Optogenetic stimulation of PV interneurons yielded both antiepileptic and ictogenic effects. During seizures, opto-stimulation of PV interneurons prematurely terminated ongoing seizures by suppressing activity in pyramidal neurons. In contrast, during the quiescent interictal period opto-stimulation of PV interneurons robustly initiated seizures, due to secondary synchronized postinhibitory rebound activation of pyramidal neurons. Thus opto-stimulation of PV interneurons holds both the potential for improving and worsening of seizure control and supports the role of synchronized activation of PV interneurons in seizure initiation.
Keywords: epilepsy, interneurons, neocortex, optogenetics, neurostimulation
Abstract
Parvalbumin (PV)-expressing interneurons exert powerful inhibitory effects on the normal cortical network; thus optogenetic activation of PV interneurons may also possess antiepileptic properties. To investigate this possibility we expressed channelrhodopsin 2 in PV interneurons by locally injecting the Cre-dependent viral vector AAV2/1-EF1a-DIO-ChETA-EYFP into the S1 barrel cortex of PV-Cre mice. Approximately 3–4 wk later recurrent electrographic seizures were evoked by local application of the chemoconvulsant 4-aminopyridine (4-AP); the ECoG and unit activity were monitored with extracellular silicone electrodes; and PV interneurons were activated optogenetically during the ictal and interictal phases. Five- to ten-second optogenetic activation of PV interneurons applied during electrographic seizures (ictal phase) terminated 33.7% of electrographic seizures compared with only 6% during sham stimulation, and the average electrographic seizure duration shortened by 38.7 ± 34.2% compared with sham stimulation. In contrast, interictal optogenetic activation of PV interneurons showed powerful and robust ictogenic effects. Approximately 60% of interictal optogenetic stimuli resulted in electrographic seizure initiation. Single-unit recordings revealed that presumptive PV-expressing interneurons markedly increased their firing during optogenetic stimulation, while many presumptive excitatory pyramidal neurons showed a biphasic response, with initial suppression of firing during the optogenetic pulse followed by a synchronized rebound increase in firing at the end of the laser pulse. Our findings indicated that ictal activation of PV-expressing interneurons possesses antiepileptic properties probably due to suppression of firing in pyramidal neurons during the laser pulse. However, in addition interictal activation of PV-expressing interneurons possesses powerful ictogenic properties, probably due to synchronized postinhibition rebound firing of pyramidal neurons.
NEW & NOTEWORTHY
Optogenetic stimulation of PV interneurons yielded both antiepileptic and ictogenic effects. During seizures, opto-stimulation of PV interneurons prematurely terminated ongoing seizures by suppressing activity in pyramidal neurons. In contrast, during the quiescent interictal period opto-stimulation of PV interneurons robustly initiated seizures, due to secondary synchronized postinhibitory rebound activation of pyramidal neurons. Thus opto-stimulation of PV interneurons holds both the potential for improving and worsening of seizure control and supports the role of synchronized activation of PV interneurons in seizure initiation.
approximately 30% of epilepsy patients suffer from drug-resistant epilepsy and continue to experience seizures despite appropriate antiepileptic drug treatment (Kwan et al. 2011). In recent years, cortical neurostimulation is emerging as a promising treatment modality for drug-resistant epilepsy (Fisher 2012; Fisher and Velasco 2014). Typically, neurostimulators generate trains of electrical pulses, which are intended to terminate developing or ongoing seizures (Bergey 2013; DeGiorgio and Krahl 2013; Fisher 2012; Fisher and Velasco 2014; Sun and Morrell 2014). A potential alternative to conventional electrical stimulation is optogenetic stimulation, where light-gated ion channels and pumps are expressed in target neurons via viral vectors, and activated by light pulses at the appropriate wavelength (Chow et al. 2012; Fenno et al. 2011; Tye and Deisseroth 2012; Yizhar et al. 2011). Recent studies have shown that optogenetic stimulation possesses antiepileptic properties in vitro and in vivo (Berglind et al. 2014; Chiang et al. 2014; Krook-Magnuson et al. 2014, 2015; Ledri et al. 2014; Paz et al. 2013; Paz and Huguenard 2015a; Ritter et al. 2014). Moreover, the ability to target specific cell types and directly hyperpolarize neurons may endow optogenetic stimulation with higher antiepileptic efficacy than electrical stimulation paradigms.
Inhibitory interneurons play a critical role in controlling cortical excitability, as well as in the process of ictogenesis and seizure propagation (de Curtis and Gnatkovsky 2009; Gnatkovsky et al. 2008; Krook-Magnuson et al. 2015; Paz and Huguenard 2015b; Shiri et al. 2015; Trevelyan and Schevon 2013; Uva et al. 2015). PV-expressing inhibitory interneurons are the most common subclass of inhibitory interneurons in the neocortex, and constitute ∼40% of all neocortical inhibitory interneurons (DeFelipe et al. 2013; Markram et al. 2004; Rudy et al. 2011; Sultan et al. 2013). As such, PV-expressing interneurons serve as a promising potential target for treating epilepsy. Consistent with this possibility previous studies have shown that optogenetic stimulation of GABAergic interneurons in hippocampal brain slices in vitro or PV-expressing interneurons in the thalamus in vivo possesses antiepileptic properties (Ledri et al. 2014; Pa et al. 2013). On the other hand, synchronized activation of interneurons may have a downside as well. Previous studies have suggested that intense activation of GABAergic interneurons may play an important role in seizure initiation (DeFelipe et al. 2013; Markram et al. 2004), and synchronized optogenetic activation of PV-expressing interneurons may in fact facilitate initiation of seizurelike events in vitro (Shiri et al. 2015; Yekhlef et al. 2015).
In this study, we investigated the consequences of optogenetic activation of PV-expressing interneurons during both the interictal and ictal phases in an acute chemoconvulsant model of neocortical epilepsy in vivo and used single-unit recordings to elucidate the underlying mechanisms and dynamics of these optogenetic-induced effects.
METHODS
Animals, viral vector injection, and surgery.
All experiments were performed on PV-IRES-Cre mice (Jackson Laboratory). We confirmed the genotype of individual PV-Cre mice with PCR testing of DNA obtained from the tail of the mice. Seven- to ten-week-old PV-IRES-Cre mice were anesthetized with isoflurane and placed in a stereotactic frame (Kopf Instruments, Tujunga, CA). Lidocaine (2%) was locally injected into the scalp region to minimize pain during surgery. After shaving the fur the skin covering the skull was opened, the skull was exposed, and a small craniotomy (1-mm diameter) was drilled over the right S1 barrel cortex (AP 1 mm, ML 3 mm). After the small craniotomy was opened, Cre-dependent viral vectors containing the gene of the fast ChETA variant of channelrhodopsin 2 (ChR2) (AAV2/1-EF1a-DIO-ChETA-EYFP, UNC Vector Core, Chapel Hill, NC) were injected into the S1 cortex at two depths of 0.35 mm and 0.7 mm with a glass pipette driven by a microinjector mounted on a micromanipulator (Narishige).
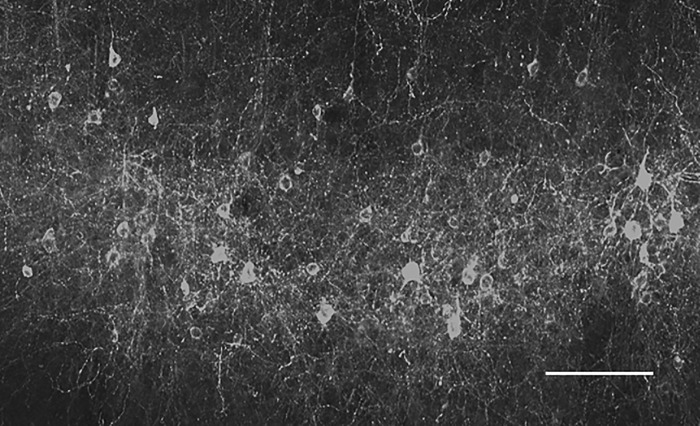
Three to five weeks after viral injection the mice were anesthetized again with intraperitoneal urethane (10 ml/kg of 20% urethane dissolved in normal saline), and lidocaine (2%) was locally injected into the scalp region. After the mice were anesthetized, a short metal rod was glued to the posterior aspect of the skull with dental cement and was used to hold the head in position during the experiment. Next, we reopened the skin, exposed the skull, and constructed a dental cement well that was used to contain aCSF solution with and without 4-aminopyridine (4-AP) during the experiments. After construction of the dental cement well a larger craniotomy (2 mm diameter) was drilled through the skull over the right S1 region (same coordinates used for viral vector injections). The craniotomy was drilled within the borders of the dental cement well. Throughout all experiments, body temperature was maintained at 37.5°C with a closed-loop animal blanket system (FHC), and the response to pain and spontaneous whisking was monitored. Additional urethane was applied if mice developed spontaneous whisking or response to pain. At the end of the experiments, we removed the brain after heart perfusion first with saline and then with 4% paraformaldehyde in 0.1 M phosphate buffer. Later, we fixed and sliced the brain (50–200 μm thick) and visualized it with a fluorescent microscope to confirm expression of the ChETA ChR2 and determine the spatial extent of expression (Fig. 1). Typically, the two injections yielded expression of ChETA in a region with a length of 0.5 mm, width of 0.5 mm, and depth of 0.5–0.8 mm. Thus viral injections extended from 100–200 μm up to 700-1,000 μm from the pial surface. Thus we transfected PV interneurons in layers 2–5 of the neocortex.
Fig. 1.
Imaging ChETA expression in PV interneurons. PV-IRES-Cre mice were injected with the viral vector AAV2/1-EF1a-DIO-ChETA-EYFP into the S1 barrel cortex at a depth of 350 μm. Figure presents a confocal z-stack fluorescent image of YFP of the cortex at the injected site. Note in the image the nonpyramidal neurons expressing YFP (and concomitantly the ChETA variant of ChR2). Scale bar, 50 μm.
Electrographic seizure induction.
Electrographic seizures were induced by local application of the convulsant 4-AP onto the neocortical surface. The convulsant 4-AP was dissolved in aCSF (3 mM) and the 4-AP-containing aCSF solution was introduced into the dental cement well surrounding the craniotomy, after the well was emptied of the control solution it contained. After topical 4-AP application, recurrent spontaneous electrographic seizures developed in all mice examined (see results).
Electrophysiological recordings.
A single-shaft 16-contact silicon electrode (A16 probe with intercontact distance of 50 μm; NeuroNexus, Ann Arbor, MI) was inserted into the S1 barrel cortex with a micromanipulator (TSE systems, Bad Homburg, Germany). The reference electrode was placed in the aCSF-containing well surrounding the craniotomy. Recordings were performed with the ME-16 amplifier and MC-Rack software (Multichannel systems, Reutlingen, Germany). During the recordings, which were obtained at a sampling rate of 25 KHz, the recorded signals were amplified (×1,000) and filtered with two different band-pass filters: 0.1–300 Hz to record the local ECoG signal and 1–5 kHz to obtain unit activity for online viewing of the local ECoG and unit activity. In addition, we stored the data filtered at 0.1–10,000 Hz on the acquisition computer for further offline analysis.
Analysis of the data was performed for the most part offline. After completion of the experiments, the recorded data were replayed and filtered with two different band-pass filters, 0.1–300 Hz to obtain the local ECoG and 1–5 KHz to obtain spikes. The spike data were sorted to single-unit activity with the offline spike sorter OS-3 (Plexon, Dallas, TX) and later analyzed with NeuroExplorer software (Nex Technologies, Madison, AL) and homemade MATLAB-based software (MathWorks, Natick, MA). Further analysis of the data and plotting of graphs were performed with IGOR software (WaveMetrics, Portland, OR). Statistical analysis was performed with Student's t-test. Recordings were discontinued if a very long electrographic seizure (>15 min) occurred.
Optogenetic stimulation.
Optogenetic stimulation was performed with a 473-nm blue laser (OEM Laser Systems). The laser was connected to a light guide (diameter of 200 μm), which was held so that the end of the light guide was ∼1–1.5 cm above the cortical surface, such that the light spot covered the entire exposed cortical surface. Laser pulses were controlled with a stimulus generator (STG4, Multichannel systems). Optogenetic stimulation was activated manually after visual identification of electrographic seizures (for details see results). In our experiments we compared laser pulses with sham opto-stimulation, in which laser intensity was set to 0 mW.
To estimate the depth of our light penetration we characterized direct activation of ChR2-expressing PV interneurons by optogenetic pulses. Direct activation was defined as significant increase in action potential firing that occurred within 5 ms of the laser pulse onset. Unit recordings were performed with a 16-channel single-shaft electrode (NeuroNexus, Ann Arbor, MI) extending between 200 and 950 μm from the pia. The 473-nm laser pulses (20–50 ms) resulted in direct activation (within 5 ms) of putative PV interneurons up to ∼500 μm from the pia. The Technion Institutional Animal Ethics Committee approved all experiments.
RESULTS
4-Aminopyridine-induced neocortical electrographic seizures in vivo.
In this project, we investigated the antiepileptic effects of optogenetic stimulation of PV-expressing inhibitory interneurons on acute partial (focal) neocortical electrographic seizures. As our seizure model we used topical application of the potassium channel blocker 4-AP onto the pial surface of the S1 barrel cortex. To specifically activate PV interneurons we expressed the fast E123T ChR2 variant ChETA in PV interneurons of PV-IRES-Cre transgenic mice using the Cre-dependent ChETA gene containing viral vector AAV-EF1a-DIO-ChETA-EYFP (Fig. 1; for details see below).
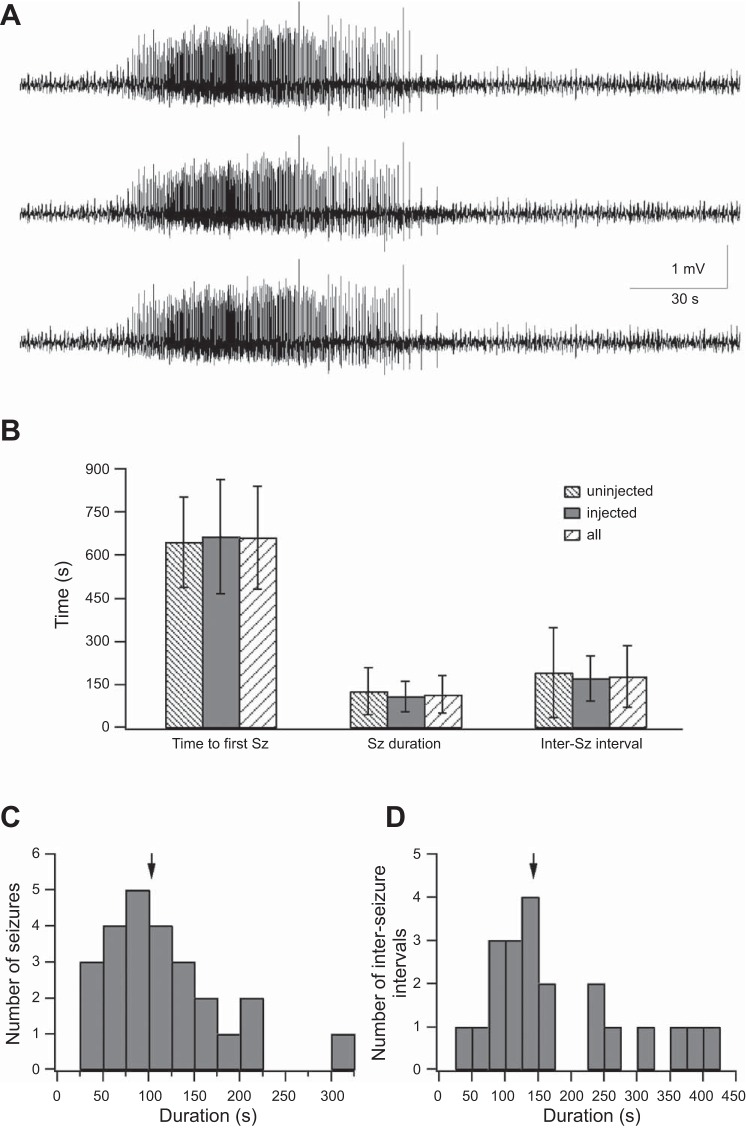
The first step in this project was to characterize the effect of the chemoconvulsant 4-AP in PV-IRES-Cre mice. The chemoconvulsant 4-AP (3 mM) was topically applied onto the surface of the S1 barrel cortex, and electrographic seizure activity was monitored with silicon-based electrodes (for details see methods). Similar to rats and wild-type mice 4-AP evoked recurrent electrographic seizures separated by interseizure quiescent periods also in PV-IRES-Cre mice (Fig. 2). Electrographic seizures were evoked in all 10 PV-IRES-Cre mice examined (3 control mice and 7 mice expressing the ChETA variant of ChR2 in PV interneurons). The average time interval between 4-AP application and onset of the first electrographic seizure was 659.8 ± 177.2 s (range of 468-1,005 s; Fig. 2B), the average electrographic seizure duration was 117 ± 65 s (Fig. 2C), and the average interseizure interval was 179.7 ± 106.7 s (Fig. 2D; 25 electrographic seizures). In some cases electrographic seizures evoked by local application of 4-AP onto the cortical surface of S1 were accompanied by visible clinical features. Semirhythmic whisker movements accompanied at times by twitching of the whisker pads were observed during 21.4% of electrographic seizures (6 of 28 electrographic seizures in 4 PV-Cre mice).
Fig. 2.
4-AP induced seizures in the S1 barrel cortex of PV-Cre mice. A: ECoG recorded simultaneously in 3 channels from the S1 barrel cortex of a PV-Cre mouse during a seizure recorded after application of 3 mM 4-AP onto the neocortical surface. B: average time to first seizure (Sz) (left), seizure duration (center), and interseizure interval (right) are presented in control PV-Cre mice uninjected with the viral vector (4 mice), in PV-Cre mice injected with the viral vector AAV2/1-EF1a-DIO-ChETA-EYFP (5 mice, injected), and in the combined group of both injected and uninjected mice. C: duration of 25 individual seizures. Arrow marks the average value. D: duration of individual interictal intervals. Arrow marks the average value.
Optogenetic activation of PV-expressing interneurons during electrographic seizures.
PV-expressing neurons are a major class of cortical inhibitory interneurons. As such, activation of PV-expressing interneurons is expected to inhibit neighboring excitatory neurons and exert powerful antiepileptic effects. To explore this possibility we investigated the effect of optogenetic activation of PV-expressing interneurons to terminate on 4-AP-induced electrographic seizures. In these experiments, we locally injected the AAV-EF1a-DIO-ChETA-EYFP viral vector into the S1 barrel cortex of PV-IRES-Cre transgenic mice. As PV-IRES-Cre mouse PV interneurons coexpress the enzyme Cre recombinase, and our viral vector is Cre-dependent, the fast ChR2 variant ChETA is specifically expressed in the Cre-expressing PV interneurons (Fig. 1). In turn, these neurons can be optogenetically activated with 473-nm light pulses.
We tested the antiepileptic effects of two stimulation paradigms applied during electrographic seizures, 5- to 10-s pulses (8 mice), and trains of 10 ms pulses (9 mice). The stimulation trains were applied manually once the seizures were identified visually. The timing of stimulation relative to the onset of electrographic seizures was analyzed offline at the end of the experiments, and stimulation trains that were applied >5 s after electrographic seizure onset were excluded from analysis. On average stimulation was initiated 2.9 ± 1.7 s (0.6–5 s) after the onset of electrographic seizures. In these experiments, the duration and fraction of electrographic seizures terminated during the optogenetic stimulation were compared with sham stimulation (in which laser intensity was set to 0 mW).
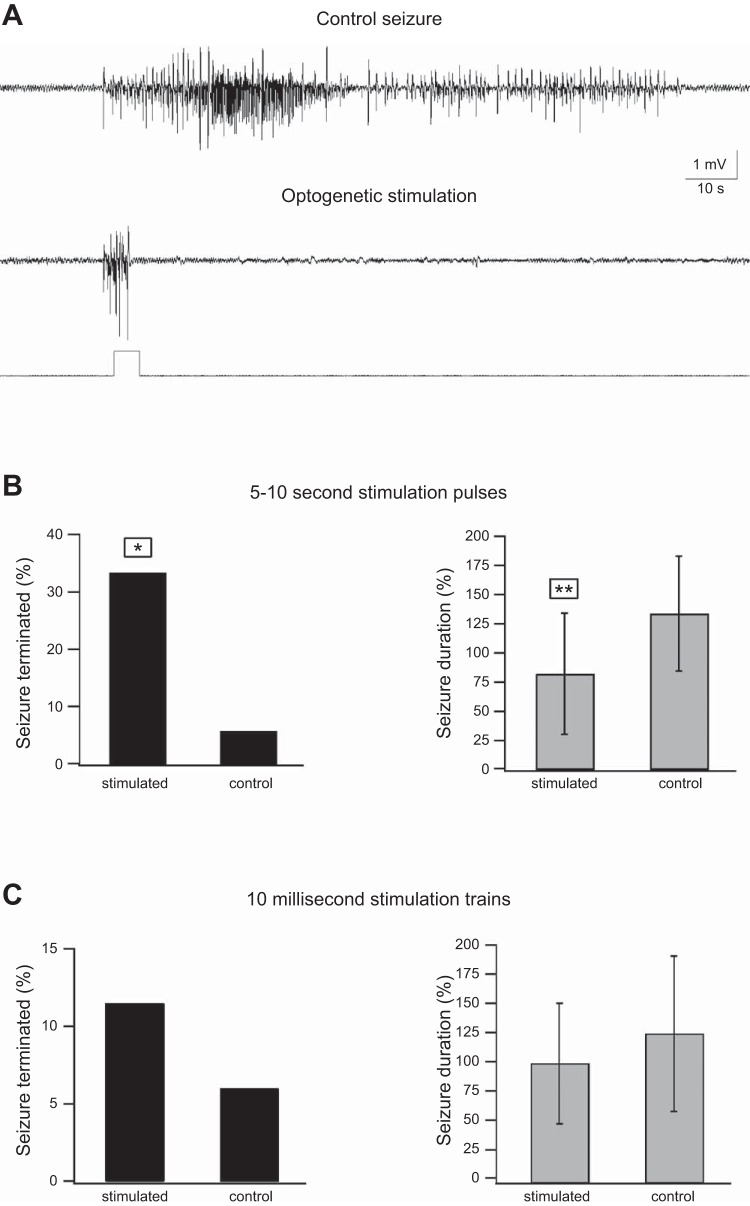
The results of these experiments revealed that during 5- to 10-s optogenetic stimulation pulses 33.7% of electrographic seizures terminated compared with only 5.6% during similar sham stimulation (Fig. 3B, left). Moreover, the average duration of seizures that were not terminated during the optogenetic stimulation pulse was shortened by 38.6% compared with sham stimulation (Fig. 3B, right). In contrast, short 10-ms opto-stimulation pulses applied either in low-frequency trains (5 pulses at 0.4 Hz) or in high-frequency trains (10-s, 10-Hz trains repeated 5 times at 0.2 Hz) showed no significant effects on either the fraction of electrographic seizures terminated during stimulation or electrographic seizure duration (Fig. 3C).
Fig. 3.
Ictal optogenetic stimulation of PV-expressing interneurons. Seizures were evoked by local application of 3 mM 4-AP onto the S1 barrel cortex, and optogenetic stimulation of PV-expressing interneurons was applied during seizures. A: example of a control unstimulated seizure (top) and a seizure terminated by 5-s optogenetic stimulation (bottom). In these experiments 2 different ictal stimulation paradigms were examined: 5- to 10-s pulses (A and B; 36 seizures in 8 rats, 18 ontogenetically stimulated and 18 sham stimulated) and trains of 10-ms pulses (C; either 5 individual 10-ms pulses applied at 0.4 Hz or 5 10-Hz, 1 s trains of 10-ms pulses applied at 0.2 Hz; 52 seizures in 9 rats, 26 optogenetically stimulated and 26 sham stimulated). The effect of the 2 ictal optogenetic stimulation paradigms is presented as % of seizures terminated during stimulation (right) and the average (mean ± SD) seizure duration of seizures that persisted beyond the duration of the optogenetic pulse (left). *P < 0.05, **P < 0.01 by either χ2 (fraction of seizures terminated) or Student's t-test (mean seizure duration). Note that 5- to 10-s optogenetic stimulation pulses of PV-expressing interneurons significantly increased the fraction of seizures terminated during the opto-stimulation and significantly decreased the average seizure duration, while 10-ms pulse trains showed no significant effects on these parameters.
Optogenetic activation of PV-expressing interneurons during the interictal phase.
We next examined the effect of optogenetic stimulation of PV-expressing interneurons during the quiescent interictal period between electrographic seizures (Fig. 2, B and D). We again investigated both short (10 ms) and longer (5–10 s) stimulation pulses and compared them to sham stimulation (0-mW laser intensity). Interictal stimulation was applied manually based on visual inspection of the electrophysiological recordings. The relative time difference between onset of stimulation and termination of the last seizure was determined offline at the end of the experiments, and cases in which stimulation was initiated <1 min from termination of the last electrographic seizure were excluded from analysis. On average, stimulation was initiated 107.9 ± 48.1 s from the end of the last seizure. Cases in which stimulation was applied prior to initiation of the first seizure were not included in calculation of this average value.
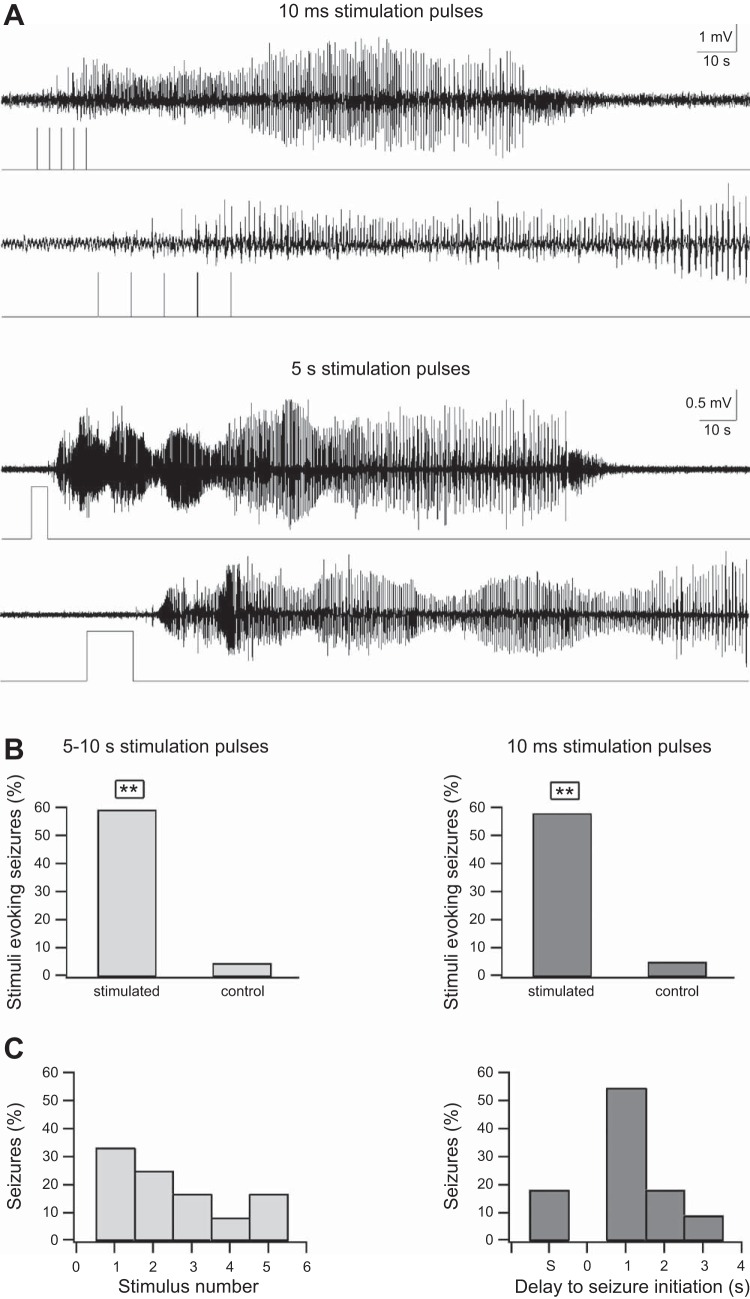
To our surprise both short (10-ms pulses repeated 5 times at 0.4 Hz) and longer (5–10 s) optogenetic stimulation pulses applied during the interictal period resulted in electrographic seizure initiation (Fig. 4). In both stimulation paradigms, slightly more than half of stimuli generated electrographic seizures compared with only ∼5% of sham stimulations (Fig. 4, A and B). In the case of 10-ms trains electrographic seizures initiated after one to four stimulation pulses (Fig. 4C). In the case of 5- to 10-s optogenetic pulses electrographic seizures typically initiated within 1–3 s after termination of the laser pulse (Fig. 4D).
Fig. 4.
Interictal optogenetic stimulation of PV-expressing interneurons. A: examples of optogenetic stimulation of PV-expressing interneurons activated during the interictal quiescent period between seizures. Two optogenetic paradigms were used: a 0.4-Hz train of five 10-ms pulses (top) and a 5-s pulse (bottom). For each example, the traces are shown at an expanded timescale in the lower trace. Note that both interictal optogenetic stimulation paradigms evoked a seizure and that for the 5-s pulse the seizure initiated after the laser pulse ended. B: % of interictal stimuli that evoked seizures during the 5- to 10-s stimuli (left) and the 0.4-Hz trains of five 10-ms optogenetic stimuli (right). **P < 0.01. The results of the optogenetic stimulation trains are compared with similar sham stimulation trains (control). C: relative timing between optogenetic stimulation and seizure initiation. Left: data for the 5- to 10-s optogenetic stimulation. Right: data for the 0.4-Hz trains of five 10-ms optogenetic pulses; S on the x-axis designates seizures that were terminated during stimulation.
Optogenetic stimulation of PV interneurons evoked electrographic seizures when applied both prior to initiation of the first electrographic seizure (at least 5 min after topical application of the 4-AP) and during the quiescent interictal intervals between electrographic seizures (at least 1 min after termination of the previous electrographic seizure). Optogenetic stimulation of PV interneurons during control conditions did not evoke electrographic seizures (7 mice). However, in one of seven mice we observed an oscillatory response that outlasted the laser pulse by several seconds after the optogenetic activation (data not shown).
Mechanism underlying electrographic seizure initiation by optogenetic activation of PV-expressing interneurons.
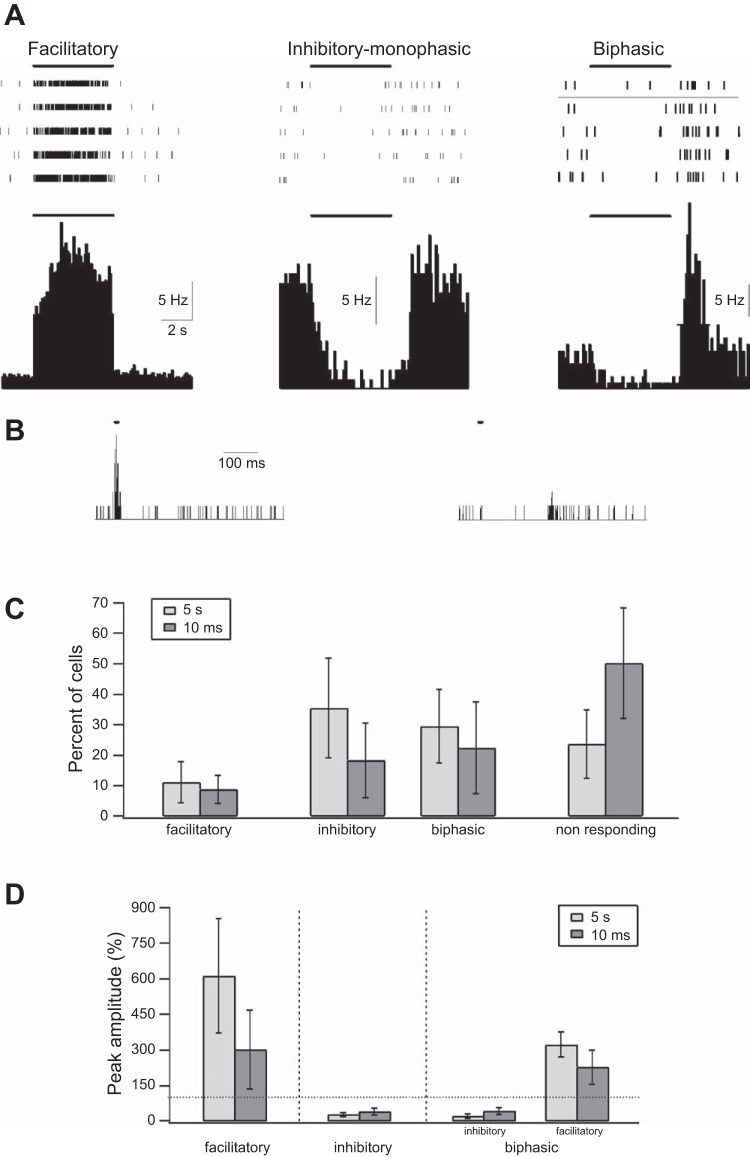
To investigate the mechanisms underlying the antiepileptic and ictogenic effects of PV interneuron opto-stimulation we performed single-unit recordings during optogenetic activation of PV interneurons. The experiments were performed both under control conditions and in the presence of 4-AP. Under control conditions we were able to repeat stimulation multiple times to generate more reliable peristimulus histograms (PSTHs), while in the presence of 4-AP more than half of the optogenetic stimulation pulses were followed by electrographic seizures, and thus we were limited in averaging only a few stimuli. Under control conditions, neurons showed two fundamentally different responses to the laser pulses. The first subset of recorded neurons, which probably represented PV interneurons, responded to the opto-stimulation with a rapid large increase in firing that lasted throughout the duration of the laser pulse. In these neurons, which constituted ∼10% of recorded neurons (8.9 ± 4.6% and 11.2 ± 6.8% for 10-ms and 5- to 10-s pulses), firing increased within 2–3 ms of laser onset and the peak response reached an average of 302 ± 165% for 10-ms pulses and 614 ± 241% for 5-s pulses (Fig. 5). These neurons probably represented PV interneurons that expressed ChR2 in a Cre-dependent manner. As a criterion for putative PV interneurons directly activated by the laser pulses we used a significant increase in firing within 5 ms of the laser onset. Interestingly, 68% of these putative PV interneurons showed a narrow spike waveform (<0.5 ms between trough and peak), further supporting the fact these neurons represented PV interneurons (Barthó et al. 2004).
Fig. 5.
Single-unit response to optogenetic activation under control conditions. A: response of 3 individual single units located in the S1 barrel cortex during a 5-s optogenetic activation of PV-expressing interneurons. Top: raster plots from 5 individual trials during opto-stimulation. Bottom: cumulative peristimulus histograms (PSTHs) of 3 individual neurons to the 5-s opto-stimulation (time bin of 0.1 s). The 3 neurons shown present examples of the different responses to 5-s optogenetic stimulation pulses. The first (left) shows a facilitatory response with rapid increase of firing that probably occurs in PV interneurons directly activated by the laser pulse. The second (center) shows a monophasic reduction in firing, probably occurring in pyramidal neurons secondarily inhibited by PV interneurons. The third (right) shows a biphasic response consisting of an initial suppression of firing followed by rebound-increased firing after the end of the optogenetic stimulation. The thick line marks the time of optogenetic stimulation. B: PSTH during 10-ms optogenetic stimuli is shown for 2 neurons (time bin of 1 ms). The first (left) responded with a rapid increase of firing to the optogenetic stimulus and represented a putative inhibitory interneuron, while the second (right) responded with a biphasic response and probably represented an excitatory pyramidal neuron. C: % of recorded neurons showing facilitatory, inhibitory monophasic, and biphasic inhibitory-excitatory responses and no significant responses during 10-ms and 5-s optogenetic stimulations. D: average peak amplitude of the different responses to the 10-ms and 5-s optogenetic pulses. The dotted line marks the prestimulus baseline value.
A second larger subset of neurons consisting of 49.8 ± 18.1% (10-ms pulses) and 76.3 ± 11.2% (5-s pulses) of recorded neurons responded to opto-stimulation with a reduction in firing. On average in these neurons the firing frequency nadir reached 42.5 ± 14.6% and 26 ± 8.9% of the control value for the 10-ms and 5-s pulses and the onset of suppression of firing was typically observed only 10–30 ms after onset of the laser pulse (Fig. 5). This response probably occurred in both pyramidal neurons and other interneurons secondarily inhibited by the optogenetically activated PV-expressing interneuron. Consistent with this possibility, all but four neurons with inhibitory responses had wider spike waveforms (>0.5 ms) (Barthó et al. 2004). In this respect it is important to stress that the majority of neurons innervated by PV-expressing interneurons are excitatory pyramidal neurons, yet other subtypes of inhibitory interneurons are also directly innervated by PV interneurons. The remaining neurons showed no significant change in firing following opto-stimulation of PV interneurons (Fig. 5C). Interestingly, about half of the presumptive pyramidal neurons, which demonstrated an initial suppression of firing during optogenetic stimulation, developed a second delayed phase in which firing markedly increased at the end of the laser pulse. This biphasic suppression-excitation response was observed in 22.5 ± 15.1% and 29.6 ± 12.1% of neurons after 10-ms and 5-s optogenetic pulses, respectively (Fig. 5). In all neurons, enhanced firing developed after optogenetic stimulation ended (for example see Fig. 5A). On average, the late enhanced firing response that occurred at the end of opto-stimulation had a peak of 228.7 ± 72.3% and 323.3 ± 52.4% of the average control prestimulus firing rate after 10-ms and 5-s optogenetic pulses. The average duration (half-width) of the late rebound firing response was 0.82 ± 0.48 s and 1.13 ± 0.54 s after 10-ms and 5-s optogenetic pulses. Interestingly, rebound increased firing was synchronized between different presumptive excitatory pyramidal neurons. When we calculated the pairwise joint PSTH we found that almost all pairs of neurons with delayed rebound responses showed a significant positive peak at the end of opto-stimulation (data not shown).
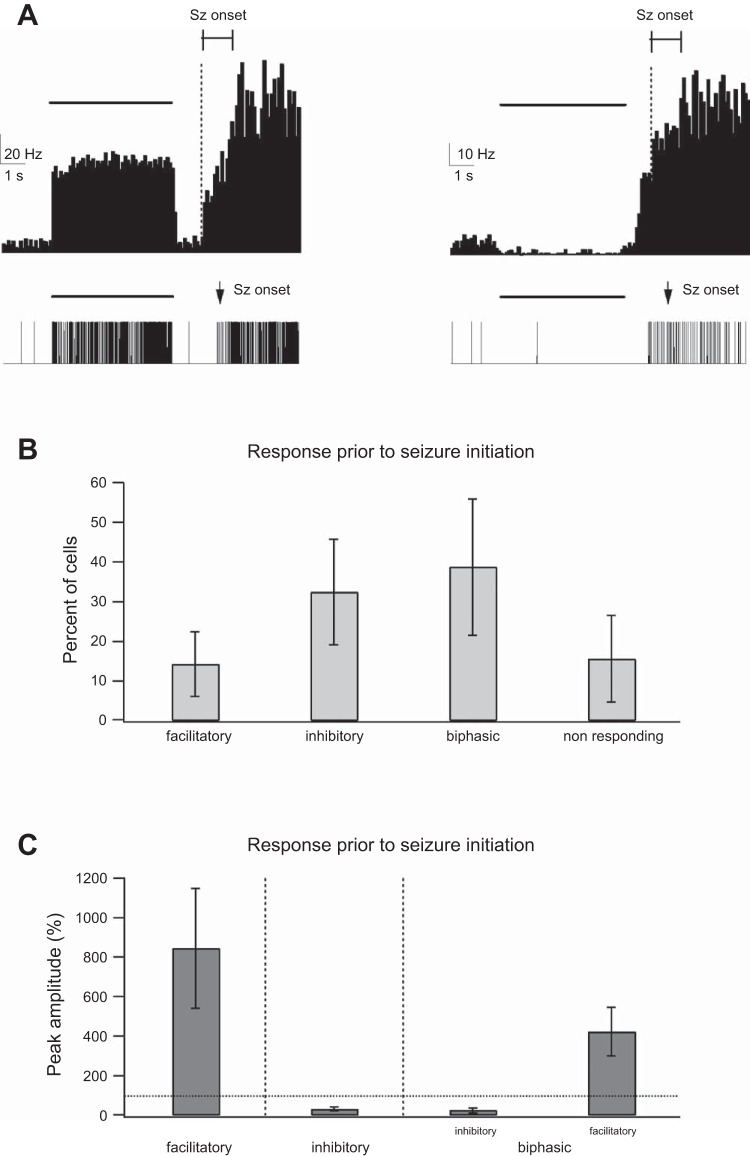
We repeated single-unit analysis in the presence of 4-AP. As mentioned above, in the presence of 4-AP we were limited in the number of repetitions, as more than half of the optogenetic stimuli induced electrographic seizures. Despite the small number of repetitions (typically 5–10 per mouse), similar to control conditions we observed several subtypes of responses to optogenetic stimulation in the presence of 4-AP. Presumptive PV interneurons, which constituted 14.3 ± 8.2% of recorded neurons, responded with a rapid increase in firing during the 5-s laser pulses (Fig. 6). At the end of the laser pulse firing frequency decreased in presumptive PV interneurons but rapidly increased again in the cases where electrographic seizures initiated (Fig. 6A). In the remaining neurons, we observed three additional subtypes of responses. First, we observed presumptive pyramidal neurons that decreased their firing during the laser pulse (monophasic inhibitory response) and rapidly increased firing if electrographic seizures initiated. Second, we observed presumptive pyramidal neurons that decreased their firing during laser activation, followed by increased firing at the end of the laser pulse (biphasic inhibitory-excitatory response), and further increased firing if electrographic seizures initiated. Third, we observed neurons that did not respond significantly during the laser pulse but rapidly increased their firing in cases where electrographic seizures initiated. Figure 6B describes the relative percentage of the different neuronal responses during optogenetic activation in the presence of 4-AP. It is important to stress that the unit response to electrographic seizures in our averaged response is artificially graded and reduced as only a fraction of laser pulse-initiated electrographic seizures (and hence a reduction in the response amplitude) and electrographic seizure initiation was not synchronized (and hence the graded response).
Fig. 6.
Single-unit response to optogenetic activation in the presence of 4-AP. A, top: peristimulus histogram (PSTH) of 2 individual single units located in the S1 barrel cortex during optogenetic activation of PV-expressing interneurons (the PSTH averages 7 optogenetic stimuli that led to seizure initiation). Bottom: single-unit activity of the 2 neurons during a single seizure. The individual neurons present examples of 2 different responses to 5-s optogenetic stimulation pulses in the presence of 4-AP (3 mM applied onto the neocortical surface). The first (left) is of a neuron responding with a facilitatory response to the optogenetic stimulation. When seizures initiated, the firing frequency rapidly increased. The second (right) shows a neuron with a biphasic inhibitory-excitatory response consisting of an initial suppression of firing followed by rebound-increased firing after the end of the optogenetic stimulation, which preceded seizure onset. When seizures initiated, firing further increased. The thick horizontal lines mark the timing of optogenetic stimulation. In both cases, a fraction of optogenetic stimulations evoked seizures. The thin horizontal line with boundaries shows the time window in which seizures initiated in the different optogenetic stimuli, and the vertical dotted line marks the initiation of the earliest seizure. It is important to stress that while the onset of individual seizures was associated with an abrupt increase in the firing frequency, the averaged response of several seizures showed a graded onset, as seizures initiated at different times relative to the opto-stimulation. B: % of recorded neurons showing facilitatory, monophasic inhibitory, and biphasic inhibitory-excitatory responses and no significant response during a 5-s optogenetic stimulation. C: average peak amplitude of the different responses to the 5-s optogenetic pulses. The dotted line marks the prestimulus baseline value.
DISCUSSION
In this study, we investigated the effect of optogenetic activation of PV-expressing interneurons during the ictal and interictal phases of 4-AP-induced epileptic electrographic seizures in vivo. The two main findings of this study are as follows. First, 5- to 10-s optogenetic activation of PV interneurons during electrographic seizures (ictal phase) possessed antiepileptic effects, manifesting as both termination of approximately a third of electrographic seizures and shortening of the average electrographic seizure duration by almost 40%. Single-unit recordings revealed that these ictal antiepileptic effects probably result from suppression of firing in excitatory pyramidal neurons during the optogenetic pulse. Second, activation of PV interneurons during the interictal phase resulted in effective and robust initiation of electrographic seizures. Single-unit recordings revealed that the powerful ictogenic effect of interictal PV interneuron activation probably resulted from rebound enhancement of firing of excitatory pyramidal neurons at the end of the optogenetic stimulation pulse.
To stimulate PV interneurons in our study we relied on the specificity of Cre recombinase expression in PV interneurons of the PV-Cre transgenic mouse line we used. We did not directly confirm the overlap between PV interneurons and viral expression with immunohistochemical staining of PV in our mice. Previous studied have examined this question and described a very high degree of specificity of Cre recombinase expression to PV-expressing interneurons in the PV Cre transgenic mouse line we worked on (Runyan et al. 2010; Sohal et al. 2009; Tanahira et al. 2009; Yi et al. 2014). Nevertheless, we cannot exclude the possibility that ChR2 is also expressed in some excitatory pyramidal neurons or other subtypes of inhibitory interneurons.
A second issue in our experiments deals with the efficacy of ChR2 expression in PV interneurons. Again, we did not obtain direct data from the mice of our experiments, yet recently experiments have been performed in the lab addressing this question in another set of mice. In these experiments, ChR2 was expressed in PV-Cre mice with a Cre-dependent viral vector, and PV expression imaged with immunohistochemical staining (red) was compared with ChR2 expression in individual neurons (EYFP). These experiments revealed large inter- and intramouse variability in ChR2 expression. Specifically, within the same mouse the percentage of neurons expressing ChR2 markedly declined in the periphery of the ChR2-expressing region. Moreover, the percentage of PV neurons expressing ChR2, at the region of maximal expression, varied between 36% and 68% in different mice (n = 4; J. Schiller, personal communication). These findings are consistent with previously published data (Sohal et al. 2009). Considering these findings, it is possible that increasing the efficacy of ChR2 expression or the brain volume in which ChR2 is expressed will enhance the antiepileptic efficacy of optogenetic stimulation of PV interneurons.
In this study we did not investigate the cellular mechanisms underlying the rebound firing in pyramidal neurons following synchronized activation of PV interneurons, yet we hypothesize participation of three synergistic mechanisms in this phenomenon. First, and probably most important, is the fact the synchronized inhibitory postsynaptic potential is gradually diminishing and, as a result, the E/I balance is shifted toward excitation and action potential firing in the population. Second, the diminishing hyperpolarization can reduce the threshold for action potential initiation because of effects on voltage-gated sodium channel. Third, the declining hyperpolarization can secondarily activate h channels and t-type voltage-gated calcium channels that in turn can assist in action potential initiation.
Cortical neurostimulation is an emerging new therapy for drug-resistant epilepsy (Fisher 2012; Fisher and Velasco 2014). While conventional neurostimulation utilizes electrical stimulation, recent developments raise the possibility that optogenetic stimulation can serve as an alternative to conventional electrical stimulation. Optogenetic stimulation holds several advantages over conventional electrical stimulation. It can generate either excitatory or inhibitory effects on the stimulated neurons depending on the light-sensitive channel or pump used (Chow et al. 2012; Fenno et al. 2011; Tye et al. 2012). In addition, optogenetic stimulation can target specific cell types such as excitatory pyramidal neurons or subtypes of inhibitory interneurons (Yizhar et al. 2011). In this study, we used the advantage of the optogenetic stimulation and specifically activated PV-expressing interneurons.
PV-expressing interneurons are the most common subtype of interneurons in the neocortex. They tend to innervate the soma, axon, and proximal dendrites of excitatory pyramidal and stellate neurons, and as such PV interneurons play a critical role in controlling cortical excitability (Rudy et al. 2011; Sultan et al. 2013). In this study, we first investigated the antiepileptic effects of ictal optogenetic activation of PV-expressing interneurons. We found that activation of PV-expressing interneurons during electrographic seizures prematurely terminated ongoing electrographic seizures and significantly shortened the average electrographic seizure duration. We further found that prolonging optogenetic pulses increased their antiepileptic efficacy. While trains of short (10-ms) pulses showed no significant antiepileptic effects, longer stimuli lasting several seconds did prematurely terminate and shortened ongoing electrographic seizures.
The antiepileptic effects of PV interneuron opto-stimulation are consistent with previous in vitro studies that reported elimination of 4-AP-induced seizurelike events in hippocampal-entorhinal brain slices by optogenetic activation of either PV-expressing interneurons or all subclasses of inhibitory interneurons (Ledri et al. 2014). In this study, the antiepileptic efficacy of optogenetic stimulation was much larger when all interneurons were stimulated, compared with selective stimulation of PV-expressing interneurons (Ledri et al. 2014). It is important to stress that in contrast to the study presented by Ledri et al. (2014), which was performed in brain slices in vitro, we performed our experiments in vivo.
In contrast to the antiepileptic effects of ictal optogenetic activation, interictal activation of PV interneurons possessed powerful ictogenic effects. Our findings are consistent with the results of recent studies that have shown initiation of seizurelike events in hippocampal-entorhinal brain slices in vitro by optogenetic activation of PV-expressing interneurons (Shiri et al. 2015; Yekhlef et al. 2015). Our findings further strengthen the ictogenic effect of PV-expressing interneurons but also add additional information to the previously published studies. First, we show the ictogenic effect of PV interneurons in the neocortex in addition to the hippocampal-entorhinal region; second, we show the ictogenic effect of PV interneuron stimulation in vivo, in contrast to the previous studies that were performed in brain slices in vitro. Third and most importantly, we add a mechanistic dynamical layer and show that the ictogenic effects of PV interneuron activation probably result from postinhibition rebound increased firing in excitatory neurons.
The finding regarding the ictogenic effect of PV-expressing interneurons holds important implications for the pathogenesis of seizure initiation on the one hand and for the potential therapeutic use of this stimulation paradigm on the other.
Previous studies have suggested the role of synchronized activation of interneurons in the interictal to ictal state transition (seizure initiation) (Avoli and de Curtis 2011; de Curtis et al. 2009; Gnatkovsky et al. 2008; Gonzalez-Sulser et al. 2012; Shiri et al. 2015; Uva et al. 2015). According to this hypothesis, synchronized firing of inhibitory interneurons is followed by activation of excitatory pyramidal neurons and in turn self-sustained ictal synchronized hyperactivity (de Curtis et al. 2009; Avoli and de Curtis 2011). Consistent with this hypothesis are previous studies that reported increased firing in interneurons prior to initiation of chemoconvulsant-induced electrographic seizures in vitro (Avoli and de Curtis 2011; Gnatkovsky et al. 2008; Gonzalez-Sulser et al. 2012; Uva et al. 2015) and the ability to initiate seizurelike events in vitro by optogenetic activation of PV- (and somatostatin)-expressing interneurons (Shiri et al. 2015; Yekhlef et al. 2015). However, it is important to stress that although the data obtained, including from our work, do prove that synchronized activation of inhibitory interneurons can initiate seizures in vitro and in vivo, it still has to be proven with additional experiments that this mechanism participates in initiation of spontaneous seizures in chronic epilepsy. Moreover, a recent study has reported that PV interneurons probably participate in generation of pilocarpine-evoked electrographic seizures in the hippocampus by induction of a depolarization block in PV interneurons (Yi et al. 2015).
Optogenetic stimulation is an emerging potential treatment modality for drug-resistant epilepsy (Berglind et al. 2014; Chiang et al. 2014; Krook-Magnuson et al. 2014, 2015; Ledri et al. 2014; Paz et al. 2013; Paz and Huguenard 2015a; Ritter et al. 2014). An especially attractive approach is to suppress neocortical excitability by selectively activating inhibitory interneurons. Our findings show that this approach indeed has antiepileptic effects. However, it also carries the potential for significant adverse events of initiating seizures when activated in the interictal period. As seizure detection and prediction algorithms have false positive alarms, this adverse effect may be a significant one. Further studies are required to develop safer interneuron stimulation paradigms that retain the ictal antiepileptic efficacy but eliminate their interictal ictogenic effects.
GRANTS
This study was supported by the Israel Science Foundation (ISF), the Adelis Foundation, and the Prince Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.A. performed experiments; F.A. and Y.S. analyzed data; F.A. and Y.S. interpreted results of experiments; F.A. and Y.S. prepared figures; Y.S. conception and design of research; Y.S. drafted manuscript; Y.S. edited and revised manuscript; Y.S. approved final version of manuscript.
REFERENCES
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol 95: 104–132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92: 600–608, 2004. [DOI] [PubMed] [Google Scholar]
- Bergey GK. Neurostimulation in the treatment of epilepsy. Exp Neurol 244: 87–95, 2013. [DOI] [PubMed] [Google Scholar]
- Berglind F, Ledri M, Sørensen AT, Nikitidou L, Melis M, Bielefeld P, Kirik D, Deisseroth K, Andersson M, Kokaia M. Optogenetic inhibition of chemically induced hypersynchronized bursting in mice. Neurobiol Dis 65: 133–141, 2014. [DOI] [PubMed] [Google Scholar]
- Blauwblomme T, Jiruska P, Huberfeld G. Mechanisms of ictogenesis. Int Rev Neurobiol 114: 155–185, 2014. [DOI] [PubMed] [Google Scholar]
- Chiang CC, Ladas TP, Gonzalez-Reyes LE, Durand DM. Seizure suppression by high frequency optogenetic stimulation using in vitro and in vivo animal models of epilepsy. Brain Stimul 7: 890–899, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Boyden ES. Genetically encoded molecular tools for light-driven silencing of targeted neurons. Prog Brain Res 196: 49–61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: The role of low-voltage fast activity. Epilepsia 50: 2514–525, 2009. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvárday Z, Kubota Y, Lewis DA, Marín O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamás G, Thomson A, Wang Y, Yuste R, Ascoli GA. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14: 202–216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio CM, Krahl SE. Neurostimulation for drug-resistant epilepsy. Continuum (Minneap Minn) 19: 743–755, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 34: 389–412, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS. Therapeutic devices for epilepsy. Ann Neurol 71: 157–168, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol 10: 261–270, 2014. [DOI] [PubMed] [Google Scholar]
- Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol 64: 674–686, 2008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sulser A, Wang J, Queenan BN, Avoli M, Vicini S, Dzakpasu R. Hippocampal neuron firing and local field potentials in the in vitro 4-aminopyridine epilepsy model. J Neurophysiol 108: 2568–2580, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention Inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro 1: e.2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Soltesz I. Beyond the hammer and the scalpel: selective circuit control for the epilepsies. Nat Neurosci 18: 331–338, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med 365: 919–926, 2011. [DOI] [PubMed] [Google Scholar]
- Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J Neurosci 34: 3364–3377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. [DOI] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci 16: 64–70, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Huguenard JR. Optogenetics and epilepsy: past, present and future. Epilepsy Curr 15: 34–38, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Huguenard JR. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat Neurosci 28: 351–359, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Golshani P, Takahashi K, Dufour S, Valiante T, Kokaia M. WONOEP appraisal: optogenetic tools to suppress seizures and explore the mechanisms of epileptogenesis. Epilepsia 55: 1693–1702, 2014. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71: 45–61, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron 67: 847–857, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol 77: 541–546, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan KT, Brown KN, Shi SH. Production and organization of neocortical interneurons. Front Cell Neurosci 21: 221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics 11: 553–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahira C, Higo S, Watanabe K, Tomioka R, Ebihara S, Kaneko T, Tamamaki N. Parvalbumin neurons in the forebrain as revealed by parvalbumin-Cre transgenic mice. Neurosci Res 63: 213–223, 2009. [DOI] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13: 251–266, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology 69: 45–54, 2013. [DOI] [PubMed] [Google Scholar]
- Uva L, Breschi GL, Gnatkovsky V, Taverna S, de Curtis M. Synchronous inhibitory potentials precede seizure-like events in acute models of focal limbic seizures. J Neurosci 35: 3048–3055, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in the mouse medial entorhinal cortex. J Neurophysiol 113: 1616–1630, 2015. [DOI] [PubMed] [Google Scholar]
- Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL, Holloway BB, Johnston AD, Nathanson NM, Deisseroth K, Gerber DJ, Tonegawa S, Lawrence JJ. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol 592: 3463–3494, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, DeCan E, Stoll K, Marceau E, Deisseroth K, Lawrence JJ. Muscarinic excitation of parvalbumin-positive interneurons contributes to the severity of pilocarpine-induced seizures. Epilepsia 56: 297–309, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron 71: 9–34, 2011. [DOI] [PubMed] [Google Scholar]