Abstract
Background. Development of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia after a respiratory viral infection is frequently fatal in children. In mice, S. aureus α-toxin directly injures pneumocytes and increases mortality, whereas α-toxin blockade mitigates disease. The role of α-toxin in pediatric staphylococcal-viral coinfection is unclear.
Methods. We enrolled children across 34 North American pediatric intensive care units with acute respiratory failure and suspected influenza virus infection. Serial serum anti-α-toxin antibody titers and functional α-toxin neutralization capacity were compared across children coinfected with MRSA or methicillin-susceptible S. aureus (MSSA) and control children infected with influenza virus only. MRSA isolates were tested for α-toxin production and lethality in a murine pneumonia model.
Results. Influenza virus was identified in 22 of 25 children with MRSA coinfection (9 died) and 22 patients with MSSA coinfection (all survived). Initial α-toxin–specific antibody titers were similar, compared with those in the 13 controls. In patients with serial samples, only MRSA-coinfected patients showed time-dependent increases in anti-α-toxin titer and functional neutralization capacity. MRSA α-toxin production from patient isolates correlated with initial serologic titers and with mortality in murine pneumonia.
Conclusions. These data implicate α-toxin as a relevant antigen in severe pediatric MRSA pneumonia associated with respiratory viral infection, supporting a potential role for toxin-neutralizing therapy.
Keywords: influenza, pneumonia, bacteria, intensive care unit, pediatric, respiratory failure
Methicillin-resistant Staphylococcus aureus (MRSA), previously an uncommon pathogen in children, has been associated in the past decade with rising numbers of cases of severe pneumonia and other invasive infections [1]. Upper respiratory tract viral illness commonly precedes the development of staphylococcal pneumonia, suggesting that viral-induced alteration of host immunity predisposes the host to bacterial infection. Influenza virus–S. aureus coinfection in children is often fatal as compared to isolated infection with S. aureus or influenza virus [2–4]. During the 2009 influenza A(H1N1) pandemic, coinfection with MRSA was associated with an overall 3-fold increase in pediatric mortality, with an 8-fold increase among previously healthy children [5]. In most cases, mortality occurred despite vancomycin administration, suggesting that antistaphylococcal therapy is insufficient to ameliorate disease. These studies indicate that children infected with influenza virus and, likely, those infected with other respiratory viral pathogens are a high-risk group for poor outcome when secondarily infected by MRSA.
A roadblock to the successful development of S. aureus vaccines and therapeutics has been limited insight on the role of staphylococcal virulence factors in human disease. The elicitation of robust antibody responses to candidate vaccines has not correlated with human protection [6], implying that both vaccine targets and the patient populations evaluated in clinical trials may be suboptimally identified. S. aureus α-toxin (α-hemolysin [Hla]), a secreted pore-forming cytotoxin, contributes to severe alveolar injury and lethal staphylococcal pneumonia in animal models [7–9]. α-toxin is required for alveolar injury in experimental pneumonia, where increased α-toxin production on a strain-specific basis directly correlates with worsened outcome [8, 10, 11]. Passive and active immunization approaches yielding α-toxin–neutralizing antibodies are highly efficacious in preventing lethal pneumonia in mice [7, 8, 12], prompting the investigation of these approaches in human clinical trials [13–15]. To date, the applicability of these findings in the setting of viral coinfection remains uninvestigated.
If α-toxin contributes to S. aureus coinfection in children with influenza or other severe viral respiratory infections, toxin-neutralizing antibody therapies may decrease morbidity and mortality. To this end, we evaluated the serologic response to α-toxin in critically ill children with MRSA or methicillin-susceptible S. aureus (MSSA) infection who had respiratory failure resulting from suspected or proven influenza virus coinfection, compared with the response in a matched cohort of children with influenza only. Given the observed clinical severity of MRSA coinfection, we hypothesized that MRSA-coinfected patients would display a prominent response to α-toxin, indicative of exposure during infection.
METHODS
Study Population
Children (<21 years of age) with suspected community-acquired influenza virus infection admitted to a participating pediatric intensive care unit (PICU) in the Pediatric Acute Lung Injury and Sepsis Investigator's (PALISI) Pediatric Intensive Care Influenza (PICFlu) Study Group were enrolled during each influenza season from December 2008 to June 2014. Blood samples were collected after enrollment and, starting in November 2009, were collected serially approximately 0, 3, 7, and 14 days after enrollment. The institutional review board at each of the 34 sites approved the study, and informed consent was obtained from the parent or legal guardian of all subjects. The Supplementary Data describe criteria for inclusion and exclusion (Supplementary Table 1), microbiologic testing methods, and clinical definitions.
Human Serum Analyses
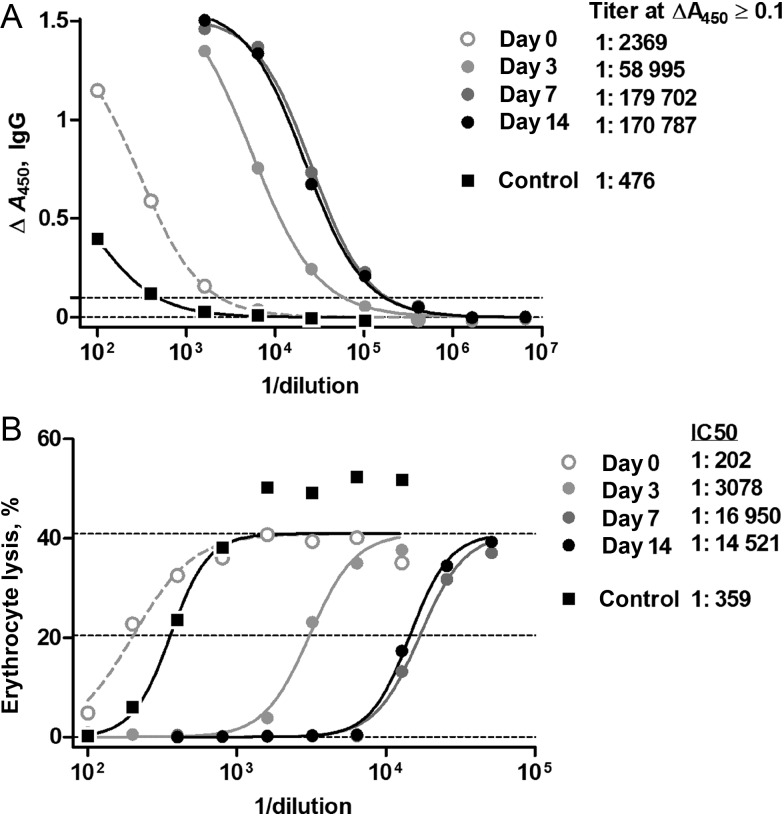
Specific immunoglobulin G (IgG) against plate-bound recombinant Escherichia coli–derived nontoxigenic α-toxin mutant, HlaH35L, and Panton-Valentine leukocidin (PVL) subunits LukF-PV and LukS-PV were measured by standard indirect enzyme-linked immunosorbent assay (ELISA) on serially diluted sera. Specific titers were defined as the interpolated dilution of serum sufficient to give 0.1 Absorbance Units of specific signal (absorbance at 450 nm, the difference between antigen- and mock-coated plates), approximately one tenth of the maximum signal. Detailed methods are described in the Supplementary Data. For measurement of functional α-toxin neutralization activity, serial dilutions of sera were preincubated with 2 nM recombinant α-toxin [8] in 0.1 mL of phosphate-buffered saline (PBS; pH 7.4) with 1 g/L bovine serum albumin for 15 minutes at room temperature. Following incubation of these samples for 1 hour with 5 × 107 freshly washed rabbit erythrocytes (Hemostat Laboratories, Dixon, California) in 0.1 mL of PBS, intact cells and debris were centrifuged (at 700×g for 10 minutes), and supernatant absorbance was measured at 450 nm to estimate the level of dissolved hemoglobin. The percentage of specific lysis was computed using no toxin and 2.5% Triton X-100 control supernatants. The half-maximal inhibition (IC50) was estimated by 4-parameter logistic regression, with forced common minima and maxima. The mouse anti-HlaH35L monoclonal antibody 7B8 [16] was confirmed to have an IC50 of 72 ng/mL (approximately 0.5 nM), confirming the theoretical 2:1 antigen/antibody stoichiometry (data not shown).
Bacteriologic Studies
Clinical isolates available from infected patients were shipped to the Bubeck Wardenburg laboratory in the University of Chicago. Microbiologic techniques are detailed in the Supplementary Data.
Murine Pneumonia Model
Animal experiments were approved by the University of Chicago Animal Care and Use Committee. Seven-week-old female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were infected with 2–4 × 108 colony-forming units of S. aureus in 30 µL of PBS delivered via intranasal inoculation, with inoculum preparation and animal monitoring performed as previously described [17].
Statistics
Between-group comparisons were performed using the Mann–Whitney test or 1-way analysis of variance for continuous variables, the Fisher exact test for categorical variables, and Pearson or Spearman correlations, as appropriate. The Xyplot function of the Trellis graphics in R, version 3.1.1, with the lattice graphics and nlme packages [18–20] was used to explore the rise of reciprocal titers of specific IgG against α-toxin, LukF-PV, and LukS-PV and α-toxin neutralization activity (1/IC50). We used a hierarchical linear mixed-effects model to model log10-transformed reciprocal titers with time as a linear effect, random among patients and fixed between groups and including a group-time interaction term to examine the effects by testing the null hypothesis that the time profiles for the 3 groups are parallel (Supplementary Data). Other analyses were done using SAS, version 9.1 (SAS Institute, Cary, North Carolina), and Prism5 (GraphPad, La Jolla, California). The Bonferroni method of adjusting for multiple comparisons was used for comparisons of toxin-specific levels. For other comparisons, significance was set at a P value of < .05.
RESULTS
We enrolled 356 critically ill children suspected of having influenza virus infection. Influenza virus was confirmed in 276 (77.5%), other viral respiratory pathogens in 91 (25.6%), and no viral pathogen in 26 (7.3%). Coinfection with S. aureus was clinically diagnosed in 54 children; 45 had sufficient sera available for testing (22 coinfected with MRSA and influenza virus, 3 coinfected with MRSA and other viruses, and 20 coinfected with MSSA and influenza virus). The S. aureus–coinfected patients were on average approximately 10 years old, whereas those with isolated influenza virus infection were significantly younger, with a mean age of 7.4 years. From 170 children with isolated influenza virus infection, we identified 26 control patients who we could match for age, sex, ethnicity, race, and illness severity, 13 of whom had sufficient sera available for testing (Table 1). Patients coinfected with MRSA or MSSA were more likely to be previously healthy and to receive invasive mechanical ventilator support, compared with influenza virus–monoinfected controls. MRSA-coinfected patients were more severely ill than those with MSSA coinfection or isolated influenza, displaying a higher frequency of acute respiratory distress syndrome, extracorporeal membrane oxygenation support, and longer PICU stay. All deaths (involving 9 of 25 subjects [36%]) occurred in the MRSA group (P < .001). Coinfection with multiple viruses was common, and other, additional pathogenic bacteria were isolated from 4 patients (Supplementary Table 2).
Table 1.
Demographic Characteristics, Illness Severity, and Clinical Course of Critically Ill Children Coinfected With A Virus and Methicillin-resistant Staphylococcus aureus (MRSA), Coinfected With Influenza and Methicillin-susceptible S. aureus (MSSA), and Influenza Virus Controls
| Characteristic | MRSA-Viral Coinfectiona (n = 25) | MSSA-Influenza Coinfection (n = 20) | Influenza Controlsa (n = 13) | P Valueb |
|---|---|---|---|---|
| Female sex | 9 (36) | 8 (40) | 6 (46) | .89 |
| Hispanic ethnicity | 7 (28) | 2 (10) | 2 (15) | .33 |
| Race | ||||
| White | 21(84) | 18 (90) | 8 (61) | |
| Black | 2 (8) | 1 (5) | 4 (30) | |
| Other | 2 (8) | 1 (5) | 1 (8) | .23 |
| Age, y | 10.3 ± 4.7 | 9.6 ± 5.9 | 9.7 ± 5 | .88 |
| Health status | ||||
| Previously healthy | 20 (80) | 15 (75) | 6 (46) | |
| Underlying health conditions | 5 (20) | 5 (25) | 7 (54) | .08 |
| Mild-moderate asthma only | 2 (8) | 1 (5) | 2 (15) | |
| Other chronic conditions | 3 (16) | 4 (20) | 5 (38) | |
| Supportive care and complications | ||||
| Mechanical ventilation | .01 | |||
| None | 0 (0) | 1 (5) | 0 (0) | |
| Noninvasive only | 1 (4) | 0 (0) | 4 (31) | |
| Any invasive | 24 (96) | 19 (95) | 9 (69) | |
| Acute lung injury/ARDS | 21 (84) | 10 (50)c | 5 (39) | .01 |
| Shock receiving vasopressors | 21 (84) | 11 (55)c | 7 (54) | .03 |
| Extracorporeal life support | 13 (52) | 1 (5)d | 2 (15) | .02 |
| PRISM score | 20.3 ± 14 | 11.5 ± 9.2c | 7 ± 5.1 | .002 |
| Duration PICU stay, h | 592 ± 746 | 339 ± 445 | 364 ± 407 | .30 |
| PICU mortality | 9 (36) | 0 (0)d | 0 (0) | <.001 |
Data are no (%) of children or mean value ± SD, unless otherwise indicated.
Abbreviations: ARDS, acute respiratory distress syndrome; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality.
a A total of 22 patients were infected with influenza virus, and 3 patients were infected with other viruses, identified as parainfluenza type 4, human metapneumovirus with rhinovirus, or rhinovirus; the influenza control group had no clinical diagnosis of bacterial coinfection.
b By the Fisher exact test (for categorical variables) and 1-way analysis of variance (for continuous variables).
c P < .05, compared with MRSA coinfection.
d P < .01, compared with MRSA coinfection.
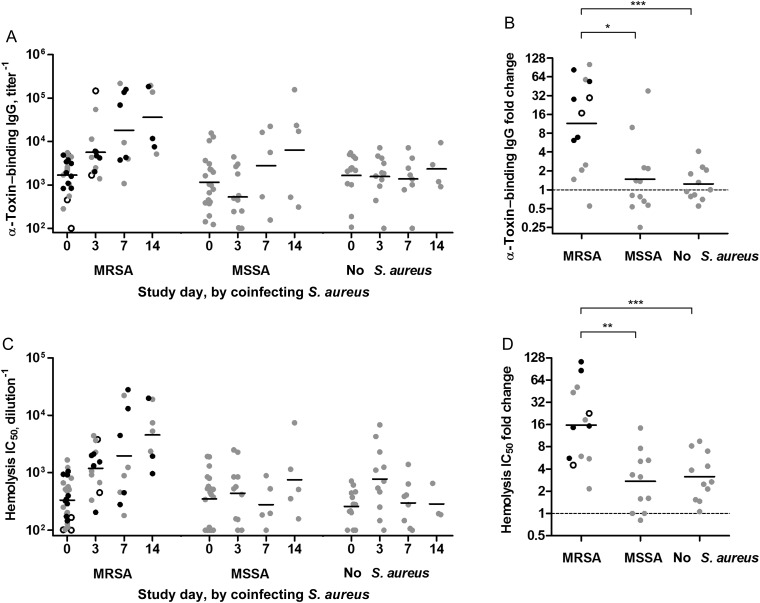
Sera from infected patients were examined for α-toxin–specific IgG antibody by indirect ELISA and subjected to an in vitro toxin neutralization assay to measure titers of functional serum antibody capable of preventing α-toxin–induced hemolysis of rabbit erythrocytes (Figure 1). There were no differences in α-toxin titers between cohorts at enrollment (day 0 sera in Figure 2A and Supplementary Figure 1), but a time-dependent increase in anti-α-toxin activity was observed in patients coinfected with MRSA. Geometric mean titers increased approximately 10-fold during the first 2 weeks of PICU stay, in some patients exceeding 100-fold (Figure 2B). The distribution of the sample timing is included in the Supplementary Data. To compensate for the inability to define the precise onset of human infection, incomplete samples, and uneven sampling at each time point, we used a linear mixed model to analyze the data. As shown in Table 2 and Figure 2A and 2B, the resulting model demonstrated that the observed increase in anti-α-toxin titer was larger in the MRSA-coinfected cohort than in MSSA-coinfected patients (borderline significance after Bonferroni correction) or noncoinfected patients (highly significant after Bonferroni correction). Similarly, the serum dilution resulting in 50% neutralization of toxin hemolytic activity (IC50) increased prominently in MRSA-coinfected patients (Table 2 and Figure 2C). Among the MRSA-coinfected cohort, 9 of 13 patients with serial samples displayed a >4-fold rise in anti-α-toxin titer (Figure 2B and 2C). In contrast, >4-fold increases were observed in only 2 of 12 MSSA-coinfected patients, and 1 of 11 influenza virus–control patients. Indicative of the specificity of the anti-α-toxin response in MRSA-coinfected patients, there was no significant difference in the serologic response to LukF-PV and LukS-PV in the MRSA and MSSA cohorts (Table 2 and Supplementary Figures 2 and 3).
Figure 1.
α-Toxin–specific antibody measurements on a representative patient coinfected with methicillin-resistant Staphylococcus aureus (MRSA) and influenza virus. Four serial serum samples from 1 MRSA-coinfected patient, a 2.7-year-old male presenting with shock and acute lung injury, were subjected to the assays as described in “Methods” section; a healthy adult donor control serum is included. A, α-Toxin–binding antibody as measured by enzyme-linked immunosorbent assay (ELISA), using an immunoglobulin G (IgG)–specific secondary detector. The x-axis denotes the reciprocal dilution of patient serum. The dotted line denotes the threshold for computation of the end point titer, arbitrarily defined at 0.1 specific absorbance units. B, Residual hemolytic activity of 2 nM recombinant α-toxin incubated with serial dilutions of patient serum. Lines denote minimum, maximum, and half-maximum hemolytic activities. The computed titers and inhibitory concentration for half-inhibition (IC50) levels are as listed.
Figure 2.
Methicillin-resistant Staphylococcus aureus (MRSA) coinfection elicited a prominent anti-α-toxin response relative to methicillin-susceptible S. aureus (MSSA) coinfection. A, α-Toxin–specific antibody level displayed as a function of study day and by coinfection with MRSA, MSSA, or neither. B, Ratio of highest convalescent titer vs acute titers for each patient when serial specimens were available, classified by coinfection status. C, α-Toxin–specific antibody assessed by functional neutralization of recombinant α-toxin in a rabbit erythrocyte lysis assay. “IC50” indicates the inhibitory concentration of diluted serum that results in 50% inhibition of 2 nM recombinant α-toxin. D, Ratio of highest neutralization titer in convalescent sera vs acute sera for each patient when serial specimens were available, classified by coinfection status. Linear mixed-effects model analysis on log-transformed data revealed that the time profiles for MSRA are significantly different from those for MSSA and no S. aureus for α-toxin–binding immunoglobulin G (IgG) and α-toxin–neutralizing IC50, as indicated above. Black symbols indicate patients who died during admission, while open symbols indicate patients with influenza-like illness who had an alternate viral diagnosis. Bars indicate geometric means. *P < .05, **P < .01, and ***P < .001.
Table 2.
Results of Comparisons of the Time Progression of Toxin-Specific Antibody Levels Among Children With Suspected Influenza Virus Monoinfection and Those Coinfected With Influenza Virus and Methicillin-Resistant Staphylococcus aureus (MRSA) or Methicillin-Susceptible S. aureus (MSSA)
| Comparison of Reciprocal Titers or IC50 Values (Log10) | MRSA Coinfection vs MSSA Coinfection | MRSA Coinfection vs Monoinfection | MSSA Coinfection vs Monoinfection |
|---|---|---|---|
| Anti-α-toxin IgG | .02 | .0007 | .29 |
| α-Toxin neutralization activity | .006 | .0001 | .12 |
| Anti-PVL LukF-PV subunit IgG | .84 | .001 | .005 |
| Anti-PVL LukS-PV subunit IgG | .07 | .0001 | .02 |
| Estimated anti-α-toxin increase | MRSA Coinfection | MSSA Coinfection | Monoinfection |
| Log10 IgG anti-α-toxin titer per unit time (95% CI) | 0.0046 (.003–.006) | 0.0017 (−2.252–2.255) | 0.0003 (−2.288–2.289) |
| Log10 IC50 α-toxin neutralization activity per unit time (95% CI) | 0.0041 (.003–.005) | 0.0014 (−2.292–2.295) | −0.0002 (−2.303–2.302) |
Data are P values unless otherwise indicated and were corrected by the Bonferroni test, with values of < .017 considered statistically significant. Analysis was done by linear mixed-effects modeling as described in “Methods” section.
Abbreviations: CI, confidence interval; IC50, half-maximal inhibitory concentration; IgG, immunoglobulin G; PVL, Panton-Valentine leukocidin.
Admission sera analysis demonstrated α-toxin–specific antibody titers ranging from <1:100 to 1:16 000 and neutralization IC50 from <1:100 to 1:2000 (study day 0 data in Figure 2A and 2C). There was weak correlation between the 2 assays (Pearson r2 = 0.26 over log10-transformed data; P < .0001; Supplementary Figure 4A), consistent with the production of a substantial fraction of noninhibitory antibody against α-toxin. An increased correlation was observed, however, when convalescent sera were analyzed (Pearson r2 = 0.68; P < .0001; Supplementary Figure 4B), reflecting the strong anti-α-toxin humoral response in MRSA coinfection. Initial serum titer and toxin-neutralization capacity were independent of age (Supplementary Figure 5).
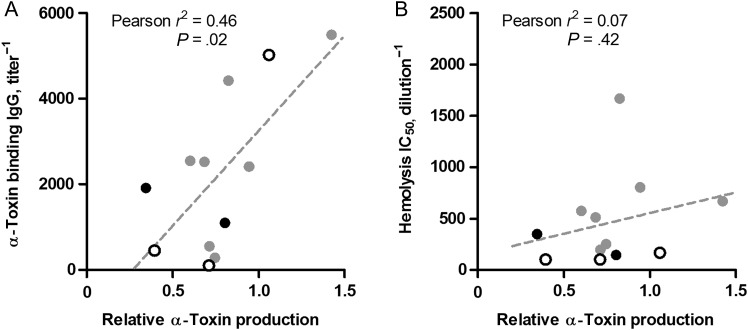
To examine whether α-toxin production by individual bacterial isolates during infection relates to the magnitude of elicited antitoxin response, we examined available S. aureus isolates from 12 patients, all of whom were MRSA coinfected. Despite identical growth characteristics in vitro (Supplementary Figure 6), variation in α-toxin production was observed between individual strains (Figure 4). Toxin levels ranged from 0.3 to 1.4 times that produced by strain LAC, a well-characterized epidemic USA300 MRSA isolate [8]. A low but significant correlation between α-toxin production and toxin-binding activity from the corresponding initial patient sera (Figure 3A) was observed. This correlation was not evident for α-toxin–neutralizing activity (Figure 3B) or subsequent titers (data not shown).
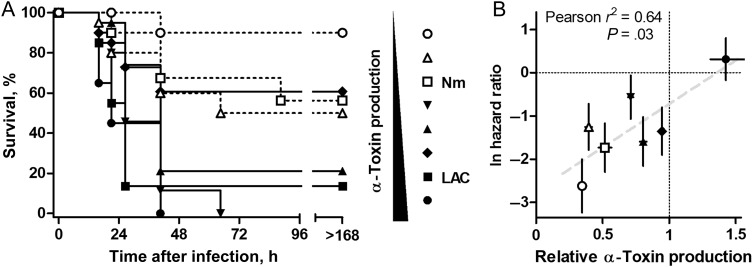
Figure 4.
α-Toxin production by clinical methicillin-resistant Staphylococcus aureus (MRSA) isolates correlated with disease severity in experimental mouse S. aureus pneumonia. A, Kaplan–Meier survival curves of C57Bl/6 mice infected with various S. aureus strains (n = 19–20/group), including control strains LAC (MRSA USA300) and Newman (Nm; methicillin-susceptible S. aureus). Strains are arrayed in the legend as a function of increasing in vitro α-toxin production. B, Loge-transformed hazard ratios of each strain against S. aureus LAC, graphed against strain in vitro production of α-toxin. The measures for strain LAC are 0 and 1, respectively (lines). Error bars indicate standard errors of the mean of each measure. Deming linear regression is shown.
Figure 3.
α-Toxin production by clinical methicillin-resistant Staphylococcus aureus (MRSA) isolates correlated with admission α-toxin titer. MRSA isolates from 12 patients were analyzed for in vitro α-toxin production and normalized against S. aureus strain LAC, a known high producer of the toxin. α-Toxin–binding antibody titer (A) or toxin neutralization activity (B) of the corresponding patient's initial sera displayed as a function of in vitro α-toxin production. Mean toxin production from 3 independent determinations is shown. Black symbols indicate fatal cases, while open symbols indicate patients with influenza-like illness who had an alternate viral diagnosis. Abbreviations: IC50, half-maximal inhibitory concentration; IgG, immunoglobulin G.
In this study, 23 of 25 patients with MRSA coinfection received vancomycin, and 11 of 25 patients received clindamycin, an antimicrobial agent commonly used empirically to treat disease due to community-acquired MRSA and for its potential to reduce toxin production. In this small sample of patients, receipt of clindamycin did not significantly decrease mortality (odds ratio, 0.24; 95% confidence interval, .02–1.8; P = .21), even though most clinical MRSA isolates were found clindamycin susceptible (24 of 25 were susceptible; 1 was not tested). Likewise, clindamycin therapy was not associated with alteration of anti-α-toxin antibody levels over time (Supplementary Figure 7), suggesting that toxin elaboration may occur early in the course of bacterial infection. The small sample size and critically ill state of the patients mandated the inclusion of patients who received intravenous IgG (7 of 25 patients in the MRSA cohort and 2 of 20 of the MSSA cohort) or frozen plasma (8 and 3 patients, respectively). However, our measurements of anti-α-toxin antibody levels in commercially available preparations of pooled human IgG suggest that the observed increases in patients' toxin-specific IgG titers exceeded the theoretical contribution of exogenous antibody in intravenous IgG (Supplementary Table 3).
The complex disease state and relative rarity of life-threatening pediatric S. aureus coinfection in the setting of respiratory tract infection with influenza virus or another virus precludes an assessment of whether toxin production by S. aureus correlates directly with clinical outcomes. Likewise, existing animal model systems of influenza virus–S. aureus coinfection have not yet been evaluated for their ability to discern the role of specific staphylococcal virulence factors in pathogenesis, as host death from coinfection neither requires the administration of live staphylococci nor correlates well with S. aureus virulence [21, 22]. We therefore tested the ability of 6 clinical S. aureus isolates—with a range of levels of α-toxin expression from our patient cohort—to contribute to lung injury in a well-characterized mouse model of lethal pneumonia that permits discrimination of strains on the basis of molecular pathogenesis attributable to specific virulence factors [23]. This model permits a sensitive readout of toxin-associated injury, wherein lethality following pulmonic challenge in C57BL/6 mice is positively correlated with in vitro α-toxin production [7, 11]. Groups of inbred mice were infected via the intranasal route with a fixed inoculum of selected MRSA isolates, in addition to the clinical MRSA isolate LAC/USA300 and MSSA isolate Newman, both of which have been well-characterized in this model [7, 23]. On measuring hazard ratios of each infecting strain, we documented a significant correlation (r2 = 0.64; P = .03) between in vitro α-toxin production and in vivo lethality (Figure 4).
DISCUSSION
Identifying therapies to prevent fatality and severe lung injury from S. aureus coinfection in children with influenza and other severe viral respiratory illnesses is hindered by a lack of understanding of disease progression on a molecular level and the challenges associated with conducting detailed human clinical studies in sporadic, fulminant disease. Enrollment of patients from 2008 to 2014 across 34 North American pediatric centers, coupled with mechanistic studies, provides support for the role of S. aureus α-toxin in the pathogenesis of MRSA coinfection following infection with influenza virus, and likely with other viral pathogens. MRSA-coinfected children exhibited a worsened disease outcome relative to MSSA-coinfected children, simultaneously displaying a significant increase in anti-α-toxin IgG titer and functional neutralization capacity but a similar response to PVL toxin. Together, these findings support a link between in vivo α-toxin exposure, disease progression, and the host immunologic response to α-toxin.
The recent development of an ex vivo model of human lung infection with S. aureus provides novel insight on the role of α-toxin in injury to the human lung, demonstrating the ability of the toxin to cause profound necrotizing damage to the alveolar epithelium [24]. These findings confirm prior molecular studies on α-toxin–mediated injury, also demonstrating the direct correlation between strain-specific levels of toxin expression and the magnitude of injury. While small-animal models of S. aureus pneumonia have failed to conclusively identify a role of PVL in disease progression [7, 25, 26], PVL was demonstrated to correlate with the formation of empyema in the ex vivo human model [24]. These molecular observations may explain the more prominent anti-α-toxin IgG response observed over the course of MRSA coinfection in children—in whom the disease course was markedly worse than in MSSA-coinfected children—while the rise in the anti-PVL response was unable to distinguish between the 2 groups. Pathologic analysis of lung tissue from children with an antecedent viral illness who died of fulminant S. aureus infection reveals severe tissue necrosis consistent with the effects of α-toxin on human lung tissue [27]. With the relatively small number of MRSA isolates available for study and the unavailability of MSSA strains, we were unable to determine whether MSSA strains produced comparable amounts of α-toxin or the role of clonal lineages in toxin production.
Neither vancomycin nor clindamycin therapy modulated the response to α-toxin in our patient population despite documented efficacy against the clinical isolates associated with disease. Studies in both mouse models and an ex vivo human lung infection model demonstrated that α-toxin–induced tissue injury occurs very early following S. aureus infection, in advance of clinical evidence of infection [24, 28]. Children with viral–S. aureus coinfection therefore likely present to clinical attention after acute toxin-mediated lung injury has been established.
These data complement the study by Fritz et al, which demonstrated that children with invasive S. aureus infection had lower preexisting α-toxin–specific antibody titers yet higher convalescent levels as compared to children with either primary or recurrent skin or soft-tissue infection [29]. Similarly, these findings are congruent with those of 2 studies in adult populations demonstrating that individuals who survived invasive staphylococcal infection had higher anti-α-toxin titers than healthy controls [30] and that lower levels of serum antibody to α-toxin along with other staphylococcal toxins is associated with increased risk for sepsis [31]. A limitation of these prior studies was that serologic analyses were semiquantitative and did not investigate functional α-toxin neutralization capacity. We have demonstrated that neutralizing anti-α-toxin IgG is indeed produced in vivo during human infection—possibly too late to ameliorate the initial lung damage induced in MRSA infection. These data suggest that α-toxin neutralization by either passive or active immunization may be considered as a strategy to reduce the mortality and morbidity of invasive MRSA pneumonia in the setting of influenza virus infection or influenza-like upper respiratory tract infection.
The sample size of this study is relatively small, potentially limiting interpretation of the data. This reflects the difficulty in the recruitment of severely ill patients, especially those who were previously healthy. In the entire cohort, children coinfected with MRSA or MSSA were older than children who became critically ill from influenza virus without bacterial coinfection. This markedly limited the sample of similarly aged patients to choose as controls. However, the strength of our study is the availability of serial samples from patients enrolled from November 2009 onward, allowing us to define both the interpatient variation of antibody levels early in infection, as well as intrapatient changes during disease progression. An additional limitation is the lack of a gold standard for distinguishing codetection of S. aureus from invasive coinfection due to this bacterium. We therefore had to depend on clinical diagnoses and the isolation of S. aureus from a sterile site (Supplementary Table 2).
Our data suggest that MRSA coinfection induces a rapid anti-α-toxin antibody response in most patients, consistent with the known role of this toxin in early molecular pathogenesis of lung injury [28]. While this response bears a modest dose-dependent relationship with the α-toxin production of the infecting S. aureus strain, the paucity of functional neutralizing capacity in the earliest phase of illness may allow for unmitigated disease progression. This consideration may be of specific relevance in the pediatric population, which is less likely to have long-term exposure to S. aureus as a colonized host, or in individuals with minor skin and soft-tissue infection. To date, detailed analysis of the development of the antistaphylococcal immune response as a function of developmental age has not been conducted on a population level; such a study may prove informative for its ability to provide insight on developmental windows of time in which active or passive immunotherapy may substantially augment the host response against S. aureus.
Our findings have important implications for the design of clinical studies and potential therapeutic intervention in the pediatric population, suggesting a potential role for early prevention of toxin-mediated injury in high-risk patients with influenza or other viral respiratory infections. Essential to the clinical validation and implementation of these approaches will be the development of sensitive animal models in which the role of α-toxin can be quantitatively assessed in the setting of influenza virus–S. aureus coinfection. Similarly, the preclinical efficacy of antitoxin prophylaxis or therapy will need to be examined in experimental model systems to provide a rational basis for the development of focused clinical trials.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge the collaboration of the PALISI PICFlu Study Site Investigators, specified as follows who enrolled patients and made other major contributions to this study: Ronald C. Sanders, MD, and Glenda Hefley, RN, MNsc (Arkansas Children's Hospital, Little Rock); David Tellez, MD, Courtney Bliss, MS, Aimee Labell, MS, RN, Danielle Liss, BA, and Ashley L. Ortiz, BA (Phoenix Children's Hospital, AZ); Katri Typpo, MD, and Jen Deschenes, MPH (Banner Children's/Diamond Children's Medical Center, Tucson, AZ); Barry Markovitz, MD, Jeff Terry, MBA, and Rica Sharon P. Morzov, RN, BSN, CPN (Children's Hospital Los Angeles, CA); Ana Lia Graciano, MD, and Melita Baldwin, BS (Children's Hospital Central California, Madera); Nick Anas, MD, Adam Schwarz, MD, Chisom Onwunyi, RN, BSN, MPH, CCRP, Stephanie Osborne, RN, CCRC, Tiffany Patterson, BS, CRC, and Ofelia Vargas-Shiraishi, BS, CCRC (Children's Hospital of Orange County, Orange, CA); Anil Sapru, MD, Maureen Convery, BS, and Victoria Lo, BA (UCSF Benioff Children's Hospital, San Francisco, CA); Heidi Flori, MD, Becky Brumfield, RCP, and Julie Simon, RN (UCSF Benioff Children's Hospital Oakland, CA); Angela Czaja, MD, Peter Mourani, MD, Valeri Batara Aymami, RN, MSN-CNS, Susanna Burr, CRC, Megan Brocato, CCRC, Stephanie Huston, BS, RPSGT, CRC, Emily Jewett, and Danielle Loyola, RN, BSN, MBA (Children's Hospital Colorado, Aurora); Christopher Carroll, MD, MS, Kathleen Sala, MPH, and Sherell Thornton-Thompson, CCRP (Connecticut Children's Medical Center, Hartford); John S. Giuliano Jr, MD, and Joana Tala, MD (Yale–New Haven Children's Hospital, CT); Gwenn McLaughlin, MD (Holtz Children's Hospital, Miami, FL); Matthew Paden, MD, Chee-Chee Manghram, Stephanie Meisner, RN, BSN, CCRP, and Cheryl L. Stone, RN (Children's Healthcare of Atlanta at Egleston, GA); Juliane Bubeck Wardenburg, MD, PhD, Neethi Pinto, MD, MS, and Andrea DeDent, PhD (The University of Chicago Medicine Comer Children's Hospital, IL); Vicki Montgomery, MD, FCCM, Janice Sullivan, MD, Tracy Evans, RN, Kara Richardson, RN, and Melissa Thomas, RN, BSN, CCRC (Kosair Children's Hospital, Louisville, KY); Adrienne Randolph, MD, MSc, Anna A. Agan, BA, Ryan M. Sullivan, RN, BSN, CCRN, and Stephanie Cobb, BA (Boston Children's Hospital, MA); Melania Bembea, MD, MPH, and Elizabeth D. White, RN, CCRP (Johns Hopkins Children's Center, Baltimore, MD); Stephen Kurachek, MD, Angela A. Doucette, CCRP, and Erin Olson, RN, CCRP (Children's Hospitals and Clinics of Minnesota, Minneapolis); Mary Hartman, MD, and Rachel Jacobs, BA (St. Louis Children's Hospital, MO); Edward Truemper, MD, and Machelle ZinkDawson, RN, BSN, MEd, CCRC (Children's Hospital of Nebraska, Omaha); Daniel L. Levin, MD, and J. Dean Jarvis, MBA, BSN (Children's Hospital at Dartmouth-Hitchcock, NH); Chhavi Katyal, MD (The Children's Hospital at Montefiore, Bronx, NY); Kate Ackerman, MD, L. Eugene Daugherty, MD, and Laurel Baglia, PhD (Golisano Children's Hospital, Rochester, NY); Mark W. Hall, MD, Kristin Greathouse, BSN, MS, and Lisa Steele, RN, BSN, CCRN (Nationwide Children's Hospital, Columbus, OH); Neal Thomas, MD, Jill Raymond, RN, MSN, and Debra Spear, RN (Penn State Children's Hospital, Hershey, PA); Julie Fitzgerald, MD, Mark Helfaer, MD, Scott Weiss, MD, Jenny L. Bush, RNC, BSN, Mary Ann Diliberto, RN, Brooke B. Park, RN, BSN, and Martha Sisko, RN, BSN, CCRC (Children's Hospital of Philadelphia, PA); Frederick E. Barr, MD (Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN); Renee Higgerson, MD, and LeeAnn Christie, RN (Dell Children's Medical Center of Central Texas, Austin); Cindy Darnell, MD, and Shanda Johnson, RRT, MHA, CCRP (Children's Medical Center of Dallas, TX); Laura L. Loftis, MD, Nancy Jaimon, RN, MSN-Ed, and Ursula Kyle, MS (Texas Children's Hospital, Houston); Rainer Gedeit, MD, Briana E. Horn, Kate Luther, MPH, and Kathy Murkowski, RRT, CCRC (Children's Hospital of Wisconsin, Milwaukee); Douglas F. Willson, MD, and Robin L. Kelly, RN (University of Virginia Children's Medical Center, Charlottesville); Philippe A. Jouvet, MD, and Anne-Marie Fontaine, BSc (Centre Hospitalier Universitaire Sainte-Justine, Montreal, Canada); and Marc-André Dugas, MD (Centre Hospitalier de l'Université Laval, Quebec, Canada).
Financial support. This work was supported by the National Institutes of Health (grants AI084011 [to A. G. R.] and AI097434 [to J. B. W.]) and the Centers for Disease Control and Prevention.
Potential conflicts of interest. J. B. W. has the potential to receive royalties from Novartis Vaccines and Diagnostics in relation to patents owned by the University of Chicago. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: on behalf of the PALISI PICFlu Study Group, David Tellez, Courtney Bliss, Aimee Labell, Danielle Liss, Ashley L. Ortiz, Katri Typpo, Jen Deschenes, Barry Markovitz, Jeff Terry, Rica Sharon P. Morzov, Ana Lia Graciano, Melita Baldwin, Nick Anas, Adam Schwarz, Chisom Onwunyi, Stephanie Osborne, Tiffany Patterson, Ofelia Vargas-Shiraishi, Anil Sapru, Maureen Convery, Victoria Lo, Heidi Flori, Becky Brumfield, Julie Simon, Angela Czaja, Peter Mourani, Valeri Batara Aymami, Susanna Burr, Megan Brocato, Stephanie Huston, Emily Jewett, Danielle Loyola, Christopher Carroll, Kathleen Sala, Sherell Thornton-Thompson, John S. Giuliano, Joana Tala, Gwenn McLaughlin, Matthew Paden, Chee-Chee Manghram, Stephanie Meisner, Cheryl L. Stone, Juliane Bubeck Wardenburg, Neethi Pinto, Andrea DeDent, Vicki Montgomery, Janice Sullivan, Tracy Evans, Kara Richardson, Melissa Thomas, Adrienne Randolph, Anna A. Agan, Ryan M. Sullivan, Stephanie Cobb, Melania Bembea, Elizabeth D. White, Stephen Kurachek, Angela A. Doucette, Erin Olson, Mary Hartman, Rachel Jacobs, Edward Truemper, Machelle ZinkDawson, Daniel L. Levin, J. Dean Jarvis, Chhavi Katyal, Kate Ackerman, L. Eugene Daugherty, Laurel Baglia, Mark W. Hall, Kristin Greathouse, Lisa Steele, Neal Thomas, Jill Raymond, Debra Spear, Julie Fitzgerald, Mark Helfaer, Scott Weiss, Jenny L. Bush, Mary Ann Diliberto, Brooke B. Park, Martha Sisko, Frederick E. Barr, Renee Higgerson, LeeAnn Christie, Cindy Darnell, Shanda Johnson, Laura L. Loftis, Nancy Jaimon, Ursula Kyle, Rainer Gedeit, Briana E. Horn, Kate Luther, Kathy Murkowski, Douglas F. Willson, Robin L. Kelly, Philippe A. Jouvet, Anne-Marie Fontaine, and Marc-André Dugas
References
- 1.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010; 23:616–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed C, Kallen AJ, Patton M et al. . Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J 2009; 28:572–6. [DOI] [PubMed] [Google Scholar]
- 3.Finelli L, Fiore A, Dhara R et al. . Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008; 122:805–11. [DOI] [PubMed] [Google Scholar]
- 4.Hall MW, Geyer SM, Guo CY et al. . Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 2013; 41:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randolph AG, Vaughn F, Sullivan R et al. . Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics 2011; 128:e1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol 2012; 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 2007; 13:1405–6. [DOI] [PubMed] [Google Scholar]
- 8.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205:287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel) 2013; 5:1140–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy AD, Otto M, Braughton KR et al. . Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A 2008; 105:1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeo FR, Kennedy AD, Chen L et al. . Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc Natl Acad Sci U S A 2011; 108:18091–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua L, Hilliard JJ, Shi Y et al. . Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 2014; 58:1108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uniformed Services University of the Health Sciences, Nabi Biopharmaceuticals. Staphylococcus aureus toxoids phase 1–2 vaccine trial. Bethesda, MD: National Library of Medicine; https://clinicaltrials.gov/ct2/show/NCT01011335.NLM Accessed 31 December 2015. [Google Scholar]
- 14.Aridis Pharmaceuticals. Safety, pharmacokinetics and efficacy of KBSA301 in wevere pneumonia (S. aureus) Bethesda, MD: National Library of Medicine, 2012. https://clinicaltrials.gov/ct2/show/NCT01589185.NLM Accessed 31 December 2015. [Google Scholar]
- 15.Medimmune LLC. Study of the efficacy and safety of MEDI4893 (SAATELLITE). Bethesda, MD: National Library of Medicine, 2014. https://clinicaltrials.gov/ct2/show/NCT02296320.NLM Accessed 31 December 2015. [Google Scholar]
- 16.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 2009; 77:2712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berube BJ, Sampedro GR, Otto M, Bubeck Wardenburg J. The psmα locus regulates production of Staphylococcus aureus alpha-toxin during infection. Infect Immun 2014; 82:3350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team. R: a language and environment for statistical computing. Vienna, Austria:R Foundation for Statistical Computing, 2014. http://www.R-project.org. Accessed July 4 2016. [Google Scholar]
- 19.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer, 2000. [Google Scholar]
- 20.Pinheiro JC, Bates D, DebRoy S, Sarkar D; R Core Team. nlme: linear and nonlinear mixed effects models. R package. Ver 3.1-120, 2015. http://CRAN.R-project.org/package=nlme. Accessed July 4 2016. [Google Scholar]
- 21.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis 2011; 203:880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis 2010; 201:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 2007; 75:1040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mairpady Shambat S, Chen P, Nguyen Hoang AT et al. . Modelling staphylococcal pneumonia in a human 3D lung tissue model system delineates toxin-mediated pathology. Dis Model Mech 2015; 8:1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis 2008; 198:1166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labandeira-Rey M, Couzon F, Boisset S et al. . Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science 2007; 315:1130–3. [DOI] [PubMed] [Google Scholar]
- 27.Adem PV, Montgomery CP, Husain AN et al. . Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med 2005; 353:1245–51. [DOI] [PubMed] [Google Scholar]
- 28.Inoshima I, Inoshima N, Wilke GA et al. . A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 2011; 17:1310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz SA, Tiemann KM, Hogan PG et al. . A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin Infect Dis 2013; 56:1554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensson B, Hedstrom SA, Kronvall G. Antibody response to alpha- and betahemolysin from Staphylococcus aureus in patients with staphylococcal infections and in normals. Acta Pathol Microbiol Immunol Scand B 1983; 91:351–6. [DOI] [PubMed] [Google Scholar]
- 31.Adhikari RP, Ajao AO, Aman MJ et al. . Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206:915–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.