change from the lower to the higher workload (P = 0.93). Similarly, increasing EMG ∼60% from the low to the high workload caused an increase in normalized RF MEP (P < 0.01) and RF CMEP (P < 0.05) but no change in MEP/CMEP (P = 0.26; Figs. 3 and 4).
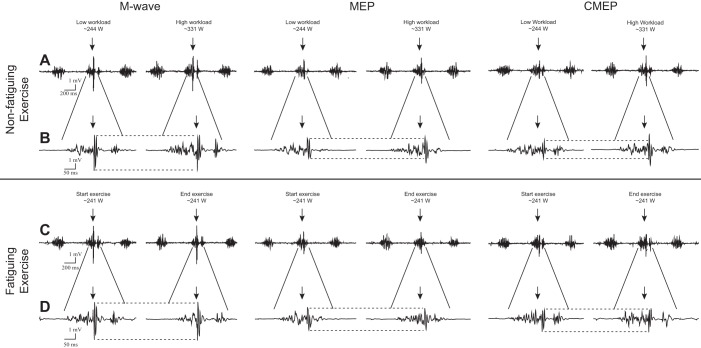
Fig. 3.
Representative raw electromyographic responses (M wave, MEP, and CMEP) in the vastus lateralis during the low- and high-workload bouts of the nonfatiguing cycling trial (A and B) and at the start and end of the fatiguing cycling trial (C and D). Arrows and dotted lines represent the point of stimulation and size of the initial response, respectively. B and D represent a ×4 magnification Y-axis view for individual responses depicted in A and C, respectively. Note that M waves were unchanged across each protocol. MEPs and CMEPs increased from the low to high workload in the nonfatiguing trial but remained unchanged during the fatiguing trial.
Fig. 4.
Changes in the excitability of the motor pathway (area of MEP normalized to Mmax; A and D), the motoneuron pool (CMEP normalized to Mmax; B and E), and the motor cortex (MEP/CMEP ratio; C and F) associated with an ∼50% increase in EMG occurring in the absence (nonfatiguing cycling) and presence (fatiguing cycling exercise) of significant central and peripheral fatigue. *P < 0.05, significant difference within trials.
Fatiguing cycling.
Mmax remained unaltered from the start of fatiguing cycling to task failure in VL (40 ± 6 vs. 41 ± 6 μV·s, respectively; P = 0.10) and RF (58 ± 13 vs. 63 ± 17 μV·s, respectively; P < 0.04). Furthermore, in the VL, normalized MEP (P = 0.27) and CMEP (P = 0.12) remained unaltered from start of fatiguing cycling to exhaustion. This was similar in the RF with both normalized MEP (P = 0.10) and CMEP (P = 0.90) remaining unaltered from start of fatiguing cycling to exhaustion (Fig. 4). Finally, MEP/CMEP remained similar throughout fatiguing in both the VL (P = 0.67) and RF (P = 0.81; Figs. 3 and 4).
DISCUSSION
The excitability of the corticospinal pathway depends on the balance between various excitatory and inhibitory processes (Gandevia 2001). The current experiments were designed to evaluate the impact of exercise-induced fatigue on the known facilitatory influence of muscle activation (estimated via EMG; Weavil et al. 2015) on the excitability of the corticospinal pathway during cycling exercise. Whereas evidence of a nearly 50% rise in muscle activation, which required an increase in workload of about 100 W, significantly facilitated the motor pathway during cycling in the absence of fatigue, corticospinal excitability was unaltered despite the same increase in muscle activation during exhaustive cycling characterized by significant central and peripheral fatigue. These findings suggest that fatigue during cycling exercise may suppress, or disfacilitate, the excitatory influence of increasing muscle activation on the corticospinal pathway.
An important assumption for the interpretation of the current findings is the sensitivity and reproducibility of EMG as a frequently used (e.g., Bigland-Ritchie et al. 1983; Lepers et al. 2002) estimate of muscle activation and/or neural input to the skeletal muscle. We conducted a set of experiments to demonstrate that the EMG assessment during cycling exercise was sensitive enough to identify changes associated with an increase in power output of 50 W. In addition, and importantly, both the increase in absolute units (mV) and the percent change in EMG from one workload to another were linear and similar between days, a critical necessity supporting our experimental design.
Corticospinal Excitability During Nonfatiguing Cycling Exercise
In agreement with previous studies based on isometric (Martin et al. 2008a; Taylor et al. 1997; Todd et al. 2003) and dynamic (Boorman et al. 1992; Weavil et al. 2015; Zehr et al. 2001) exercise, the increase in muscle activation (associated with the increase in exercise intensity from ∼240 W to ∼330 W) during brief nonfatiguing cycling significantly augmented corticospinal excitability (Figs. 3 and 4). Because the ∼40% increases in MEPs and CMEPs were similar, the overall facilitation of the corticospinal pathway may be attributed to an increased excitability of the motoneuronal pool (Gorassini et al. 1998; Martin et al. 2006a). Although the majority of previous findings have identified the spinal motoneuron as the primary site for the changes in corticospinal excitability (Martin et al. 2006a; McNeil et al. 2011a; Zehr et al. 2001), alterations within the motor cortex have also been observed (Mazzocchio et al. 1994).
Corticospinal Excitability During Fatiguing Cycling Exercise
The excitability of the corticospinal pathway during exhaustive cycling exercise characterized by a significant degree of neuromuscular fatigue remained unaltered throughout the trial (Figs. 3 and 4), a finding that confirms previous observations during constant-load locomotor exercise (Sidhu et al. 2012a). Importantly, although the unchanged cortical and spinal excitability during exhaustive cycling exercise may, at first sight, insinuate the absence of any effect of fatigue on the motor pathway projecting to knee extensors, these findings may also reflect the concurrent opposing action of both excitatory and disfacilitatory, or inhibitory, processes. Specifically, although the near 50% increase in muscle activation facilitated the excitability of the corticospinal pathway during nonfatiguing cycling, the same increase in muscle activation did not alter corticospinal excitability during cycling in the presence of fatigue (Figs. 3 and 4). The differential response to nonfatiguing and fatiguing cycling suggests that fatigue may suppress the facilitating influence of muscle activation on the corticospinal pathway. Because MEPs can be modulated at both the spinal and/or cortical level, whereas CMEPs are only modulated at a spinal level, the unchanged MEP/CMEP ratio during the exhaustive cycling bout suggests that cortical excitability is largely unaffected by fatigue during locomotor exercise. Consequently, the inhibitory influence of fatigue is likely exerted at the level of the motoneurons. This is in agreement with a recent study documenting a 20% reduction in the excitability of biceps brachii motoneurons in the presence of significant elbow flexor fatigue (McNeil et al. 2011a).
Several possible factors could contribute to the diminished motoneuronal excitability observed in the presence of significant locomotor muscle fatigue. For example, the repetitive activation of motoneurons can lead to an insufficient release, or depletion, of neurotransmitters (e.g., acetylcholine) from the synaptic vesicles and compromise synaptic efficacy (Otsuka et al. 1962; Redman and Silinsky 1994; Wu and Saggau 1997; Zucker and Regehr 2002), which has been suggested to account for some of the decrease in motoneuronal excitability during and immediately after exercise (Gandevia et al. 1999; Petersen et al. 2003). Additionally, studies including decerebrate cats have suggested that signaling from group III/IV muscle afferents can directly reduce the excitability of motoneurons in lower limb extensor muscles (Kaufman et al. 2002; Kniffki et al. 1981). Human studies utilizing postexercise cuff-occlusion techniques to activate nociceptive group III/IV muscle afferents confirm the diminishing effect of these sensory neurons on elbow extensor, but not flexor, motoneurons (Martin et al. 2006b). One potential mechanism of the muscle afferent-mediated neural depression is an increase in the afterhyperpolarization period (Kniffki et al. 1981; Schomburg et al. 1999), which reduces the likelihood for neuronal discharge (Matthews 1999). In contrast, no change (Windhorst et al. 1997) or even an enhancement (Martin et al. 2008b) in motoneuronal excitability has been reported in studies using intramuscular infusions of biochemical substances (e.g., bradykinin or serotonin) or hypertonic saline to activate group III/IV muscle afferents. This divergence in the literature may, at least in part, result from the activation of different subpopulations of group III/IV muscle afferents which preferentially respond to either noxious or nonnoxious metabolic/biochemical stimuli (Amann et al. 2014; Light et al. 2008). Regardless, the exact role of group III/IV muscle afferents in directly altering the excitability of motoneurons projecting to knee-extensor muscles during fatiguing exercise remains unclear.
Finally, feedback from large-diameter Ia (muscle spindle) afferents can facilitate the motoneuron pool (Biro et al. 2007; Hultborn et al. 1987; Macefield et al. 1993), particularly at the beginning of prolonged exercise (Macefield et al. 1991). However, group III/IV muscle afferent feedback, which is elevated during muscle contractions and particularly during fatigue (Adreani et al. 1997; Kaufman et al. 2002), has been suggested to presynaptically inhibit the Ia pathway during exercise (Rossi et al. 1999), an impact that potentially abolishes the proposed facilitatory effect. Indeed, a recent study showed that experimental increases in Ia feedback during single-joint exercise are incapable of preventing the fatigue-induced diminution of motoneuronal excitability (McNeil et al. 2011b) and questions the facilitating effect of Ia afferents on motoneuronal excitability during fatigue. These observations suggest that group III/IV muscle afferents may indirectly, via presynaptic inhibition of the Ia pathway, compromise motoneuronal excitability during exercise.
It should be considered that although EMG is frequently used as an estimate of motoneuronal output and muscle activation, its validity to reflect changes in neural drive from upstream of the motor cortex is limited. Specifically, exercise-induced decreases in corticospinal excitability (McNeil et al. 2011a), as present during fatigue (i.e., fatiguing cycling), require an increase in neural input to overcome this impairment and to ensure that muscle activation is adequate to sustain a given external workload (Martin et al. 2006b, 2008b; Mazzocchio et al. 1994). Given these circumstances, EMG potentially underestimated the magnitude of neural drive from upstream of the motor cortex at the end of fatiguing cycling. Consequently, despite a similar ΔEMG in the nonfatiguing and fatiguing cycling trial, the actual increase in neural drive might have been greater in the latter. Given the facilitating effect of neural drive on corticospinal excitability (Taylor et al. 1997; Weavil et al. 2015), this would suggest that the present findings likely underestimate the inhibitory effect of fatigue on corticospinal excitability.
Experimental Considerations
Changes in EMG can be determined by alterations in motor unit firing rates and/or the number of motor units recruited (Kukulka and Clamann 1981). Consequently, although ΔEMG in fatiguing and nonfatiguing cycling was matched, there may have been differences between these trials in terms of the neural strategy used to elicit the changes in EMG. However, during fatiguing muscle contractions, in which firing rates of active motor units actually decline, the recruitment of additional motor units is key to maintain a given force output and therefore the increase in EMG (Johnson et al. 2004). Furthermore, during nonfatiguing muscle contractions, increases in VL force output up to 80–95% of its maximal capacity are mainly achieved by increases in motor unit recruitment, whereas the modulation of firing rates has been suggested to play an ancillary role (De Luca and Contessa 2012; De Luca and Hostage 2010). Important in this context, during fatiguing cycling exercise at or near maximal aerobic capacity, the VL is only recruited up to about 50% of its maximal (task specific) capacity (Taylor and Bronks 1995). Consequently, the changes in EMG during both nonfatiguing and fatiguing cycling may have primarily been determined by changes in motor unit recruitment.
Although different factors have been identified to influence the bipolar EMG signal, amplitude cancellation is considered a key confounding variable because it may blunt the EMG signal at higher contraction intensities (Farina et al. 2004). This may threaten the linear relationship between workload and EMG, leading to an underestimation of muscle activation during exercise at higher intensities. However, as stated above, the contraction intensity of the quadriceps may only have been around 50% of its maximal capacity, suggesting that amplitude cancellation might have only played a minor role in either trial. Indeed, we have previously documented that the relationship between EMG and workload is linear during cycling at workloads up to ∼130% of V̇o2max (Weavil et al. 2015). Furthermore, the linear regression analyses based on VL/RF EMG and workload (nonfatiguing trial) and exercise time (fatiguing trial) suggest acceptable linearity in the present study. Amplitude cancellation may have therefore only resulted in minimal, if any, underestimation of muscle activation during the trials.
Conclusion
A given increase in muscle activation during nonfatiguing cycling exercise augments the excitability of the motoneuronal pool without affecting the motor cortex. This facilitatory effect is abolished, or counterbalanced, during cycling exercise in the presence of fatigue. Despite the disfacilitating, or inhibitory, effect of fatigue on motoneuronal excitability, this impact does not reduce the excitability of the corticospinal pathway to a level lower than that observed at the beginning of fatiguing cycling exercise. Consequently, the excitatory influence associated with the progressive increase in muscle activation during fatiguing constant-load cycling may prevent a reduction in corticospinal excitability as a result of fatigue and thereby maintain the efficacy of the motor pathway to relay neural drive from higher brain areas.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-103786, HL-116579, and HL-091830, Veterans Affairs Merit Grant E6910R, and Veterans Affairs SPiRE Grant E1572P.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.W., S.K.S., T.S.M., R.S.R., and M.A. conception and design of research; J.C.W., S.K.S., and T.S.M. performed experiments; J.C.W. analyzed data; J.C.W. and M.A. interpreted results of experiments; J.C.W. prepared figures; J.C.W. drafted manuscript; J.C.W., S.K.S., T.S.M., R.S.R., and M.A. edited and revised manuscript; J.C.W., S.K.S., T.S.M., R.S.R., and M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We express gratitude to the individuals who volunteered to participate in this study.
REFERENCES
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol 575: 937–952, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188: 19–23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol 50: 313–324, 1983. [DOI] [PubMed] [Google Scholar]
- Biro A, Griffin L, Cafarelli E. Reflex gain of muscle spindle pathways during fatigue. Exp Brain Res 177: 157–166, 2007. [DOI] [PubMed] [Google Scholar]
- Boorman G, Becker WJ, Morrice BL, Lee RG. Modulation of the soleus H-reflex during pedalling in normal humans and in patients with spinal spasticity. J Neurol Neurosurg Psychiatry 55: 1150–1156, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104: 1034–1046, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol 508: 625–633, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol 96: 1486–1495, 2004. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol 521: 749–759, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Romer LM, Ross EZ. Voluntary activation of human knee extensors measured using transcranial magnetic stimulation. Exp Physiol 94: 995–1004, 2009. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389: 757–772, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KV, Edwards SC, Van Tongeren C, Bawa P. Properties of human motor units after prolonged activity at a constant firing rate. Exp Brain Res 154: 479–487, 2004. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG, Adreani CM, Pickar JG. Discharge properties of group III and IV muscle afferents. Adv Exp Med Biol 508: 25–32, 2002. [DOI] [PubMed] [Google Scholar]
- Klass M, Levenez M, Enoka RM, Duchateau J. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99: 1096–1104, 2008. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H. Synaptic effects from chemically activated fine muscle afferents upon alpha-motoneurones in decerebrate and spinal cats. Brain Res 206: 361–370, 1981. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219: 45–55, 1981. [DOI] [PubMed] [Google Scholar]
- Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol 92: 1487–1493, 2002. [DOI] [PubMed] [Google Scholar]
- Levenez M, Garland SJ, Klass M, Duchateau J. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99: 554–563, 2008. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to alpha-motoneurones during sustained voluntary contractions. J Physiol 440: 497–512, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol 471: 429–443, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Butler JE, Gandevia SC, Taylor JL. Noninvasive stimulation of human corticospinal axons innervating leg muscles. J Neurophysiol 100: 1080–1086, 2008. [DOI] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol 95: 3512–3518, 2006a. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586: 1277–1289, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB. The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol 518: 867–882, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. J Physiol 474: 261–267, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589: 3533–3544, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Khan SI, Gandevia SC, Taylor JL. The reduction in human motoneurone responsiveness during muscle fatigue is not prevented by increased muscle spindle discharge. J Physiol 589: 3731–3738, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Endo M, Nonomura Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol 12: 573–584, 1962. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci 23: 7974–7980, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Silinsky EM. ATP released together with acetylcholine as the mediator of neuromuscular depression at frog motor nerve endings. J Physiol 477: 117–127, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Decchi B, Ginanneschi F. Presynaptic excitability changes of group Ia fibres to muscle nociceptive stimulation in humans. Brain Res 818: 12–22, 1999. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol 76: 159–200, 1991. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H, Kniffki KD. Contribution of group III and IV muscle afferents to multisensorial spinal motor control in cats. Neurosci Res 33: 195–206, 1999. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Bentley DJ, Carroll TJ. Cortical voluntary activation of the human knee extensors can be reliably estimated using transcranial magnetic stimulation. Muscle Nerve 39: 186–196, 2009. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Cresswell AG, Carroll TJ. Motor cortex excitability does not increase during sustained cycling exercise to volitional exhaustion. J Appl Physiol 113: 401–409, 2012a. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Hoffman BW, Cresswell AG, Carroll TJ. Corticospinal contributions to lower limb muscle activity during cycling in humans. J Neurophysiol 107: 306–314, 2012b. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M. Spinal mu-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592: 5011–5024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol 103: 560–568, 2007. [DOI] [PubMed] [Google Scholar]
- Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol 573: 511–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AD, Bronks R. Reproducibility and validity of the quadriceps muscle integrated electromyogram threshold during incremental cycle ergometry. Eur J Appl Physiol Occup Physiol 70: 252–257, 1995. [DOI] [PubMed] [Google Scholar]
- Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 16: 215–223, 2006. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res 117: 472–478, 1997. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol 490: 519–528, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol 96: 1496–1503, 2004. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol 551: 661–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol 29: 418–427, 1991. [DOI] [PubMed] [Google Scholar]
- Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol Regul Integr Comp Physiol 308: R998–R1007, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhorst U, Kirmayer D, Soibelman F, Misri A, Rose R. Effects of neurochemically excited group III–IV muscle afferents on motoneuron afterhyperpolarization. Neuroscience 76: 915–929, 1997. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 20: 204–212, 1997. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hesketh KL, Chua R. Differential regulation of cutaneous and H-reflexes during leg cycling in humans. J Neurophysiol 85: 1178–1184, 2001. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. [DOI] [PubMed] [Google Scholar]