Abstract
Background. In the United States, >40% of people infected with human immunodeficiency virus (HIV) smoke cigarettes.
Methods. We used a computer simulation of HIV disease and treatment to project the life expectancy of HIV-infected persons, based on smoking status. We used age- and sex-specific data on mortality, stratified by smoking status. The ratio of the non-AIDS-related mortality risk for current smokers versus that for never smokers was 2.8, and the ratio for former smokers versus never smokers was 1.0–1.8, depending on cessation age. Projected survival was based on smoking status, sex, and initial age. We also estimated the total potential life-years gained if a proportion of the approximately 248 000 HIV-infected US smokers quit smoking.
Results. Men and women entering HIV care at age 40 years (mean CD4+ T-cell count, 360 cells/µL) who continued to smoke lost 6.7 years and 6.3 years of life expectancy, respectively, compared with never smokers; those who quit smoking upon entering care regained 5.7 years and 4.6 years, respectively. Factors associated with greater benefits from smoking cessation included younger age, higher initial CD4+ T-cell count, and complete adherence to antiretroviral therapy. Smoking cessation by 10%–25% of HIV-infected smokers could save approximately 106 000–265 000 years of life.
Conclusions. HIV-infected US smokers aged 40 years lose >6 years of life expectancy from smoking, possibly outweighing the loss from HIV infection itself. Smoking cessation should become a priority in HIV treatment programs.
Keywords: smoking, tobacco, smoking cessation, life expectancy, HIV, United States, mathematical model
(See the editorial commentary by Althoff on pages 1618–20.)
Cigarette smoking is the leading preventable cause of death in the United States [1]. Although the prevalence of smoking among US adults decreased from 42% in 1965 to 17% in 2014 [2, 3], smoking still accounts for >480 000 deaths in the United States annually [1]. Smokers lose about a decade of life compared with nonsmokers in the general population, but they can regain much of this through smoking cessation [4].
Among HIV-infected people, smoking is also a major cause of morbidity and mortality [5–7]. Over 40% of HIV-infected people in care in the United States smoke; an additional 20% are former smokers [8]. Moreover, HIV-infected smokers appear to face higher rates of cardiovascular disease, chronic obstructive pulmonary disease, and numerous primary cancers compared with rates expected from smoking itself [9–11]. While antiretroviral therapy (ART) has improved their life expectancy [12, 13], HIV-infected people are now living long enough to develop smoking-associated diseases. Unfortunately, smoking cessation interventions have not been widely implemented in HIV care.
We sought to estimate the impact of smoking on the survival of HIV-infected people. We used a simulation model to compare the life expectancies of smokers and nonsmokers among HIV-infected people in the United States and the potential gain in life expectancy from smoking cessation.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications–US model, a validated, widely published Monte Carlo microsimulation of HIV natural history and treatment [14–17], to project the life expectancy of HIV-infected US people, based on smoking status (current, former, or never), sex, and age at entry to HIV care. We defined life expectancy as the mean age at death, for ease of comparison across sensitivity analyses. We calculated it by adding the age at entry to the model-generated mean remaining years of life from the time of entry, to provide the age at death. We simulated cohorts of 1 million individuals to achieve stable per-person estimates.
To estimate the number of years of life lost from smoking, we compared the model-generated life expectancies of current and never smokers, with both groups entering HIV care at the same age and with the same distributions of CD4+ T-cell count and viral load. We compared years of life lost from smoking with years of life lost from HIV infection (with the latter calculated as the life expectancy of HIV-negative smokers minus the life expectancy of HIV-positive smokers). To derive the potential number of years of life gained from smoking cessation, we compared the model-generated life expectancies of former and current smokers. We further estimated the cumulative potential years of life gained if 10%–25% of HIV-infected current smokers in the United States quit smoking.
Model Overview
HIV-infected, ART-naive individuals enter the model upon initial linkage to HIV care and are followed until death. Following current guidelines, all simulated individuals are eligible to initiate ART (dolutegravir/abacavir/lamivudine or a similarly efficacious regimen) immediately upon entering care, regardless of CD4+ T-cell count [18]; thus, the age at entry to care is equivalent to the age at ART initiation. For each simulated individual, the model draws randomly from user-defined initial distributions of CD4+ T-cell count and viral load and then tracks clinical outcomes as the individual transitions monthly through various states of disease progression and treatment. Probabilities of transition between health states are defined by CD4+ T-cell count, viral load, history of opportunistic disease, and ART use. Treatment efficacy (ie, the probability of viral suppression) is positively correlated with an individual's adherence; the latter is negatively correlated with the probability of loss to follow-up. Without effective ART, the CD4+ T-cell count falls, raising mortality risks related to opportunistic diseases and chronic AIDS. The model also allows the user to specify non–AIDS-related mortality by age and sex.
Smoking Status and Non–AIDS-Related Mortality
Within each simulation, all individuals had the same smoking status. We set mortality probabilities for current and former smokers equal to those for never smokers until age 40 years, assuming that few deaths prior to that age are smoking related [4, 19]. Starting at age 40 years, we used different age- and sex-specific monthly probabilities of non–AIDS-related death, based on smoking status.
We assumed, in the base case, that never and current smokers remained in their respective smoking status until death. In each cohort of former smokers, all individuals stopped smoking at the same age (upon entering HIV care, unless otherwise specified) and remained abstinent; we relaxed this continuous abstinence assumption in a sensitivity analysis. We did not evaluate those who had quit smoking prior to entering care (except in a sensitivity analysis of years since cessation) because we sought to estimate the potential survival benefit if a smoking cessation intervention were provided as part of HIV care. Because the full benefits of smoking cessation do not manifest immediately, we conservatively assumed that former smokers had the same mortality risk as current smokers until 5 years after cessation; this reflected studies where former smokers were those who had not smoked in the previous 5 years and data from large US cohort studies indicating that the all-cause mortality risk in men who quit smoking does not fall below that of current smokers until 5 years after cessation [4, 20].
Our base case analysis included imperfect ART adherence and retention in care, which are important modifiers of survival. To compare smoking-associated and HIV-associated life expectancy losses and to validate our model with data from published studies, we additionally simulated, stratified by smoking status, (1) HIV-uninfected people and (2) HIV-infected people with perfect ART adherence and retention in care [4, 21, 22].
ART and Other Model Details
Details regarding ART efficacy, adherence, loss to follow-up, and other model specifications are described in the Supplementary Materials and elsewhere [17, 23]. Model figures, flow charts, state definitions, protocols for data compilation, user guides, and other details are available at: http://www.massgeneral.org/mpec/cepac/.
Input Parameters
Cohort Characteristics
We simulated HIV-infected people reflective of those initiating care in the United States [20, 24–32] (Table 1). Parameters were derived from North American AIDS Cohort Collaboration on Research and Design data, where the mean CD4+ T-cell count (±SD) at entry to care was 360 ± 280 cells/µL, and the median age at presentation was 43 years [24]. In the base case, we assumed the same CD4+ T-cell count distribution regardless of age at entry to care; we relaxed this assumption in a 2-way sensitivity analysis.
Table 1.
Base Case Input Parameters and Ranges for a Simulation of Smoking and Smoking Cessation Among Human Immunodeficiency Virus (HIV)–Infected Persons in the United States
| Parameter | Base Case |
Rangea | Reference(s) | |
|---|---|---|---|---|
| HIV- and ART-related parameter | ||||
| CD4+ T-cell count at entry to care, cells/µL | [24] | |||
| Mean ± SD | 360 ± 280 | |||
| Rangea | 50–500 | |||
| Received first-line suppressive ARTb and had <50 copies/mL at 48 wk, % | 93 | [25, 26] | ||
| Adherence rate, %, mean ± SD | 89 (14) | [23, 27, 28] | ||
| Virologic failure rate for patients who received suppressive ART,b % per mo | 0.1 | [29] | ||
| Loss to follow-up rate per 100 person-years, by adherence ratec | [23, 30] | |||
| >95% | 0.1 | … | ||
| <50% | 84.5 | … | ||
| Return to care rate, per 100 person-years | 18.1 | [23, 31] | ||
| Smoking- and non–AIDS-related parameter | Men | Women | ||
| Unadjusted monthly non–AIDS-related mortality probability, ×10−4, among never smokers, by aged | [4, 20] | |||
| 30 y | 0.58 | 0.48 | … | … |
| 40 y | 1.75 | 0.79 | … | … |
| 50 y | 3.27 | 2.24 | … | … |
| 60 y | 4.78 | 3.67 | … | … |
| 70 y | 17.76 | 11.03 | … | … |
| 80 y | 36.10 | 24.15 | … | … |
| Mortality risk ratio vs never smokers (starting at age 40 y) | ||||
| Current smokerse,f | 2.8 | 2.8 | 1.9–4.0 | [20, 21] |
| Former smokers, by age at cessationf,g,h | [4, 21] | |||
| <35 y | 1.0 | 1.0 | 1.0–2.4 | … |
| 35–44 y | 1.1 | 1.3 | 1.0–2.4 | … |
| 45–54 y | 1.5 | 1.6 | 1.0–2.4 | … |
| 55–64 y | 1.7 | 1.8 | 1.0–2.4 | … |
| SMR, adjusted for mortality risk factors associated with both HIV risk behavior group and smoking | [8, 20, 32] | |||
| Men who have sex with men | 1 | … | … | … |
| Women who have sex with men | … | 1.97 | … | … |
| Men who have sex with women only | 1.80 | … | … | … |
Abbreviations: ART, antiretroviral therapy; SD, standard deviation; SMR, standardized mortality ratio.
a This is the range of input values used in sensitivity analyses. Mortality risk ratio ranges are based on confidence intervals in published studies.
b Defined as dolutegravir/abacavir/lamivudine.
c Loss to follow-up rates for those with adherence rates between 50% and 95% are based on interpolation between the loss to follow-up rates for those with adherence rates of <50% and those with adherence rates of >95%.
d In the absence of age- and smoking-stratified non–AIDS-related mortality rates for HIV-infected people, these probabilities were adapted from data reported in US general population studies [4, 20].
e Sex-specific mortality risk ratios from a general population study, used in the base case, had been adjusted for age, race, and educational level [20].
f Mortality risk ratios from a study of HIV-infected people, used in a sensitivity analysis, had been adjusted for age, sex, route of HIV transmission, baseline CD4+ T-cell count, AIDS at ART initiation, duration of ART, and calendar year of ART initiation [21].
g Sex-specific mortality risk ratios from a general population study, used in the base case, had been adjusted for age, education level, alcohol use, and body mass index [4].
Smoking Status and Mortality
For never smokers, we applied non–AIDS-related mortality probabilities based on published all-cause mortality rates for never smokers in the US general population (Supplementary Materials and Supplementary Table 1) [4, 20]. For current smokers, starting at age 40 years, we set non–AIDS-related mortality probabilities equal to those of never smokers, multiplied by a published multivariable-adjusted risk ratio (RR) of 2.8 [20]. For former smokers, starting at age 40 years (and at least 5 years after cessation), we set non–AIDS-related mortality probabilities equal to those of never smokers, multiplied by a published multivariable-adjusted RR ranging from 1.0 to 1.8, depending on age at cessation (Table 1) [4]. We assumed that future mortality probabilities for those who quit smoking before age 35 years were equal to those of never smokers [4].
Although non–AIDS-related mortality risks may be greater in HIV-infected versus HIV-uninfected persons, owing to factors besides smoking (such as other behavioral factors and depression), we limited the base case to changes in only smoking because of inherent limitations in the available data to estimate risks attributable to other behavioral factors [32, 33]. In sensitivity analysis, we accounted for mortality differences by behavioral risk group, incorporating these data.
ART
Efficacy of ART (viral suppression to below detection at 48 weeks) was 93%. Details regarding ART and other input parameters are in Table 1 and Supplementary Text.
Model Validation
We internally validated our model by comparing projections with results reported in the US general population study from which base case non–AIDS-related mortality rates were derived [4]. We externally validated model projections by comparing them with studies of smoking-associated survival reduction in HIV-infected people in (1) a Kaiser Permanente US cohort [22] and (2) a largely European cohort [21].
Sensitivity Analysis
We performed sensitivity analyses to examine the robustness of the results with alternative assumptions and estimates (Supplementary Text). These included (1) HIV risk behaviors, using standardized mortality ratios, to account for potentially higher non–HIV-related and non–smoking-related mortality risks among HIV-infected people, compared with the general population (Supplementary Table 2) [32]; (2) alternative smoking-associated mortality risks (eg, mortality RR of 1.9–4.0 for HIV-infected current vs never smokers [21]), to account for a potential differential impact of smoking on HIV-infected people versus the general population; (3) raw mortality rates for current and former smokers [4, 20], instead of RRs, to account for different smoking-associated mortality risks across ages; (4) delay of smoking cessation after entering HIV care, as some current smokers will quit after their initial entry into HIV care; (5) years since cessation for former smokers [20], since residual smoking-related risks likely depend not only on age at cessation, but also time since cessation; (6) immediate mortality reduction upon smoking cessation, in light of studies showing relatively rapid decreases in cardiovascular disease risk following cessation [34, 35]; (7) anticipated smoking relapse after loss to follow-up, assuming that those who had quit smoking would experience relapse upon being lost to follow-up and that some (25%) would quit smoking again upon returning to care; (8) perfect ART adherence and no loss to follow-up, as the impact of smoking and cessation might be greater when competing mortality risks from HIV infection are lower; (9) CD4+ T-cell count at presentation (range, 50–500 cells/µL), wherein competing mortality risks from HIV infection are varied; and 10) age and CD4+ T-cell count at presentation in a 2-way sensitivity analysis.
Population-Level Impact
Using Centers for Disease Control and Prevention data [8, 36] and model results, we estimated the cumulative potential years of life gained if 10%–25% of HIV-infected current smokers quit smoking. We assumed that there were 743 420 HIV-diagnosed people aged 30–64 years in the United States, with 77.5% in care [37] and a smoking prevalence of 38.9%–46.5%, depending on age, yielding an overall estimate of 247 586 HIV-infected smokers (Supplementary Text).
RESULTS
Base Case
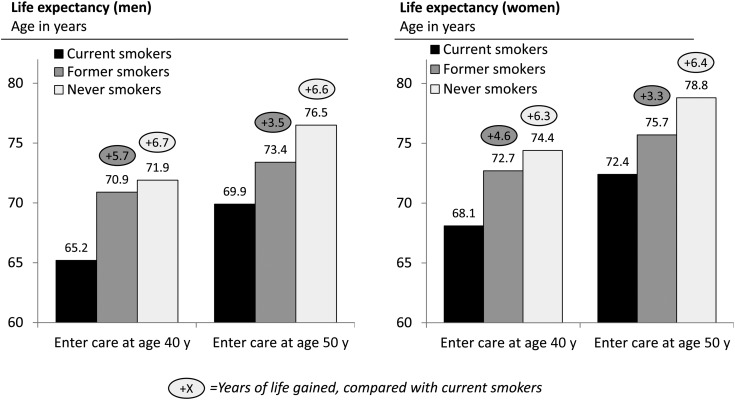
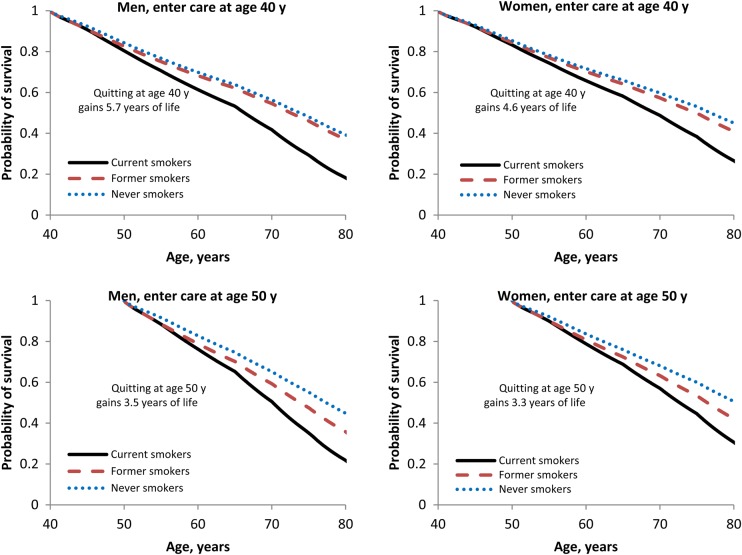
Life expectancy for men entering HIV care at age 40 years was 65.2 years, 70.9 years, and 71.9 years for current, former, and never smokers; for women, it was 68.1 years, 72.7 years, and 74.4 years, respectively (Figure 1; the depicted life expectancy was greater for those entering care at older ages, because of a survivor effect – their years of life remaining were fewer than those for younger people). Men and women who quit smoking upon entering HIV care at age 40 years gained 5.7 years and 4.6 years of life expectancy, respectively, compared with those who continued to smoke. Smoking cessation at younger ages yielded survival that more closely resembled that of never smokers (Figure 2 and Supplementary Figure 1). Gains from smoking cessation decreased with age, but even smokers who quit upon entering care at age 60 years had a substantial gain in life expectancy (2.5 years and 2.4 years for men and women, respectively; Supplementary Table 3).
Figure 1.
Life expectancies for people entering human immunodeficiency virus (HIV) care, based on smoking status. These simulations assumed that current smokers continued smoking until death and that former smokers quit smoking upon entering HIV care and remained abstinent. Life expectancy values are indicated as mean age at time of death for a simulated cohort of 1 million persons of the same sex, age, and smoking status. The vertical axis begins at age 60 years. Circled numbers indicate the gain in life expectancy, compared with current smokers, from stopping smoking or never smoking.
Figure 2.
Model-generated survival curves. Current smokers (solid line) continued smoking until death, former smokers (dashed line) quit smoking upon entering human immunodeficiency virus (HIV) care and remained abstinent, and never smokers (dotted line) smoked <100 cigarettes in their lifetime. The difference in life expectancy between former smokers and current smokers is indicated on each plot as the gain in life expectancy from smoking cessation. Vertical panels indicate differences in age at presentation (40 years and 50 years). The left side represents men; the right side represents women.
Life Expectancy Losses From Smoking and From HIV Infection
Simulated 40-year-old, HIV-uninfected current smokers had a life expectancy of 72.1 years (for men) and 76.4 years (for women), losing 10.7 years and 10.6 years of life expectancy, respectively, compared with HIV-uninfected never smokers. We compared the life expectancy losses from smoking and from HIV infection among 40-year-old HIV-infected current smokers (with imperfect ART adherence and retention in care, as per the base case [Supplementary Text]): the losses associated with smoking and with HIV infection were 6.7 years and 6.9 years, respectively, for men, and 6.3 years and 8.3 years, respectively, for women. When we simulated HIV-infected current smokers with perfect ART adherence and retention in care, life expectancy was 68.6 years (for men) and 72.1 years (for women); the life expectancy losses associated with smoking and HIV infection were 8.6 years and 3.5 years, respectively, for men, and 8.2 years and 4.3 years, respectively, for women (Supplementary Table 4).
Model Validation
The model-generated life expectancy gain from smoking cessation among 40 year-old HIV-uninfected people was similar to that reported in a prior US study (7.7–9.0 years [depending on sex] in the model vs 9 years in the prior study) [4]. Model-generated life expectancy losses from smoking and from HIV infection were similar to those reported in a Kaiser Permanente cohort [22] and in a largely European cohort in which loss to follow-up was low [21] (complete validation comparisons are available in Supplementary Table 5).
Sensitivity Analysis
We report sensitivity analyses where input data were most uncertain and/or where variation in the underlying parameter materially impacted our findings. Details, as well as results of other sensitivity analyses that did not differ substantially from those of the base case (eg, anticipated smoking relapse after loss to follow-up), are in the Supplementary Text and Table 6.
HIV Risk Behaviors
Differences between standardized mortality ratio–adjusted and base case analyses were <1.0 years regarding life expectancy losses from smoking and gains from cessation. In standardized mortality ratio–adjusted analyses, male and female current smokers entering HIV care at age 40 years lost 7.2 years and 7.0 years, respectively, compared with never smokers; those who quit upon entering care regained 5.9 years and 4.9 years, respectively, compared with those who continued to smoke (Supplementary Table 7).
Alternative Smoking-Associated Mortality Risks
When applying alternative smoking-associated mortality RRs from general population studies, the life expectancy loss associated with current (continued) smoking, compared with never smoking, for those entering HIV care at age 40 years ranged from 6.4 years to 6.9 years for men and from 6.1 years to 6.6 years for women, compared with base case losses of 6.7 years and 6.3 years, respectively (Supplementary Table 8).
When using mortality RRs from a study of HIV-infected people, life expectancy losses from smoking and gains from cessation had wide ranges, but point estimates were similar to those of the base case (Supplementary Table 9). The mean (range) loss for current smokers entering care at age 40 years, compared with never smokers, was 6.2 years (range, 4.1–9.1 years) for men, compared with 6.7 years in the base case, and 5.9 years (range, 3.8–8.7 years) for women, compared with 6.3 years in the base case.
Delay of Smoking Cessation
Smoking cessation 5 or 10 years after entering HIV care still resulted in substantial life expectancy gains (Table 2). Compared with those who continued to smoke, men who entered care at age 40 years and quit smoking 0, 5, and 10 years later gained 5.7 years (base case), 3.3 years, and 2.9 years, respectively; women gained 4.6 years (base case), 3.1 years, and 2.8 years, respectively.
Table 2.
Effect of Smoking Cessation 0, 5, or 10 Years After Entering Human Immunodeficiency Virus (HIV) Care Among HIV-Infected Persons in the United States
| Variable | Men |
Women |
||||
|---|---|---|---|---|---|---|
| Life Expectancy, Age, y | YLG, Compared With Current Smokers | Alive at Age 70 y, % | Life Expectancy, Age, y | YLG, Compared With Current Smokers | Alive at Age 70 y, % | |
| Enter care at age 30 y | ||||||
| Current smokers | 61.3 | … | 37 | 63.9 | … | 44 |
| Quit at age 40 y | 66.4 | 5.1 | 49 | 68.1 | 4.2 | 51 |
| Quit at age 35 y | 66.8 | 5.5 | 50 | 68.3 | 4.4 | 52 |
| Quit at age 30 y | 67.3 | 6.0 | 51 | 69.6 | 5.7 | 54 |
| Never smokers | 67.3 | 6.0 | 51 | 69.6 | 5.7 | 54 |
| Enter care at age 40 y | ||||||
| Current smokers | 65.2 | … | 42 | 68.1 | … | 49 |
| Quit at age 50 y | 68.1 | 2.9 | 49 | 70.9 | 2.8 | 54 |
| Quit at age 45 y | 68.5 | 3.3 | 50 | 71.2 | 3.1 | 55 |
| Quit at age 40 y | 70.9 | 5.7 | 55 | 72.7 | 4.6 | 57 |
| Never smokers | 71.9 | 6.7 | 56 | 74.4 | 6.3 | 60 |
| Enter care at age 50 y | ||||||
| Current smokers | 69.9 | … | 51 | 72.4 | … | 57 |
| Quit at age 60 y | 71.9 | 2.0 | 55 | 74.3 | 1.9 | 60 |
| Quit at age 55 y | 72.2 | 2.3 | 56 | 74.6 | 2.2 | 61 |
| Quit at age 50 y | 73.4 | 3.5 | 59 | 75.7 | 3.3 | 63 |
| Never smokers | 76.5 | 6.6 | 65 | 78.8 | 6.4 | 68 |
These simulations assumed that, after smoking cessation, former smokers remained abstinent. The relative mortality risk for former smokers was based on age at the time of smoking cessation. Life expectancy is reported as age at death.
Abbreviation: YLG, years of life gained.
Age and CD4+ T-Cell Count at Presentation: 2-Way Sensitivity Analysis
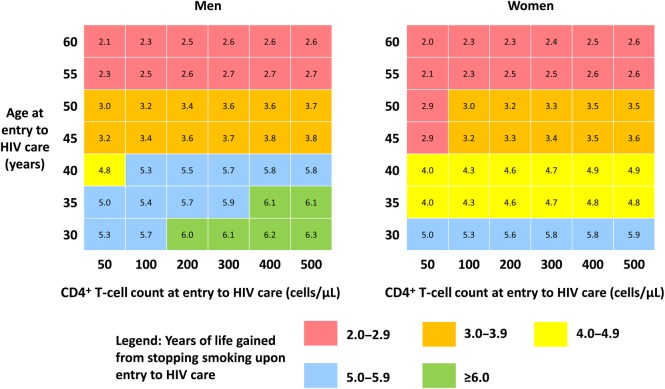
Results were sensitive to both age and CD4+ T-cell count at entry to care; smoking cessation improved life expectancy across strata of both variables. Younger individuals with higher initial CD4+ T-cell counts gained the most from cessation (Figure 3).
Figure 3.
Two-way sensitivity analysis of impact of smoking cessation, based on initial CD4+ T-cell count and age at entry to human immunodeficiency virus (HIV) care. Numbers in each box represent the mean years of life gained by those who quit smoking upon entering HIV care and remained abstinent, compared with those who continued to smoke. Colors correspond to the magnitude of the gain. Relative mortality risks were based on age at the time of smoking cessation.
Population-Level Impact of Smoking Cessation
Among HIV-infected people in care in the United States, smoking cessation and sustained abstinence by 10%–25% of current smokers aged 30–64 years would result in 106 000–265 000 expected years of life gained (Supplementary Tables 10 and 11).
DISCUSSION
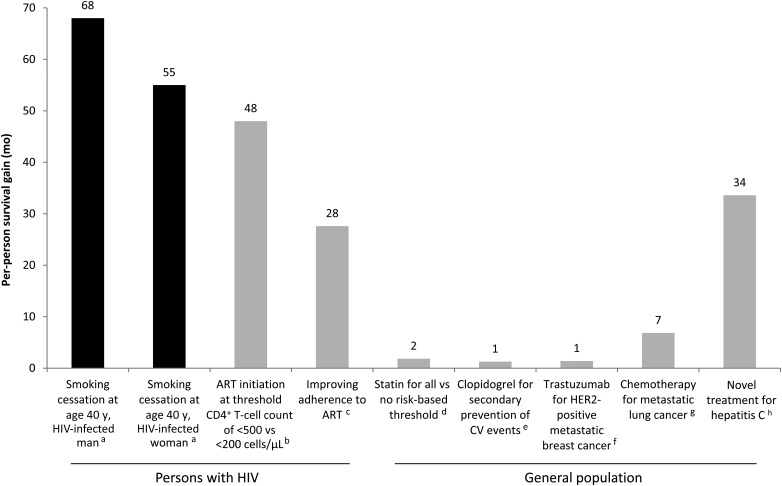
Using a simulation model of HIV disease, we found that smoking substantially reduces the life expectancy of HIV-infected people in the United States who are linked to care and that smoking cessation could have a major impact on survival. For men initially linked to care, the life expectancy loss associated with smoking is similar to the loss associated with HIV. For both men and women who are adherent to ART and remain in care, the loss from smoking is about double that from HIV. The survival benefit for an HIV-infected individual who quits smoking upon entering care at age 40 years exceeds that of other common interventions (Figure 4) [38–44]. Further, at the population level, smoking cessation could lead to a substantial number of years of life gained, given the very high prevalence of smoking among HIV-infected people.
Figure 4.
Per-person survival gains with various interventions. aGains are for those who quit smoking upon entering HIV care at age 40 years and remained abstinent, compared with those who continued to smoke until death. These model-generated outcomes reflected a scenario in which adherence to ART and retention in care were imperfect; bFor those entering HIV care at age 40 years; assumes an initial viral load of 100 000 copies/mL, 90% adherence to ART, and minimal toxicity from ART [38]; cAssumes that adherent and nonadherent patients consume 98% and 55% of prescribed ART doses. Improvement in survival represents the difference between an ideal scenario, reflecting ideal ART use observed in clinical trials, and a typical scenario, where the percentage of adherent patients declines over time to 52% at 18.6 months [39]; dBased on a representative US population aged 40–75 years. “Statin for all” refers to treating 100% of adults with a statin for primary prevention of CV disease. “No risk-based threshold” refers to an American College of Cardiology/American Heart Association treatment strategy that does not include an atherosclerotic CV disease risk-based criterion; about 8% of adults would still be eligible for statin treatment via other criteria, such as a history of CV disease or diabetes or an elevated low-density lipoprotein cholesterol level. This is the most conservative treatment strategy reported by Pandya et al; a “no statin” strategy is not reported [40]; eClopidogrel plus aspirin vs aspirin alone for secondary prevention of CV events [41]; fTrastuzumab plus chemotherapy vs chemotherapy alone [42]; gPaclitaxel plus cisplatin vs best supportive care [43]; hSofosbuvir plus ledipasvir plus ribavirin vs no treatment for hepatitis C virus genotype 1 infection [44]. Abbreviations: ART, antiretroviral therapy; CV, cardiovascular; HIV, human immunodeficiency virus.
Unfortunately, to this point, HIV-infected US people have been less likely to quit smoking, compared with HIV-uninfected people, a pattern consistent across age groups: of those who have ever smoked, 32% of HIV-infected people and 52% of other US adults quit smoking [8]. These strikingly low cessation rates underpin the need for novel cessation strategies. The rate of desire to quit is similar between HIV-infected and HIV-uninfected people [45]. Few studies have examined smoking cessation interventions in HIV-infected people; they have generally shown low abstinence rates [46]. It is unclear whether the success of cessation interventions differs between HIV-infected people and others.
ART has transformed the course of HIV infection, such that virally suppressed persons now have a life expectancy approaching that of uninfected persons [13]. However, life expectancy gaps persist [22], due partly to smoking-associated comorbidities that HIV-infected people are increasingly experiencing. Studies of HIV-infected people in the ART era consistently show higher mortality among smokers, compared with nonsmokers [5–7, 21, 47, 48]. Recent cohort studies of HIV-infected people in Denmark [47] and Western Europe and North America [21], in which accessibility and adherence to ART were high, found that smoking was associated with more years of life lost than HIV infection (only approximately 5% of the subjects in the latter study were in North America).
Our model accounts for nonadherence to ART and loss to follow-up, which occur more frequently in the United States than in European cohorts and which increase the risk of AIDS-related death, thereby potentially dampening the impact of smoking and the benefit of cessation. Nonetheless, even in this simulated US population, the life expectancy loss from continued smoking among HIV-infected people entering care at age 40 years is extremely high, at >6 years per person.
We used smoking-stratified mortality rates from the US general population in our simulations of non–AIDS-related mortality risks. Smoking might be more harmful in HIV-infected as compared with HIV-uninfected persons, particularly regarding the risk of myocardial infarction [9], so the actual impact of smoking and cessation on the life expectancy of HIV-infected persons may be even greater than we have shown in the base case. However, 2 large studies did not detect a mortality interaction between smoking and HIV infection (ie, the mortality RR associated with smoking was similar for HIV-infected and HIV-uninfected people) [47] or an interaction between smoking and CD4+ T-cell count (ie, the mortality RR associated with smoking was similar in those with high and low baseline CD4+ T-cell counts) [21].
The robustness of our results in the face of alternative assumptions about smoking-associated mortality risks is notable (Supplementary Table 6). Use of the upper and lower bounds of RR confidence intervals from a study of HIV-infected people [21] had the greatest effect on the impact of smoking and of cessation. The confidence intervals in that study were wide because of the small number of deaths. The smoking-associated mortality RR in that study was slightly lower than that in a US general population study [20], likely because former smokers were combined with current smokers in generating the HIV-specific estimate.
HIV-infected people might face higher non–AIDS-related mortality risks than HIV-uninfected people due to depression, substance use (besides tobacco), and high-risk behaviors; these may be more common in HIV-infected smokers compared with HIV-uninfected smokers [49]. In a sensitivity analysis, we sought to account for potentially higher competing mortality risks among smokers and among people at risk for HIV infection, compared with the general population, by using standardized mortality ratios. Although standardized mortality ratio–adjusted life expectancies were lower than base case results, the differences in the effects of smoking and cessation on survival were small. Data used to inform US standardized mortality ratios were limited by small sample size, short follow-up period, and relatively young patients [32].
While the harmful effects of smoking depend on the quantity of tobacco consumed (eg, pack-years), we could not explicitly account for this in our model. Reliable and precise clinical histories of smoking quantity are generally not reported in cohort studies because they are challenging to elicit. The data we used for smoking-associated RRs are based on studies of large numbers of subjects with varying pack-year histories; use of the average of these histories is common in the smoking literature for both general populations and HIV-infected people [4, 8, 21], although the average tobacco exposure might differ between the 2 populations. We believe that these published averages, combined with our simulations of large numbers of individuals and our wide-ranging sensitivity analyses, provide reasonable estimates for population-based analyses.
Similar to other model-based investigations, our analysis is limited by uncertainties in the input parameters and by some inevitable simplifications in our representation of risk factors and disease. Key uncertainties and limitations include exclusion of possible increases in AIDS-related mortality due to smoking; assumption that those who quit smoking would remain abstinent (relaxed in a sensitivity analysis); lack of differentiation between current, former, and never smokers regarding initial CD4+ T-cell count and viral load, ART adherence, and loss to follow-up; the assumption, in the base case, that the initial CD4+ T-cell count distribution (and, hence, distribution of time since HIV infection) was the same regardless of age at entry to HIV care; and population projections based on a cross-sectional survey of HIV-infected people in care, in which the prevalence of injection drug users was low (2%) [8]. Former smokers in published cohorts might have adopted other healthy lifestyle modifications in addition to smoking cessation; our estimates of survival gains from smoking cessation similarly reflect these changes.
In conclusion, smokers in HIV care may now lose as much or more life expectancy from smoking as from HIV infection, but cessation can greatly improve survival. Providers caring for HIV-infected people should address smoking during every patient encounter and offer guideline-based behavioral and pharmacologic treatments for tobacco use [50]. Novel intervention strategies targeting this important population are needed. Smoking cessation should be a major priority in HIV care programs.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Amy Zheng for her technical assistance.
Disclaimer. The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, NIH (grants T32 AI007433, R01 AI420006, and R37 AI093269); and Massachusetts General Hospital (Research Scholars Award to R. P. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention, Office on Smoking and Health. Smoking and tobacco use: fast facts. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/ Accessed 5 May 2016.
- 2.Centers for Disease Control and Prevention, Office on Smoking and Centers for Disease Control and Prevention, Office on Smoking and Health. Smoking and tobacco use: trends in current cigarette smoking among high school students and adults, United States, 1965–2011. Available at: http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/ Accessed 5 May 2016.
- 3.Jamal A, Homa DM, O'Connor E et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2015; 64:1233–40. [DOI] [PubMed] [Google Scholar]
- 4.Jha P, Ramasundarahettige C, Landsman V et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013; 368:341–50. [DOI] [PubMed] [Google Scholar]
- 5.Crothers K, Goulet JL, Rodriguez-Barradas MC et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev 2009; 21:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifson AR, Neuhaus J, Arribas JR et al. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health 2010; 100:1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pines H, Koutsky L, Buskin S. Cigarette smoking and mortality among HIV-infected individuals in Seattle, Washington (1996–2008). AIDS Behav 2011; 15:243–51. [DOI] [PubMed] [Google Scholar]
- 8.Mdodo R, Frazier EL, Dube SR et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen LD, Helleberg M, May MT et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis 2015; 60:1415–23. [DOI] [PubMed] [Google Scholar]
- 10.Crothers K, Huang L, Goulet JL et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011; 183:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helleberg M, Gerstoft J, Afzal S et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS 2014; 28:1499–508. [DOI] [PubMed] [Google Scholar]
- 12.Walensky RP, Paltiel AD, Losina E et al. The survival benefits of AIDS treatment in the United States. J Infect Dis 2006; 194:11–9. [DOI] [PubMed] [Google Scholar]
- 13.Samji H, Cescon A, Hogg RS et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedberg KA, Losina E, Weinstein MC et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001; 344:824–31. [DOI] [PubMed] [Google Scholar]
- 15.Paltiel AD, Weinstein MC, Kimmel AD et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med 2005; 352:586–95. [DOI] [PubMed] [Google Scholar]
- 16.Losina E, Schackman BR, Sadownik SN et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis 2009; 49:1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walensky RP, Sax PE, Nakamura YM et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Accessed 6 May 2016.
- 19.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thun MJ, Carter BD, Feskanich D et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368:351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helleberg M, May MT, Ingle SM et al. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS 2015; 29:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus JL, Chao CR, Leyden WA et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross EL, Weinstein MC, Schackman BR et al. The clinical role and cost-effectiveness of long-acting antiretroviral therapy. Clin Infect Dis 2015; 60:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Althoff KN, Gange SJ, Klein MB et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis 2010; 50:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walmsley SL, Antela A, Clumeck N et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 26.Raffi F, Rachlis A, Stellbrink H-J et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–43. [DOI] [PubMed] [Google Scholar]
- 27.Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PLoS One 2012; 7:e31591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch JD, Gonzales M, Rosenquist A, Miller TA, Gilmer TP, Best BM. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Manag Care Pharm 2011; 17:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raffi F, Jaeger H, Quiros-Roldan E et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 30.Fleishman JA, Yehia BR, Moore RD, Korthuis PT, Gebo KA, HIV Research Network. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr 2012; 60:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helleberg M, Engsig FN, Kronborg G et al. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS 2012; 26:741–8. [DOI] [PubMed] [Google Scholar]
- 32.Seage GR, Holte SE, Metzger D et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol 2001; 153:619–27. [DOI] [PubMed] [Google Scholar]
- 33.Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 2012; 41:433–45. [DOI] [PubMed] [Google Scholar]
- 34.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA 2003; 290:86–97. [DOI] [PubMed] [Google Scholar]
- 35.Kawachi I, Colditz GA, Stampfer MJ et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med 1994; 154:169–75. [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. HIV Surveillance Report, 2013; vol. 25. http://www.cdc.gov/hiv/library/reports/surveillance/ Accessed 6 May 2016.
- 37.Centers for Disease Control and Prevention. CDC VitalSigns - new hope for stopping HIV. 2011. Available at: http://www.cdc.gov/vitalsigns/hivtesting/index.html Accessed 6 May 2016.
- 38.Braithwaite RS, Roberts MS, Goetz MB et al. Do benefits of earlier antiretroviral treatment initiation outweigh harms for individuals at risk for poor adherence? Clin Infect Dis 2009; 48:822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munakata J, Benner JS, Becker S, Dezii CM, Hazard EH, Tierce JC. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care 2006; 44:893–9. [DOI] [PubMed] [Google Scholar]
- 40.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA 2015; 314:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Bhatt DL, Dunn ES et al. Cost-effectiveness of clopidogrel plus aspirin versus aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA trial. Value Health 2009; 12:872–9. [DOI] [PubMed] [Google Scholar]
- 42.Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC. HER-2 testing and trastuzumab therapy for metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 2004; 22:854–63. [DOI] [PubMed] [Google Scholar]
- 43.Berthelot JM, Will BP, Evans WK, Coyle D, Earle CC, Bordeleau L. Decision framework for chemotherapeutic interventions for metastatic non-small-cell lung cancer. J Natl Cancer Inst 2000; 92:1321–9. [DOI] [PubMed] [Google Scholar]
- 44.Najafzadeh M, Andersson K, Shrank WH et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med 2015; 162:407–19. [DOI] [PubMed] [Google Scholar]
- 45.Shahrir S, Tindle HA, McGinnis KA et al. Contemplation of smoking cessation and quit attempts in human immunodeficiency virus-infected and uninfected veterans. Subst Abuse 2016; 37:315–22. [DOI] [PubMed] [Google Scholar]
- 46.Pacek LR, Cioe PA. Tobacco use, use disorders, and smoking cessation interventions in persons living with HIV. Curr HIV/AIDS Rep 2015; 12:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Helleberg M, Afzal S, Kronborg G et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis 2013; 56:727–34. [DOI] [PubMed] [Google Scholar]
- 48.Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific mortality among HIV-infected individuals, by CD4(+) cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS 2014; 28:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benard A, Bonnet F, Tessier J-F et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDs 2007; 21:458–68. [DOI] [PubMed] [Google Scholar]
- 50.Siu AL, U.S. Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015; 163:622–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.