We explored plantar sensitivity, lower limb cutaneous reflexes, and postural stability in healthy young and older adults. Plantar insensitivity and balance instability have been reported in older adults previously; however, these changes have not been measured in the same individuals. Furthermore, age-related changes in cutaneous reflexes have never been investigated. Rather than using nonspecific electrical stimulation, we used mechanical stimulation to evoke muscle responses, relying on natural cutaneous mechanotransduction.
Keywords: aging, cutaneous, reflex, psychophysics, balance
Abstract
Age-related changes in the density, morphology, and physiology of plantar cutaneous receptors negatively impact the quality and quantity of balance-relevant information arising from the foot soles. Plantar perceptual sensitivity declines with age and may predict postural instability; however, alteration in lower limb cutaneous reflex strength may also explain greater instability in older adults and has yet to be investigated. We replicated the age-related decline in sensitivity by assessing monofilament and vibrotactile (30 and 250 Hz) detection thresholds near the first metatarsal head bilaterally in healthy young and older adults. We additionally applied continuous 30- and 250-Hz vibration to drive mechanically evoked reflex responses in the tibialis anterior muscle, measured via surface electromyography. To investigate potential relationships between plantar sensitivity, cutaneous reflex strength, and postural stability, we performed posturography in subjects during quiet standing without vision. Anteroposterior and mediolateral postural stability decreased with age, and increases in postural sway amplitude and frequency were significantly correlated with increases in plantar detection thresholds. With 30-Hz vibration, cutaneous reflexes were observed in 95% of young adults but in only 53% of older adults, and reflex gain, coherence, and cumulant density at 30 Hz were lower in older adults. Reflexes were not observed with 250-Hz vibration, suggesting this high-frequency cutaneous input is filtered out by motoneurons innervating tibialis anterior. Our findings have important implications for assessing the risk of balance impairment in older adults.
NEW & NOTEWORTHY
We explored plantar sensitivity, lower limb cutaneous reflexes, and postural stability in healthy young and older adults. Plantar insensitivity and balance instability have been reported in older adults previously; however, these changes have not been measured in the same individuals. Furthermore, age-related changes in cutaneous reflexes have never been investigated. Rather than using nonspecific electrical stimulation, we used mechanical stimulation to evoke muscle responses, relying on natural cutaneous mechanotransduction.
normal adult aging leads to changes in mechanical properties of the skin, as well as cutaneous receptor density, morphology, and physiology. Researchers have observed age-related reductions in the innervation density of fast-adapting type I and II (FAI and FAII) afferent receptors (Bolton et al. 1966; Cauna and Mannan 1958), reduced cross-sectional area of FAI receptors (Iwasaki et al. 2003), decreased elasticity of the skin (Kenshalo 1986), and decreased nerve conduction (Pratt et al. 1979). There is also a well-known decline in plantar perceptual sensitivity that accompanies adult aging (for review see Shaffer and Harrison 2007). For example, older adults exhibit greater plantar vibrotactile detection thresholds at frequencies mediated by FAI and FAII afferents (Perry 2006; Wells et al. 2003). Wells et al. (2003) examined vibrotactile detection thresholds at 55 different skin sites on the foot sole, at 4 different frequencies (25, 50, 250, and 400 Hz), and demonstrated a significant increase in vibrotactile detection thresholds levels in older adults, particularly at frequencies mediated by FAII receptors (250 Hz and above). Perry (2006) further observed that this decline in plantar sensitivity becomes most pronounced after ∼70 years of age. Thus, it is commonly argued that age-related decline in cutaneous receptor function and density results in the diminished ability to perceive stimulation applied to the foot soles.
However, another important function of plantar sensory input is its ability to evoke lower limb muscle responses through spinal reflexes. Plantar cutaneous receptors are coupled synaptically indirectly [through interneuron(s)] onto motor neurons in the ventral portion of spinal cord (Fallon et al. 2005). The diminishing quality and quantity of plantar information with age likely leads to depressed lower limb cutaneous reflexes in older adults, a possibility that has yet to be investigated. In the present study, we use mechanical vibration of the foot sole to examine lower limb cutaneo-motor coupling strength in both young and older adults. Mechanical stimulation of cutaneous receptors evokes motor responses in tibialis anterior, gastrocnemius, soleus, and biceps femoris muscles (Duysens et al. 2008; Fallon et al. 2005; Forth and Layne 2007; Kavounoudias et al. 2001). Muscle responses in the upper limbs have also been observed from plantar stimulation (Bent and Lowrey 2013). A clear advantage of using controlled mechanical vibration of the skin to measure cutaneomotor coupling is that this stimulus relies on natural mechanotransduction in the underlying cutaneous afferent population. Mechanical stimulation has another benefit over nonspecific electrical stimulation, in that the former enables the experimenter to approximately target single afferent classes by manipulating vibration frequency (Bent and Strzalkowski 2014; Bolanowski et al. 1988; Johansson and Vallbo 1979; Johnson et al. 2000; Johnson 2001; Kennedy and Inglis 2002; Löfvenberg and Johansson 1984). Note that our goal is not to simulate tactile stimulation experienced during standing; sinusoidal vibrations at 30 and 250 Hz were chosen because they strongly stimulate FAI and FAII receptors, respectively, and serve as probes for eliciting cutaneous reflexes. We expected strong cutaneomotor coupling with 30-Hz vibration in particular because 1) FAI afferents innervate the plantar surface of the foot more densely than the other receptors classes (Kennedy and Inglis 2002), 2) previous lower limb cutaneous reflex studies using mechanical stimulation (Fallon et al. 2005) have argued that FA1 receptors are most strongly coupled to motor neurons (albeit indirect coupling through polysynaptic reflex arcs), and 3) based on microneurographic recordings in the hand (Johansson and Vallbo 1979; Johnson et al. 2000; Löfvenberg and Johansson 1984) and foot (Bent and Strzalkowski 2014), FAI afferents are most sensitive to vibrations near 30 Hz; therefore, this stimulus should be highly effective at driving a FAI population response that gets passed onto spinal motoneurons.
Age-related sensorineural deterioration of cutaneous afferents has been hypothesized as a key contributor to the increased risk of falling (Gryfe et al. 1977; Inglis et al. 2002; Richardson and Hurvitz 1995), as well as pathological gait and balance problems in older adults (Brocklehurst et al. 1966; Duncan et al. 1992; Lord et al. 1991, 1994; Melzer et al. 2004; Murray et al. 1969). There are well-documented age-related changes in postural stability as measured via quiet standing on a force plate, including increased sway amplitude, center of pressure (CoP) velocity, and whole body acceleration (Kouzaki et al. 2010, 2012; Masani et al. 2007; Prieto et al. 1996). The four classes of cutaneous afferent receptors covering the plantar surface of the foot provide an important source of sensory information for balance control due to their unique ability to encode shifts in the pressure distribution underfoot (Kennedy and Inglis 2002). Plantar cutaneous information potentially might drive unconscious stabilizing postural adjustments (Perry 2006; Watanabe and Okubo 1981). Supporting a functional relationship with standing balance control, complete and partial foot sole anesthesia in healthy young adults increases anteroposterior and mediolateral postural sway and also alters CoP velocity (Hämäläinen et al. 1992; Meyer et al. 2004).
Building on previous research from our laboratory (Wells et al. 2003), we examined plantar sensitivity and mechanically evoked cutaneous reflex strength in healthy young and older adults. Furthermore, to determine if plantar sensitivity or cutaneous reflex strength measures are related to postural stability, we measured shifts in the CoP while the same participants stood quietly on a force plate, and we extracted the root-mean-square (RMS) amplitude and mean power frequency (MPF) in the anteroposterior and mediolateral planes. A priori, we hypothesized that age-related deterioration of the cutaneous periphery reduces the quality and quantity of cutaneous sensory input entering the spinal cord, and therefore we predicted that increased vibrotactile thresholds would be observed in the older adults, as well as depressed cutaneous reflexes and larger, higher frequency CoP excursions, while standing. To the extent that postural stability relies on accurately perceiving information originating from the foot soles, we hypothesized that plantar sensitivity measures should provide a predictor of postural instability. However, cutaneous reflexes might also play a substantial role in balance control, as is suggested by the finding that patterned vibration applied to the foot soles while standing leads to unconsciously driven postural sway (e.g., vibrating both heels leads to unconscious forward body sway; Kavounoudias et al. 1998, 1999). Therefore, to the extent that postural stability relies on intact cutaneous reflexes, we hypothesized that measures of cutaneous reflex strength will also provide a predictor of postural instability. Specifically, we predicted that tactile detection thresholds would correlate positively with CoP RMS amplitude and MPF, and negatively with cutaneomotor coupling measures (gain, coherence, and cumulant density) in older adults. Finally, we predicted there would be a negative correlation between cutaneomotor coupling measures and CoP RMS amplitude and MPF.
METHODS
Participants.
Nineteen young adults (8 men, 11 women) between the ages of 19 and 40 yr (25.7 ± 5.5 yr, mean ± SD) and 22 older adults (9 men, 13 women) between the ages of 58 and 86 yr (68 ± 6.9 yr), with no known history of neurological disease or injury, participated in this study. Experimental protocols were explained to each participant, and written, informed consent was obtained. All procedures conformed to the standards of the World Medical Association Declaration of Helsinki and were approved by the University of British Columbia's Clinical Research Ethics Board.
Experimental setup and plantar skin testing site.
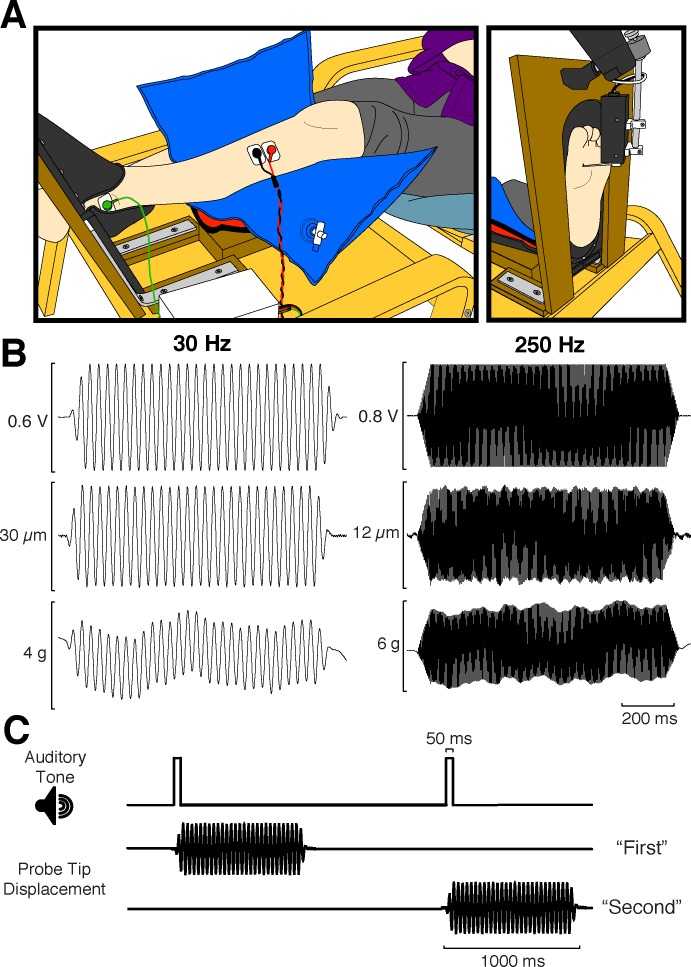
As depicted in Fig. 1A, participants were seated in a comfortable chair, with the leg being tested resting with the knee in an extended position in a custom-built leg support/footrest. Movement of the lower limb and foot was limited with this setup, which also provided unobstructed access to the foot sole for sensory and reflex testing. All sensitivity and reflex measurements were done first for one limb before switching to the other. The order in which we tested the left and right legs was randomized. A location on the medial side of the forefoot, 80% of the maximum width (measured across the ball of the foot) and 80% of the maximum length (measured from the tip of the great toe to the base of the heel), was used as the site for all sensory testing, as well as for the vibration applied during the cutaneous reflex protocol (see Fig. 1A). This corresponded approximately to the skin overtop of the first metatarsal head, a plantar skin region that was chosen because the skin properties at this location fall roughly in the middle of the spectrum in terms of skin hardness and thickness, with the heel being the hardest and thickest and the medial arch being the softest and thinnest (Strzalkowski et al. 2015). Additionally, tactile perception at the medial forefoot is reportedly more closely linked to balance function in older adults than other skin locations (Cruz-Almeida et al. 2014).
Fig. 1.
Experimental setup, vibratory stimuli, and 2IFC trial timeline. A: side schematic view of a participant's leg in the footrest/leg support setup (left) and front view of the exposed plantar surface of the foot, as well as the motor used for delivering vibrations (right). B: sample traces of 30-Hz (left) and 250-Hz vibrations (right). Top, voltage command sent to the motor; middle, probe tip displacement; bottom, contact force. C: timeline depicting the series of events that occurred on each 2IFC vibrotactile trial. The participant was presented with sequential 50-ms auditory tones denoting the 2 stimulus intervals. A 1-s vibration was delivered randomly during either the first or second interval, and the participant was required to determine which interval contained the stimulus.
Monofilament threshold estimation.
On each trial, a Semmes-Weinstein monofilament was applied perpendicularly to the skin surface. Monofilaments are precalibrated such that they bend and cease to indent the skin at a specific gram force. Monofilament application was ∼1–2 s in duration. Blindfolded participants were asked whether they felt the applied tactile stimulus. A 4-2-1–step staircase algorithm, as described previously (Dyck et al. 1993), was used to estimate pressure detection thresholds. Briefly, this staircase algorithm starts with 4-level steps in stimulus strength until a reversal point occurs (i.e., when the participant goes from responding “yes” to responding “no,” or vice versa), then switches to 2-level steps until the next reversal point, until finally settling into a 1-level step until 20 trials are given. The threshold value is taken as the mean of the 1-level step reversal points. To deter false-positive responses (as is typically done with monofilament testing), participants were specifically instructed to respond “yes” only when they were sure they had felt the stimulus and to respond “no” otherwise. To ensure participants were performing the task correctly, two to three catch trials were randomly intermixed into the testing block to confirm that the participants would respond “no” when asked if they felt any stimulation on their foot. This was done to avoid underestimation of monofilament thresholds due to participants saying “yes” even when they could not feel the stimulus. For single-interval trials such as these, catch trials are critical to ensure that the subject is performing the task correctly/honestly. Testing blocks were discounted if participants indicated sensation on more than one catch trial or reported sensation before stimulus application on multiple trials. After data were collapsed over legs and sessions, however, no participants were missing data for monofilament thresholds (see Statistical analysis).
Vibrotactile detection threshold estimation.
Sinusoidal signals (Fig. 1B) were generated using LabVIEW 10 (National Instruments) and sent as voltage values at 5 kHz via a PXI-6225 multifunctional data acquisition board (running with a PXI-8106 real-time controller in a PXI-1031 chassis; National Instruments) to a BNC 2090 output box (National Instruments). Analog voltage command signals were sent to a 300C ASI model dual-mode lever arm motor system (Aurora Scientific, Aurora, ON, Canada), which provided displacement and contact force feedback precise to 1 μm and 0.3 mN, respectively. The motor was held fixed in position on an articulating support arm (see Fig. 1A) attached to a rigid base that was decoupled from the footrest itself. The probe tip on the motorized lever arm had a diameter of 1.5 mm. Probe tip displacement, contact force, and voltage commands sent to the motor were all digitized at 5 kHz with the use of an analog-to-digital (A/D) board (Power 1401; Cambridge Electronic Design, Cambridge, UK) controlled through Spike2 (Cambridge Electronic Design) (Fig. 1C).
Participants performed a two-interval, two-alternative forced choice (2IFC) vibrotactile detection task. On each trial, participants were presented with two sequential auditory beeps demarking the two stimulus intervals and were asked to indicate whether they felt vibration following the first or second interval. Vibration was randomly delivered during either the first or second stimulus interval (Fig. 1C). Voltage commands were 1-s-long sinusoidal pulses, with 50-ms linear ramp-up and ramp-down envelops. Forty-trial blocks were completed at both 30 and 250 Hz, bilaterally. Contact force was maintained around 10 g for the entire duration of sensory testing; if the mean contact force throughout the vibratory stimulus was outside the range of 5–15 g, the trial was discounted. Trial to trial, the peak-to-peak amplitude of the voltage command was adjusted using a Bayesian adaptive procedure (Kontsevich and Tyler 1999). This psychophysical testing method efficiently estimated each participant's function, relating stimulus amplitude (in V) to his or her proportion of correct detections, and from this function a threshold level of performance can be extracted. To improve the robustness of the Bayesian procedure (Goldreich et al. 2009), we parameterized each participant's sigmoidal psychometric function online as a mixture model of the probability for a correct response by chance (0.5) given the participant had an attention lapse (δ/2) or given they were performing the task (modified Weibull function; see Tong et al. 2013), as follows:
| (1) |
and therefore,
| (2) |
where the γ parameter sets the y-intercept of the curve, the a parameter determines the lateral position of the curve along the x-axis, the b parameter determines the shape (slope) of the curve, and the δ parameter represents the lapse rate. The lapse rate term accounts for the realistic possibility of occasional attention lapses, resulting in 50% correct response probability, regardless of the peak-to-peak stimulus amplitude. The algorithm, which we programmed in LabVIEW (National Instruments), adaptively adjusted the peak-to-peak amplitude level from trial to trial, presenting the stimulus expected to yield the greatest information regarding the participant's psychometric function parameters (expected entropy minimization; Kontsevich and Tyler 1999). The γ parameter was held fixed at 0.5 for this experiment because this represents chance performance; thus the Bayesian adaptive procedure made hypotheses only on the possible a, b, and δ parameters of each participant's psychometric function and returned the joint posterior probability distribution function (PDF) over these three parameters, along with the best-estimated psychometric function.
To compute vibrotactile detection thresholds (in μm, rather than V), we reanalyzed the data offline using the actual displacements given on each trial, as measured directly from the probe tip displacement feedback trace. This method of controlling voltage online but calculating displacement thresholds offline allowed us to account for trial-to-trial variability in the actual displacement delivered due to slight variations in the precise contact force profile and the probe tip indentation angle, as well as potential variation due to inter-individual differences in mechanical skin properties. Threshold values were defined as the peak-to-peak probe tip displacement (in μm) at which the participant could correctly detect the vibration with 75% probability (halfway between chance and perfect performance). To extract the 75% correct threshold, we marginalized each participant's joint posterior PDF over the δ parameter, plotted the best-fit psychometric function for each (a, b) pair, and interpolated to find the stimulus amplitude corresponding to 75% correct performance. We then averaged the 75% correct stimulus amplitude across the (a, b) posterior PDF and took this as the participant's threshold estimate.
We screened participants for their ability to perform the task adequately with the stimulus amplitudes delivered by assessing the probability that their performance could have resulted from pure guessing on each trial relative to the probability that it could be described by a psychometric function. We call the ratio of these two probabilities the guessing Bayes factor (GBF; Goldreich et al. 2009; Peters and Goldreich 2013), which we compute as
| (3) |
where ri refers to the participant's response (correct or incorrect) on the i-th trial, t is the total number of nondiscarded trials in the testing block, and P(Ψa,b,δ) is the prior probability density over the psychometric function characterized by parameters a, b, and δ. We chose a criterion value of GBF = 1 as the cutoff above which we considered a participant being forced to guess on a given testing block. We applied this criterion on a block-by-block basis; thus we discarded from the analysis threshold data from testing blocks where it was more likely at the end of the block that the participant responded entirely randomly. Three older adults could not perform any of the 250-Hz testing blocks as indicated by their GBF values; therefore, no threshold values were available for these participants, even after data were collapsed over legs and testing sessions (see Statistical analysis).
Mechanically evoked lower limb cutaneous reflexes.
After cutaneous sensitivity testing, we assessed cutaneomotor coupling by applying continuous vibration at 30 Hz, and then 250 Hz, for 5 min (separated by at least a 2-min break) while recording surface EMG from tibialis anterior (TA) ipsilateral to the vibrated foot. The peak-to-peak amplitude of the 30- and 250-Hz sinusoidal vibrations was 200 and 20 μm, respectively (∼20 times young adult threshold amplitudes for detecting vibration at each frequency). Surface electrode pads were placed ∼2 cm apart onto the shaved, alcohol wiped, and abraded skin above the belly of TA (see Fig. 1A). Pilot testing indicated that 30 Hz provided a much stronger muscle response; therefore, this stimulus frequency was always tested first, and it was examined in all participants. Muscle responses were much weaker at 250 Hz, if present at all, and we began including 250-Hz reflex trials in the protocol only after collecting data from the first 10 participants.
EMG was bandpass filtered between 30 and 10 kHz and amplified by 10K by a preamplifier (NL824; Digitimer) and amplifier (NL820A; Digitimer), and then digitized at 5 kHz by a 16-bit A/D board (Power 1401; Cambridge Electronic Design) using Spike2 software (Cambridge Electronic Design). EMG was digitally low-pass filtered (4th-order dual-pass Butterworth filter) at 1 kHz offline for all data analyses. Throughout the continuous vibration, participants maintained a constant dorsiflexion between 10 and 15% of their maximal voluntary contraction by using biofeedback of their EMG RMS amplitude displayed in real-time through Spike2 on a 46-in. flat-panel television positioned in front of them at a distance of 1.5 m. The contraction level was not controlled for the first three older adults tested; therefore, their reflex data were excluded from the analysis. Because of variations in contact force caused by limited dorsiflexion control by the participant, we opened up our acceptable range of forces to 5 to 50 g, the maximum the motor can tolerate before disengaging and resetting.
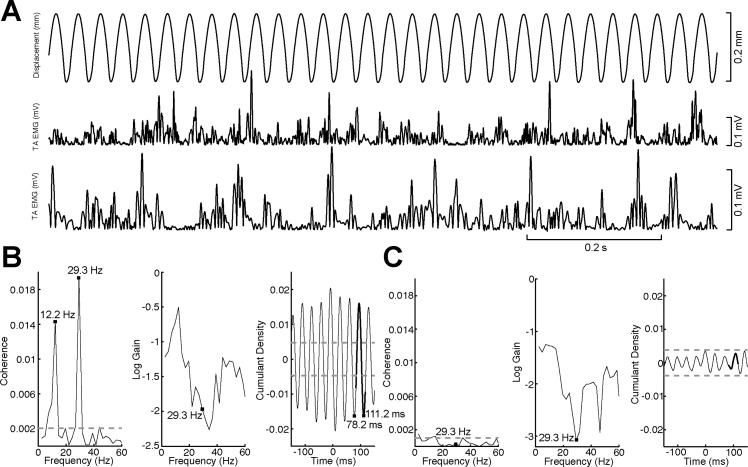
To characterize the spectral properties of the evoked cutaneous reflex response in TA in the frequency domain (gain and coherence) and the time domain (cumulant density), we used the NeuroSpec 2.0 toolkit (“sp1a.m” command) for MATLAB (The MathWorks) to estimate the linear spectrotemporal relationship between in the input (probe tip displacement) and the output (rectified EMG; see Halliday et al. 1995; Rosenberg et al. 1989). EMG was scaled into millivolts and displacement into millimeters. Three hundred-second continuous EMG and probe tip displacement traces were used in the spectral analysis (segment length = 0.4096 s; frequency resolution = 2.4414 Hz). Coherence, gain, and cumulant density estimates were considered significant, and included in further analysis, when coherence at the stimulus frequency (see below) exceeded the 95% confidence limit (see Fig. 3, dashed horizontal line). The 95% confidence limit for coherence spectra was estimated from the total segments per subject to distinguish frequencies that were significantly different from 0 (Halliday et al. 1995; Rosenberg et al. 1989).
Fig. 3.
Summary of cutaneous reflex data set. A: segment of the continuous time series data used in the spectral analysis. Top, probe tip displacement; middle and bottom, full-wave rectified EMG recorded from TA used in B and C. Participants held a 10–15% maximal voluntary contraction dorsiflexion of TA throughout the measurement. B: example from a young adult (22-yr-old female) with a strong reflex response at 30 Hz. Coherence (left), log gain (center), and cumulant density (right) were computed over the entire 600 s of concatenated data. Black squares in coherence and gain graphs denote the value that was extracted for the data analysis (29.3 Hz). The additional black square at 12.2 Hz denotes a commonly observed artifact resulting from the EMG driving changes in position due to small foot movements during the reflex protocol. Black squares in the cumulant density graph show that the oscillation is occurring at the vibratory stimulus frequency. C: example from an older adult (61-yr-old male) with nonsignificant coherence and cumulant density values calculated over 1,200 s of concatenated data; however, weak oscillations at ∼30 Hz still can be observed in the cumulant density graph. Bold black line segments overlaid on the cumulant density plots show the portion of data centered on t = 100 ms that was used in the data analysis.
In the frequency domain, we measured cutaneous reflex gain and coherence. To explore the frequency of the cutaneous reflexes evoked in TA between age groups, coherence estimates were derived. Coherence represents a measure of linear relationship between an input (i.e., mechanical tactile stimulation) and an output (i.e., muscle activity). For every frequency point, coherence varies from 0 (no linear relation) to 1 (linear system containing no noise). We extracted the coherence for the stimulus frequency bin containing the stimulus frequency applied (30 or 250 Hz). The gain function is a measure expressed in units of millivolts per millimeter and represents the magnitude of the output signal (EMG) relative to the input signal (mechanical tactile stimulation). Finally, cumulant density functions were estimated to represent time domain relationships between the continuous vibrotactile stimulation and muscle activity in TA. A cumulant density estimate derived between two signals is a correlation-like measure and is interpreted as an associative rather than a causal relationship. Accordingly, the vibration displacement-EMG cumulant density estimates represent related responses that hold no physical values. Cumulant density estimates were derived by transforming the cross spectra between probe tip displacement and rectified surface EMG signals into the time domain. The amplitudes of the cumulant density functions were then normalized by the product of the vector norms of the probe tip displacement (input) and EMG (output) signals (Dakin et al. 2010). This method of normalization essentially transforms the cumulant density values into an equivalent of a cross-correlation (r values confined between −1 and 1). The cumulant density function represents the evoked response to vibratory stimulation and exhibits similar characteristics to mechanically evoked cutaneous reflexes estimated with spike- or stimulus-triggered average techniques (Fallon et al. 2005). For the analysis, we extracted the peak-to-peak amplitude of the cumulant density over a time window equal to one cycle of the vibratory stimulus, centered at a time lag of 100 ms (see bold black line segments in Fig. 3). This time lag was chosen because it is approximately the latency of electrically evoked cutaneous reflexes in TA (Aniss et al. 1992; Zehr et al. 2014) and is also the latency of the mechanically evoked response we observed in pilot testing with a stimulus-triggered EMG average approach (unpublished observation).
Posturography.
After cutaneous sensitivity and reflex testing, quiet standing balance stability was assessed. Participants stood relaxed with their arms at their sides and eyes closed on a six-axis force plate (BP400600; Advanced Mechanical Technologies) with a stance width equal to their foot length. Normal and shear forces were sampled from the force plate amplifier at 100 Hz via an A/D board (Micro 1401; Cambridge Electronic Design). Data were low-pass filtered with a 4th-order dual-pass Butterworth filter at 5 Hz offline and used to calculate CoP in the anterior-posterior (AP) and mediolateral (ML) planes. Two 2-min trials were completed, with 1 min of rest between. RMS amplitude and MPF were calculated using the unbiased CoP signal, and these measures were subsequently averaged across all trials.
Statistical analysis.
Tests of skewness identified that the dependent variables had a tendency to be positively skewed; for young adults skewness values ranged from −0.018 to 2.502 (mean = 1.445), and for older adults skewness values ranged from −0.248 to 1.635 (mean = 0.951). Based on the Shapiro-Wilk tests, all dependent variables, except for MPF in the AP and ML planes, were found to be nonnormal for one of the age groups and were corrected using a log10 transformation before analysis. Older adult participants were tested twice on the identical testing procedure, separated by a 1-h break (for comparison with another data set). For the purpose of this study, we collapsed data over these two testing sessions by averaging over sensory threshold estimates and postural sway measures and by concatenating reflex trials. For sensory data, assessment of the within-subject effect of leg (right vs. left) and between-subject effect of age group (young vs. old) was performed using separate mixed-design repeated-measures ANOVAs for monofilament, 30-Hz, and 250-Hz detection thresholds. We further concatenated reflex trials across legs and performed independent t-tests on these and standing balance measures to assess the effect of age group. Finally, we performed pairwise Pearson's correlations between all dependent variables (and age), collapsing data over both sessions and legs, to explore the level of association between sensitivity, reflex, and standing balance measures. Statistical analyses were performed with SPSS version 23 (IBM), and in all cases we applied an alpha level of 0.05.
RESULTS
Age-related reductions in plantar skin sensitivity.
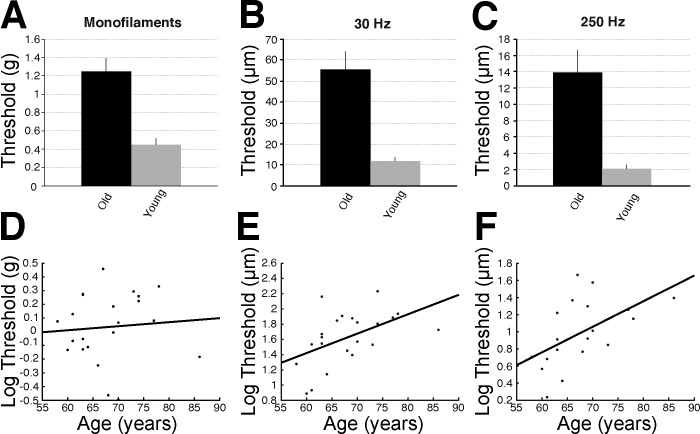
Older adults exhibited elevated detection thresholds for all stimulus types (Fig. 2). There were significant main effects of age found for monofilaments (F1,38 = 26.714; P < 0.001), 30-Hz thresholds (F1,39 = 37.592; P < 0.001), and 250-Hz thresholds (F1,34 = 36.794; P < 0.001), with higher thresholds observed in the older compared with younger group for all three measures (see Fig. 2, A–C). There were no significant interaction effects between age and leg for any of the three threshold measures. There was a main effect of leg, independent of age, for 250-Hz thresholds (F1,37 = 7.076; P = 0.012), with a significantly higher threshold found in both groups in the right compared with left leg (mean difference for young = 0.26 μm; older adult = 3.27 μm).Vibrotactile detection thresholds at 30 Hz (r = 0.500, P = 0.018) and 250 Hz (r = 0.543, P = 0.016) were correlated with age in the older adults; however, there was no correlation between age and monofilament detection thresholds (r = 0.081, P = 0.721; see Fig. 2, D–F).
Fig. 2.
Summary of sensory testing data. A–C: bar graphs showing the raw mean (±SE) difference between young and older adults in monofilament thresholds (A) and vibrotactile thresholds at 30 and 250 Hz (B and C, respectively). D–F: scatterplots depicting the age-related decline in log monofilament (D) and vibrotactile thresholds at 30 and 250 Hz (E and F, respectively) within the older adult group.
Weakened cutaneous reflex strength in older adults.
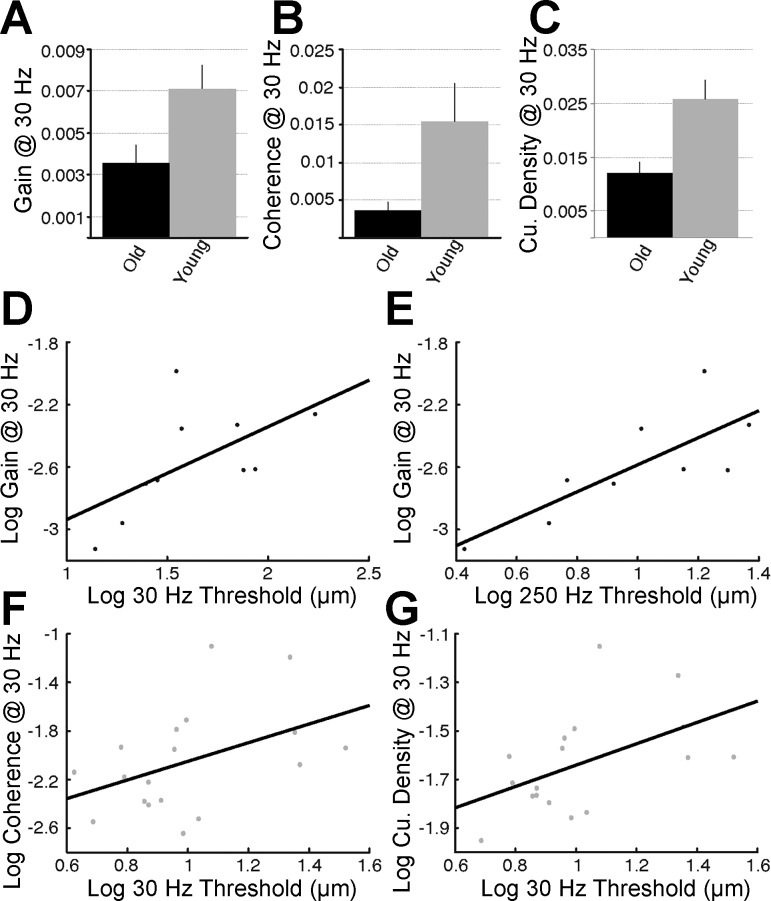
When 95% confidence limits were applied to assess the significance of coherence values at the input stimulus frequency (see Fig. 3, dashed horizontal lines), 18 of 19 (∼95%) young, but only 10 of the 19 (∼53%) older adults, had detectable cutaneous reflexes with continuous 30-Hz vibration as input stimulus. Only 1 young and 1 older adult had a significant coherence value with continuous 250-Hz vibration as input stimulus; given this, reflex responses at 250 Hz were not analyzed further. Therefore, based on the application of 95% confidence limits, it is clear that cutaneous reflexes are more strongly driven at 30 Hz than at 250 Hz, with stimulus amplitudes roughly 20 times young adult perceptual thresholds. Cutaneous reflex responses, when present to a significant degree, were also significantly depressed in older adults relative to the young (Figs. 3 and 4). Independent-samples t-tests revealed significantly lower gain (t26 = −2.674, P = 0.013), coherence (t26 = −3.422, P = 0.002), and peak-to-peak cumulant density amplitudes (t26 = −3.805, P = 0.001) at 30 Hz in older adults (Fig. 4, A–C). No correlation between age and any of the 30-Hz reflex measures within the older adults was observed; however, the validity of correlations based on 10 data points is questionable.
Fig. 4.
Age-related change in cutaneous reflexes and its relationship with plantar sensitivity. A–C: bar graphs showing the raw mean (±SE) difference between young and older adults in cutaneous reflex gain (A), coherence (B), and cumulant density (C) with 30-Hz continuous vibration as the input stimulus. D and E: correlations between log gain at 30 Hz and log 30-Hz (D) and log 250-Hz vibrotactile thresholds (E) observed within the older adults (black data points). F and G: correlations between log 30-Hz vibrotactile thresholds and log coherence (F) and log cumulant density (G) at 30 Hz observed within the young adults (gray data points). Cu., cumulative.
Age-related changes in postural stability.
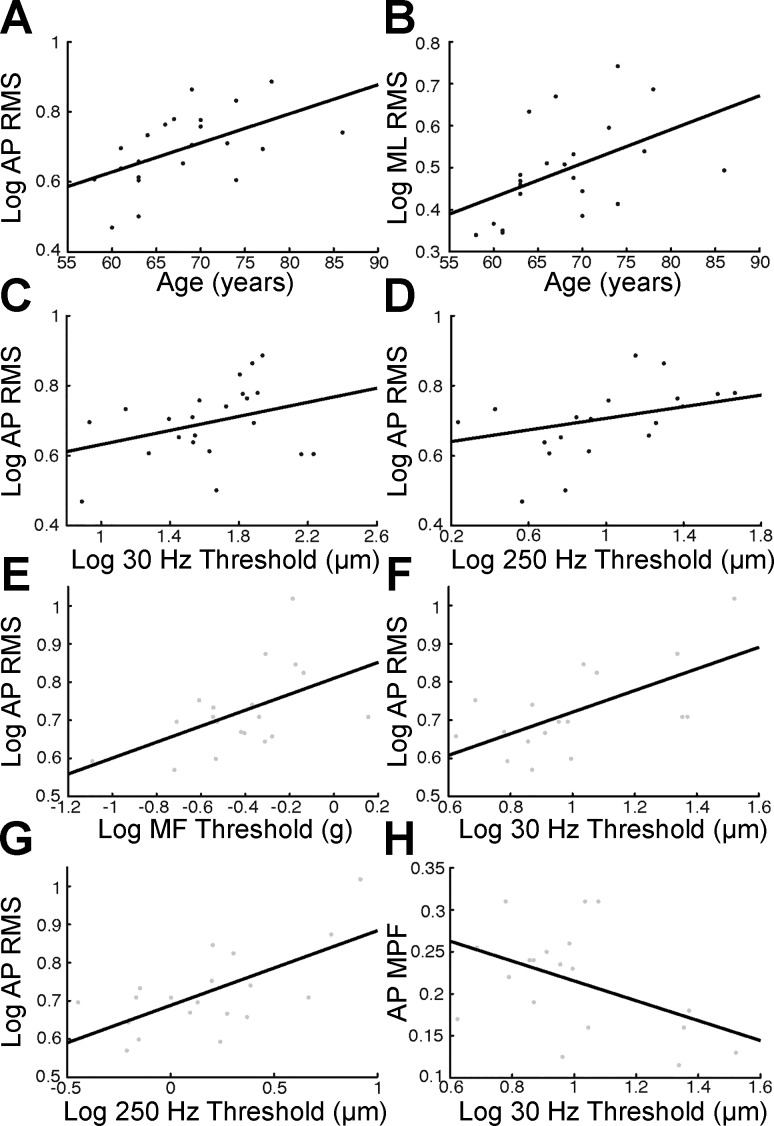
CoP MPF in the AP plane was significantly elevated in older adults (0.215 vs. 0.314 Hz; t39 = 2.846, P = 0.007); however, there were no significant differences between age groups for CoP MPF in the ML plane (t39 = 1.741, P = 0.090) or for RMS CoP amplitude in both the AP (t39 = −0.790, P = 0.434) and ML planes (t39 = 0.623, P = 0.537). However, when age was considered as a continuous variable, it was correlated with RMS CoP amplitude in the AP plane (r = 0.540, P = 0.009) and with RMS CoP amplitude in the ML plane (r = 0.487, P = 0.021) within the older adult group (Fig. 5, A and B).
Fig. 5.
Relationship between age-related decline in plantar sensitivity and postural instability. A and B: scatterplots depicting the age-related increase in log CoP RMS amplitude observed in both the AP (A) and ML planes (B) for older adults (black data points). C and D: scatterplots showing correlations between log AP CoP RMS amplitude and log 30-Hz (C) and log 250-Hz vibrotactile thresholds (D) in older adults. E–G: scatterplots showing correlations between log AP CoP RMS amplitude and log monofilament (E), log 30-Hz (F), and log 250-Hz detection thresholds (G) in young adults (gray data points). H: scatterplot displaying the relationship observed between AP CoP MPF and log 30-Hz vibrotactile thresholds in the young.
Relationships between sensitivity, reflex, and stability measures.
First, we examined the relationship between plantar sensitivity and cutaneous reflex strength. As depicted in Fig. 4E, older adults exhibited a significant correlation between 250-Hz thresholds and reflex gain at 30 Hz (r = 0.784, P = 0.012). Younger adults exhibited a significant correlation between 30-Hz detection thresholds and reflex cumulant density peak-to-peak amplitude at 30 Hz (r = 0.531, P = 0.023), as well as a marginal correlation between 30-Hz detection thresholds and coherence at 30 Hz (r = 0.445, P = 0.064; Fig. 4, F and G). However, monofilament and vibrotactile thresholds did not correlated with any other reflex measure in either age group.
Last, we wanted to determine whether plantar sensitivity or lower limb cutaneous reflex strength measures were related to postural instability. In both young and older adults, cutaneous reflex measures did not correlate with any of the postural stability measures. However, we did observe significant and trending positive correlations between CoP RMS amplitude in the AP plane and vibrotactile thresholds in the older adults (30 Hz: r = 0.333, P = 0.130; 250 Hz: r = 0.532, P = 0.019; Fig. 5, C and D). Monofilament detection thresholds also correlated with AP CoP MPF in the older adults (r = −0.449, P = 0.036). In young adults, AP CoP RMS positively correlated with monofilament (r = 0.518, P = 0.023), 30-Hz (r = 0.633, P = 0.004), and 250-Hz detection thresholds (r = 0.648, P = 0.003; Fig. 5, E–G), whereas ML CoP RMS marginally correlated with 30-Hz detection thresholds (r = 0.391, P = 0.098), but not with 250-Hz detection thresholds (r = 0.058, P = 0.813). In addition, AP CoP MPF correlated with 30-Hz detection thresholds in young adults (r = −0.462, P = 0.046; Fig. 5H). These results provide novel evidence that age-related decline in plantar skin sensitivity measures may provide a valuable predictor of postural instability.
DISCUSSION
In this study, we have provided evidence that age-related deterioration of cutaneous mechanoreceptors impacts both plantar sensitivity and cutaneous reflex coupling strength, and we have further demonstrated that changes in plantar sensitivity are correlated with the decline in postural stability in older adults. With age, monofilament and vibrotactile detection thresholds increased, and all three measures of cutaneous reflex coupling strength (gain, coherence, cumulant density) decreased. Balance stability was also found to worsen with age, as indicated by positive correlations between age and sway amplitude in both AP and ML planes, as well as an increased frequency of CoP excursions in the AP plane for older adults. We further observed relationships between perceptual sensitivity and reflex-coupling measures in both age groups; however, we did not observe any relationship between reflex coupling strength and postural stability measures. Taking the findings together, we have uncovered correlational relationships between postural stability, plantar sensitivity, and lower limb cutaneous reflexes, providing insight into a potential risk factor for balance dysfunction in older adults.
Reduced skin sensitivity with age.
Our findings replicate those of previous reports (Perry 2006; Well et al. 2003), demonstrating that human sensitivity to mechanical stimulation of the plantar skin surface declines with age. Monofilament and vibrotactile thresholds at frequencies mediated by FAI and FAII mechanoreceptors (Bolanowski et al. 1988; Johnson 2001; Strzalkowski et al. 2015) were all significantly elevated with age. Although the precise frequency bandwidths over which cutaneous input detected by these two FA mechanoreceptor afferent classes are not yet fully described for the human foot sole (Kennedy and Inglis 2002), particularly with ankle joint and foot sole under a natural standing load (Mildren et al. 2016), evidence from the glabrous skin of the hand would suggest that 30 -Hz vibrations are encoded most precisely by FAI afferents (Meissner's corpuscles) and that 250-Hz vibrations are encoded most precisely by FAII afferents (Pacinian corpuscles) (Bent and Strzalkowski 2014; Johansson and Vallbo 1979; Johnson 2001). Recently, it was suggested that human monofilament detection thresholds are most closely correlated to the mechanical threshold for evoking action potentials in human FAI afferents (Strzalkowski et al. 2015); therefore, monofilament and 30-Hz vibrotactile tests both rely on the FAI mechanoreceptor class (Meissner's corpuscles), whereas the 250-Hz vibrotactile test relies on the FAII mechanoreceptor class (Pacinian corpuscles). We support the notion of Strzalkowski et al. (2015) that monofilament thresholds are best correlated with FAI afferent responses in that correlations between monofilament and 30-Hz vibrotactile thresholds were stronger in both age groups (old: P = 0.035, r = 0.452; young: P = 0.058, r = 0.442) than correlations between monofilament and 250-Hz vibrotactile thresholds (old: P = 0.314, r = 0.244; young: P = 0.452, r = 0.183).
Between age groups, vibratory thresholds at 250 Hz increased in proportion more than vibratory thresholds at 30 Hz. Due to spatial summation in the FAII-mediated (>100 Hz) perceptual “channel,” it should be expected that reductions in peripheral afferent innervation density will impact sensitivity at 250 Hz more so than at 30 Hz. Spatial summation means that perceptual sensitivity improves when a larger population of Pacinian corpuscles is activated (e.g., by using a larger probe tip), and this property of the FAII channel was recently demonstrated in the glabrous skin of the human hand and foot (Gu and Griffin 2013). By the same logic, 250-Hz thresholds will also be most impacted by a reduction in the number of activated FAII mechanoreceptors (e.g., due to age-related loss of plantar mechanoreceptors). Spatial summation in the FAII-mediated perceptual channel makes 250-Hz thresholds our best proxy of plantar innervation density (see below). In contrast, perceptual sensitivity is not much improved by activation of a larger population of Meissner's corpuscles; therefore, a reduction in the number of FAI mechanoreceptors with age should not be expected to cause as large an effect on sensitivity, so long as at least one or a few FAI afferent terminals are still being stimulated. Nonetheless, if the morphology (Iwasaki et al. 2003) or conduction velocity (Kenshalo 1986) of the remaining FAI afferents is negatively altered with age, then age-related changes in sensitivity to monofilaments and 30-Hz vibrations would still be expected, as we observed.
Age-related depression of lower-limb cutaneous reflex amplitude.
We have observed novel evidence for age-related changes in the way that excitatory cutaneous drive to the spinal cord modulates lower motoneuron activity. Importantly, to address this question we used continuous mechanical vibration at two different stimulus frequencies, each carried predominantly by two distinct cutaneous FA afferent receptor classes (FAI and FAII), allowing us to look at receptor class-dependent effects. Overall, when present to a significant degree, cutaneous reflexes were depressed in older adults, as evidenced by lower gain and coherence at the stimulus frequency as well as smaller peak-to-peak cumulant density amplitudes. This could plausibly be the result of receptor loss or other age-related changes in cutaneous receptors, but it could also result from changes in lower limb motoneuron physiology and anatomy, altered spinal reflex connectivity strength within the ventral horn of the spinal cord, or reduced sensitivity of synaptic transmission at the neuromuscular junction. Much evidence exists on the effect of age on the degradation of afferent, efferent, and spinal local circuit components (Connelly et al. 1999; Larsson and Ansved 1995; Mynark and Koceja 2001; Terao et al. 1996). However, more research is needed to fully tease apart the multifaceted effect of normal adult aging on reflex function in terms of the distinct roles played by changes in sensory afferent, spinal interneuron, and motoneuron responses, as well as muscle force production and state of fatigue. In addition, electrically and mechanically evoked cutaneous reflexes are known to have task-, phase-, and context-dependent effects on motor control during locomotion (Lam et al. 2003; McVea and Pearson 2007; Pang and Yang 2001; Yang and Stein 1990; Zehr and Stein 1999; Zehr et al. 2014); how such reflex modulation is affected by healthy adult aging remains to be determined.
Cutaneous reflex strength was much greater at 30 Hz than it was at 250 Hz regardless of age, suggesting a greater relative contribution of FAI afferents in the evoked cutaneous reflex response. Significant reflex responses were not even evident in the young adults at 250 Hz, and given this finding, we argue that cutaneous drive from the FAI afferent population of the human foot sole plays a critical role in human lower limb cutaneous reflexes. However, human microneurography research is needed to confirm this hypothesis, as well as to further examine the role of slowly adapting (SA) type I and II afferents in lower limb cutaneous reflexes, which are receptor classes that are harder to precisely target with mechanical vibration (i.e., single-unit spikes are required to fully delineate the reflex contribution of SAI and SAII afferents). There is evidence that cutaneous reflexes can be driven by activating each of the four cutaneous receptor types (Bent and Lowrey 2013; Fallon et al. 2005); however, our findings agree with those of Fallon et al. (2005) in that FAI afferents, compared with FAII afferents, were found to be the most strongly coupled mechanoreceptor afferent type to lower limb muscles. With microneurography, Fallon et al. (2005) investigated the contribution of the four cutaneous receptor types by using manual stimulation of the planter skin to evoke cutaneous reflexes in TA and the triceps surae muscle group; in the lower limb, cutaneomotor coupling was observed most frequently with FAI afferents, followed by SAI afferents. These findings contrasted with what they had previously observed using the same technique to measure reflex coupling between upper limb muscles and cutaneous receptors of the hand: coupling was observed most frequently with SAII afferents, and SAI afferents had reportedly no influence on motoneuron activity (McNulty and Macefield 2001; McNulty et al. 1999). Differences in which afferent class(es) most strongly drive upper and lower limb motoneurons may highlight important functional roles for cutaneous input arising from the hand (i.e., dexterous object manipulation) and the foot (i.e., standing balance control) in human movement.
Lastly, it is interesting to consider the correlations we observed between our cutaneomotor coupling and plantar sensitivity measures in both young and older adults. In the young, there was a correlation observed between 30-Hz detection thresholds and reflex cumulant density peak-to-peak amplitude at 30 Hz (P = 0.023), as well as a trending correlation between 30-Hz detection thresholds and reflex coherence at 30 Hz (P = 0.064). These results are intuitive, suggesting that the ability to perceive small vibrations and the ability to transmit precise spectral and temporal information onto motoneurons at 30 Hz are related, likely because they both rely on the precise coding of 30-Hz vibration by the same FAI afferent population. Interestingly, in older adults we observed a significant correlation between 250-Hz thresholds and reflex gain at 30 Hz (P = 0.012), with only a trend for a negative correlation between 30-Hz thresholds and reflex gain at 30 Hz (P = 0.071). The stronger correlation with 250-Hz thresholds in this case, we argue, is due to this measure being our best proxy for overall plantar receptor innervation density. Even though perceptual sensitivity to 30-Hz vibrations does not improve when more FAI afferents are stimulated (i.e., no spatial summation), it seems entirely reasonable to hypothesize that reflex gain at 30 Hz would depend on the number of activated FAI afferents driving the motoneuron pool. Thus, because 250-Hz thresholds in theory serve as a proxy of innervation density for both Pacinian and Meissner's corpuscles, these values correlate better than 30-Hz thresholds with reflex gain at 30 Hz in older adults. With age-related loss of Meissner's corpuscles, the overall cutaneous drive to the motoneuron pool should be depressed (lower gain), a hypothesis we support. Innervation density is likely best captured by the gain measure, because it indicates the overall strength of the output (muscle response) relative to the overall strength of the input (skin vibration) at the stimulus frequency. Conversely, strong coherence and cumulant density values rely on precise spectral and temporal transmission of the afferent input onto motoneurons, which is evidently disrupted in older adults as well, apparently so much so that no relationship with 30-Hz thresholds, a proxy of precise coding by FAI afferents, could be observed, unlike in the young adults. However, we note that with only 10 older adults remaining in the 30-Hz reflex correlations after significance criteria were applied, our findings should not be overstated. Follow-up research is underway with larger probe-tip sizes and more powerful vibrotactile stimuli (e.g., bands of stochastic vibration) in an attempt to record significant reflex responses in the ∼47% of older adults who failed to have significant responses with the use of 30-Hz sinusoidal vibration and small probe-tip diameter (1.5 mm) in the present study.
Age-related changes in human postural control.
We observed that the RMS amplitude and mean frequency of pressure distribution excursions underfoot while standing increased with age, confirming that postural instability increases with age. Using 2-min trials, with the subjects' eyes closed and feet spaced one foot length apart, we observed that older adults, as a group, exhibited a higher mean frequency in their AP CoP excursions and, in addition, that CoP RMS amplitude in both the AP and ML planes correlated with age within the older adult group. These results are similar to those of Prieto et al. (1996), who used trials of 20 s with the subjects' eyes closed and feet in a comfortable position and observed a greater mean frequency in older adults (0.42 vs. 0.57 Hz); these values are higher than ours (0.215 vs. 0.314 Hz), likely because of their inability to resolve power at important lower frequency components due to the short trial duration used (Carpenter et al. 2001). In studies that have used durations of >60 s, age-related changes in CoP displacements include increased velocity (Mozaki and Masan 2012; Maki et al. 1990) and amplitude (Maki et al. 1990, Winter 1995).
If age-related decline in plantar receptor innervation density lies at the root of increased postural instability, we would expect 250-Hz thresholds to be the strongest correlate for balance measures. Accordingly, we observed significant correlations between AP CoP RMS amplitude and 250-Hz thresholds in both the young (P = 0.003) and older adults (P = 0.019). It is of great clinical relevance that 250-Hz thresholds serve as a predictor of AP CoP RMS across the lifespan, because it is precisely this global metric of postural stability that is elevated in older adult fallers, relative to young adults (Laughton et al. 2003). Previous research suggests that quiet standing balance control with a narrow base of support is an important diagnostic tool for identifying a heightened risk of falling; greater ML postural sway was exhibited by older adults who had experienced multiple falls, relative to those who had not (Melzer et al. 2004). The pattern of age-related stability changes in the AP or ML plane could potentially depend on the particular pattern of cutaneous mechanoreceptor loss on an individual-by-individual basis. In support of this, forefoot anesthesia mainly influences mediolateral posture control, whereas complete foot sole anesthesia has an impact on anteroposterior control (Meyer et al. 2004). Age-related receptor loss would involve the whole foot sole; thus it makes sense that 250-Hz thresholds would correlate better with CoP RMS in the AP rather than ML plane in older adults.
There is now mounting evidence that plantar cutaneous function is crucial for both static and dynamic balance control. Plantar tactile input helps with detection of foot contact during perturbations and with control of the subsequent weight transfer, thus influencing compensatory steps (Maki et al. 1999). Cutaneous information likely influences gait patterns, because vibratory thresholds have been linked to the plantar pressure distribution at specific foot sole locations in walking and running (Nurse and Nag 1999). Other studies have emphasized the role of plantar cutaneous receptors in gait (Yang and Stein 1990) and gait termination (Perry et al. 2001). Whereas FAI and FAII afferents are thought to be critical in signaling dynamic aspects of balance control such as skin slippage and dynamic changes in pressure (Kennedy and Inglis 2002; Inglis et al. 2002), as well as the different phases and direction dependence of stepping responses to unexpected perturbations (Perry et al. 2000), fast-adapting input is only half the story, and more research is needed. SAI afferents in the foot sole demonstrated the second strongest coupling to lower limb cutaneous reflexes (Fallon et al. 2005). SAI and SAII afferents are poised to provide highly pertinent information for controlling CoP position (Hämäläinen et al. 1992; Kennedy and Inglis 2002; Magnusson et al. 1990). For example, each SAI signals the continuous static pressure applied to the skin over a small area, and thus the population of SAI afferents provides a high-fidelity pressure map across the foot soles that could readily be used by either conscious or reflexive balance control mechanisms. Assuming that SA afferents play little to no role in monofilament thresholds (Strzalkowski et al. 2015), the stimuli delivered in the present study did not specifically measure the sensitivity in an SA-mediated regime, which is traditionally measured by assessing tactile spatial acuity. As with the hand (Stevens 1992), two-point discrimination thresholds, a measure of tactile spatial acuity, are also elevated in older adults on the foot sole (Lynch and Mooney 1999), strongly suggesting that SA receptors are being shed with age, as well.
Conclusion.
Our results confirm several previous reports that age-related anatomical, morphological, and physiological changes in the cutaneous periphery reduce the sensibility of the human foot sole. We provide novel correlational evidence that this deterioration might be linked to depressed cutaneous reflex coupling strength as well as decreased postural stability in older adults. These findings are of critical importance for interventions designed at reducing balance deficits in older adults, for example, the use of vibrating insoles that reportedly utilize the phenomenon of stochastic resonance to increase plantar cutaneous sensitivity and postural stability (Priplata et al. 2003).
GRANTS
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grants (to J. T. Inglis and M. G. Carpenter). R. M. Peters received salary support from NSERC funding granted to J. T. Inglis. M. D. McKeown received financial support through an NSERC Undergraduate Student Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.P., M.G.C., and J.T.I. conception and design of research; R.M.P. and M.D.M. performed experiments; R.M.P. analyzed data; R.M.P., M.D.M., M.G.C., and J.T.I. interpreted results of experiments; R.M.P. prepared figures; R.M.P. drafted manuscript; R.M.P., M.D.M., M.G.C., and J.T.I. edited and revised manuscript; R.M.P., M.D.M., M.G.C., and J.T.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Eveline Pasman and Austin Kao for assistance with the data collection and Dr. Jean-Sébastien Blouin for helpful discussions pertaining to the cutaneous reflex data analysis. We thank Dr. James J. Collins (Boston University) for loan of the motor.
REFERENCES
- Aniss AM, Gandevia SC, Burke D. Reflex responses in active muscles elicited by stimulation of low-threshold afferents from the human foot. J Neurophysiol 67: 1375–1384, 1992. [DOI] [PubMed] [Google Scholar]
- Bent LR, Lowrey CR. Single low-threshold afferents innervating the skin of the human foot modulate ongoing muscle activity in the upper limbs. J Neurophysiol 109: 1614–1625, 2013. [DOI] [PubMed] [Google Scholar]
- Bent LR, Strzalkowski ND. Cutaneous afferent sensitivity and perceptual threshold in the human foot sole. 2014 International Society for Posture and Gait Research World Congress (Oral Abstract O.2.5), Vancouver, BC, Canada, June 29-July 3, 2014. [Google Scholar]
- Bolanowski SJ Jr, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am 84: 1680–1694, 1988. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Winkelmann R, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology 16: 1–1, 1966. [DOI] [PubMed] [Google Scholar]
- Brocklehurst J, Robertson D, James-Groom P. Clinical correlates of sway in old age-sensory modalities. Age Ageing 11: 1–10, 1982. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Winter DA, Peysar GW. Sampling duration effects on centre of pressure summary measures. Gait Posture 13: 35–40, 2001. [DOI] [PubMed] [Google Scholar]
- Cauna N, Mannan G. The structure of human digital Pacinian corpuscles (corpuscula lamellosa) and its functional significance. J Anat 92: 1, 1958. [PMC free article] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol 87: 843–852, 1999. [DOI] [PubMed] [Google Scholar]
- Cruz-Almeida Y, Black ML, Christou EA, Clark DJ. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front Aging Neurosci 6: 68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT, Blouin JS. Frequency-specific modulation of vestibular evoked sway responses in humans. J Neurophysiol 103: 1048–1056, 2010. [DOI] [PubMed] [Google Scholar]
- Duncan G, Wilson J, MacLennan W, Lewis S. Clinical correlates of sway in elderly people living at home. Gerontology 38: 160–166, 1992. [DOI] [PubMed] [Google Scholar]
- Duysens J, Beerepoot V, Veltink P, Weerdesteyn V, Smits-Engelsman B. Proprioceptive perturbations of stability during gait. Clin Neurophysiol 38: 399–410, 2008. [DOI] [PubMed] [Google Scholar]
- Dyck P, O'brien P, Kosanke J, Gillen D, Karnes J. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 43: 1508–1508, 1993. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Bent LR, McNulty PA, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents from the sole of the foot and motoneurons supplying leg muscles. J Neurophysiol 94: 3795–3804, 2005. [DOI] [PubMed] [Google Scholar]
- Forth KE, Layne CS. Background muscle activity enhances the neuromuscular response to mechanical foot stimulation. Am J Phys Med Rehabil 86: 50–56, 2007. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Wong M, Peters RM, Kanics IM. A tactile automated passive-finger stimulator (TAPS). J Vis Exp 28: pii: 1374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryfe C, Amies A, Ashley M. A longitudinal study of falls in an elderly population: I. Incidence and morbidity. Age Ageing 6: 201–210, 1977. [DOI] [PubMed] [Google Scholar]
- Gu C, Griffin MJ. Spatial summation of vibrotactile sensations at the foot. Med Eng Phys 35: 1221–1227, 2013. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol 64: 237–278, 1995. [DOI] [PubMed] [Google Scholar]
- Hämäläinen H, Kekoni J, Rautio J, Matikainen E, Juntunen J. Effect of unilateral sensory impairment of the sole of the foot on postural control in man: Implications for the role of mechanoreception in postural control. Hum Mov Sci 11: 549–561, 1992. [Google Scholar]
- Inglis JT, Kennedy PM, Wells C, Chua R. The role of cutaneous receptors in the foot. Adv Exp Med Biol 508: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Goto N, Goto J, Ezure H, Moriyama H. The aging of human Meissner's corpuscles as evidenced by parallel sectioning. Okajimas Folia Anat Jpn 79: 185–189, 2003. [DOI] [PubMed] [Google Scholar]
- Johansson R, Vallbo ÅB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol 297: 405–422, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Yoshioka T, Vega-Bermudez F. Tactile functions of mechanoreceptive afferents innervating the hand. J Clin Neurophysiol 17: 539–558, 2000. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a ‘dynamometric map’ for human balance control. Neuroreport 9: 3247–3252, 1998. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci Lett 266: 181–184, 1999. [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol 532: 869–878, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PM, Inglis JT. Distribution and behaviour of glabrous cutaneous receptors in the human foot sole. J Physiol 538: 995–1002, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenshalo DR. Somesthetic sensitivity in young and elderly humans. J Gerontol 41: 732–742, 1986. [DOI] [PubMed] [Google Scholar]
- Kontsevich LL, Tyler CW. Bayesian adaptive estimation of psychometric slope and threshold. Vision Res 39: 2729–2737, 1999. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Masani K. Postural sway during quiet standing is related to physiological tremor and muscle volume in young and elderly adults. Gait Posture 35: 11–17, 2012. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve 42: 78–87, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Wolstenholme C, van der Linden M, Pang MYC, Yang JF. Stumbling corrective responses during treadmill-elicited stepping in human infants. J Physiol 553.1: 319–331, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol 45: 397–458, 1995. [DOI] [PubMed] [Google Scholar]
- Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture 18: 101–108, 2003. [DOI] [PubMed] [Google Scholar]
- Löfvenberg J, Johansson RS. Regional differences and interindividual variability in sensitivity to vibration in the glabrous skin of the human hand. Brain Res 301: 65–72, 1984. [DOI] [PubMed] [Google Scholar]
- Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol 46: 69–76, 1991. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr Soc 42: 1110–1117, 1994. [DOI] [PubMed] [Google Scholar]
- Lynch W, Mooney J. A model to assess age-related changes in two-point discrimination of plantar skin. J Am Podiatr Med Assoc 89: 383–391, 1999. [DOI] [PubMed] [Google Scholar]
- Magnusson M, Enbom H, Johansson R, Wiklund J. Significance of pressor input from the human feet in lateral postural control: the effect of hypothermia on galvanically induced body-sway. Acta Otolaryngol 110: 321–327, 1990. [DOI] [PubMed] [Google Scholar]
- Maki BE, Holliday PJ, Fernie GR. Aging and postural control: a comparison of spontaneous- and induced-sway balance tests. J Am Geriatr Soc 38: 1–9, 1990. [DOI] [PubMed] [Google Scholar]
- Maki BE, Perry SD, Norrie RG, McIlroy WE. Effect of facilitation of sensation from plantar foot-surface boundaries on postural stabilization in young and older adults. J Gerontol A Biol Sci Med Sci 54: 281–287, 1999. [DOI] [PubMed] [Google Scholar]
- Masani K, Vette AH, Kouzaki M, Kanehisa H, Fukunaga T. Larger center of pressure minus center of gravity in the elderly induces larger body acceleration during quiet standing. Neurosci Lett 422: 202–206, 2007. [DOI] [PubMed] [Google Scholar]
- McNulty PA, Macefield VG. Modulation of ongoing EMG by different classes of low-threshold mechanoreceptors in the human hand. J Physiol 537: 1021–1032, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty PA, Türker KS, Macefield VG. Evidence for strong synaptic coupling between single tactile afferents and motoneurones supplying the human hand. J Physiol 518: 883–893, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVea DA, Pearson KG. Contextual learning and obstacle memory in the walking cat. Integr Comp Biol 47: 457–464, 2007. [DOI] [PubMed] [Google Scholar]
- Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing 33: 602–607, 2004. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156: 505–512, 2004. [DOI] [PubMed] [Google Scholar]
- Mildren RL, Strzalkowski NDJ, Bent LR. Foot sole skin vibration perceptual thresholds are elevated in a standing posture compared to sitting. Gait Posture 43: 87–92, 2016. [DOI] [PubMed] [Google Scholar]
- Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. J Gerontol 24: 169–178, 1969. [DOI] [PubMed] [Google Scholar]
- Mynark RG, Koceja DM. Effects of age on the spinal stretch reflex. J Appl Biomech 17: 188–203, 2001. [Google Scholar]
- Nurse MA, Nigg BM. Quantifying a relationship between tactile and vibration sensitivity of the human foot with plantar pressure distributions during gait. Clin Biomech (Bristol, Avon) 14: 667–672, 1999. [DOI] [PubMed] [Google Scholar]
- Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol 528: 389–404, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SD. Evaluation of age-related plantar-surface insensitivity and onset age of advanced insensitivity in older adults using vibratory and touch sensation tests. Neurosci Lett 392: 62–67, 2006. [DOI] [PubMed] [Google Scholar]
- Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res 877: 401–406, 2000. [DOI] [PubMed] [Google Scholar]
- Perry SD, Santos LC, Patla AE. Contribution of vision and cutaneous sensation to the control of centre of mass (COM) during gait termination. Brain Res 913: 27–34, 2001. [DOI] [PubMed] [Google Scholar]
- Peters RM, Goldreich D. Tactile spatial acuity in childhood: effects of age and fingertip size. PLoS One 8: e84650, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt H, Amlie RN, Starr A. Short latency mechanically evoked somatosensory potentials in humans. Electroencephalogr Clin Neurophysiol 47: 524–531, 1979. [DOI] [PubMed] [Google Scholar]
- Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng 43: 956–966, 1996. [DOI] [PubMed] [Google Scholar]
- Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. Lancet 362: 1123–1124, 2003. [DOI] [PubMed] [Google Scholar]
- Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci 50: 211–215, 1995. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther 87: 193–207, 2007. [DOI] [PubMed] [Google Scholar]
- Stevens JC. Aging and spatial acuity of touch. J Gerontol 47: 35–40, 1992. [DOI] [PubMed] [Google Scholar]
- Strzalkowski NDJ, Mildren RL, Bent LR. Thresholds of cutaneous afferents related to perceptual threshold across the human foot sole. J Neurophysiol 114: 2144–2151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao S, Sobue G, Hashizume Y, Li M, Inagaki T, Mitsuma T. Age-related changes in human spinal ventral horn cells with special reference to the loss of small neurons in the intermediate zone: a quantitative analysis. Acta Neuropathol 92: 109–114, 1996. [DOI] [PubMed] [Google Scholar]
- Tong J, Mao O, Goldreich D. Two-point orientation discrimination vs. the traditional two-point test for tactile spatial acuity assessment. Front Hum Neurosci 7: 579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Okubo J. The role of the plantar mechanoreceptor in equilibrium control. Ann NY Acad Sci 374: 855–864, 1981. [DOI] [PubMed] [Google Scholar]
- Wells C, Ward LM, Chua R, Inglis JT. Regional variation and changes with ageing in vibrotactile sensitivity in the human foot sole. J Gerontol A Biol Sci Med Sci 58: 680–686, 2003. [DOI] [PubMed] [Google Scholar]
- Winter DA. Human balance and posture control during standing and walking. Gait Posture 3: 193–214, 1995. [Google Scholar]
- Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63: 1109–1117, 1990. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Nakajima T, Barss T, Klarner T, Miklosovic S, Mezzarane RA, Nurse M, Komiyama T. Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci Med Rehabil 6: 33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol 58: 185–205, 1999. [DOI] [PubMed] [Google Scholar]