Abstract
Cytomegalovirus (CMV) infection occurs frequently in young children, who, when infected, are then a major source of transmission. Oral CMV shedding by 14 infants with primary infection was comprehensively characterized using quantitative polymerase chain reaction weekly for ≥9 months. Three phases of oral shedding were identified: expansion, transition, and clearance. Viral expansion occurred over a median of 7 weeks, with a median doubling time of 3 days. During the transition phase, expansion slowed over a median of 6 weeks before peak viral load was reached. Clearance was slow (22-day median half-life), and shedding did not resolve during observation for any infant. Mathematical modeling demonstrated that prolonged oral CMV expansion is explained by a low within-host reproduction number (median, 1.63) and a delayed immune response that only decreases the infected cell half-life by 44%. Thus, the prolonged oral CMV shedding observed during primary infection can be explained by slow viral expansion and inefficient immunologic control.
Keywords: epidemiology, cytomegalovirus, transmission, mathematical model, immunology, theoretical biology
Worldwide, most primary cytomegalovirus (CMV) infections occur during early childhood, through the oral route [1]. Infected infants shed CMV orally at high, persistent levels [2–6] and are a major source of transmission, including to pregnant women, which contributes to congenital infection [3, 7–9]. Viral dynamics during primary infection are governed by properties of viral infectivity and the immune response. A detailed study of these processes could identify immunologic thresholds necessary for effective vaccination. Here, we use mathematical models to describe oral shedding dynamics during primary CMV infection in a cohort of Ugandan infants.
MATERIALS AND METHODS
Study Cohort and Data
As previously described [10], pregnant women in Kampala were enrolled, and oral swabs were collected in a standardized manner [11] from the mother and all children for quantitative polymerase chain reaction (qPCR) analysis of CMV [12]. Blood samples were collected from the infants at 6 weeks of age and every 4 months thereafter. Oral swabs and plasma samples were tested for CMV, using real-time qPCR. Primary infection was defined virologically and serologically as described elsewhere [10]. Additional information about subject selection is provided in the Supplementary Materials. This study was approved by all relevant institutional review boards in Uganda, the United States, and Canada. Written informed consent was obtained from all study participants or their guardians.
Statistical Analysis and Classification of Episodes
Episode duration was defined as the interval from the start of oral shedding until the end of observation. Correlations between same-day log10 copies of CMV in plasma and oral swabs were calculated using Pearson correlation for all values and for positive values only. To see whether positive plasma samples were associated with higher oral log10 CMV levels, we performed regression analysis, with oral swab concentration as the outcome and binary status of plasma sample as the predictor, using a mixed model with a random intercept by infant to account for within-subject correlation.
For each infant, episodes were classified into 3 phases, using 2 time points of oral shedding: time of the first viral concentration within 1 log10 of the peak and time of the peak. For each phase and each infant, log10 viral concentration was regressed on time, and linear fit was assessed using r2. Doubling time and half-life were calculated from the regression results. We used Spearman correlations to assess correlations between episode features and demographic information. Median values and confidence intervals (CIs) were reported from 10 000 bootstrap replicates for each Spearman correlation. We used linear regression to test whether maternal human immunodeficiency virus (HIV) status predicted episode features.
Estimation of Rebound Dynamics
To explore rebound dynamics, we fit the clearance phase (see Results) by using cubic linear regression with time as the predictor. Clearance deceleration times and rebound times were estimated by calculating the inflection point and local minimum from fitted polynomial parameters (Supplementary Methods).
Within-Host Mathematical Model Without Immune Pressure
We constructed our initial mathematical model based on target-cell limitation dynamics in the absence of an immune response [13, 14] and the delay between cell infection and CMV replication [15, 16]. For details, see Equation S1 in Supplementary Methods. Compartments were susceptible oral epithelial (squamous or salivary gland) cells, infected cells prior to CMV replication, infected cells with replicating CMV, and free virus. Absent infection, we used empirically derived turnover rates of susceptible cells [17]. We imputed known latency and death rates of CMV-infected cells [15, 18], replication rate of CMV in vitro [16], and clearance rate of oral EBV [19] into the model. Two remaining unknown parameters, viral infectivity and infection time, were estimated using the data (Supplementary Methods). Model parameter values are shown in Supplementary Table S1.
Mathematical Modeling of the Oral Immune Response to CMV
The immune response that eventually controls oral CMV shedding is likely multifactorial [20–24]. For simplicity, we constructed a model that assumed clearance of oral shedding results from a mounting cytolytic response based on a saturated process [25]. For details, see Equation S2 in Supplementary Methods and Supplementary Table S2. Results presented from this model incorporate combinations of fitted parameters that determine both immune timing and magnitude. For details on parameter estimation, see the Supplementary Methods. An alternative virus-mediated clearance model is discussed in the Supplementary Methods.
Basic and Effective Reproduction Numbers
The basic reproduction number, R0, defined as the average number of cells infected by a single infected cell in a fully susceptible population, describes the initial dynamics of infection and was calculated using the target-cell model incorporating only viral expansion phase (Equation S3 in the Supplementary Methods). The effective reproduction number, R, is an estimate of newly infected cells infected by a single infected cell, accounting for depleting susceptible cells and increasing immune pressure during infection (Equation S4 in the Supplementary Methods). R captures 2 important features of immune pressure: (1) times when virus is being cleared (when R < 1) and (2) the time of peak immune pressure (when minimum R occurs) [26].
Data and Code
Programming was done using the R language (CRAN). Differential equations were simulated using the lsoda algorithm in the deSolve package. Optimizations were performed using the Nelder-Mead algorithm in the nloptr package. Data and analysis code are available at: https://www.github.com/bryanmayer/CMV-Primary-Infection.
RESULTS
Primary CMV Infection
Among 32 Ugandan infants followed prospectively from birth, 20 postnatal primary CMV infections were captured over 30 months [10]. We excluded 6 infants following acquisition, owing to limited data (Supplementary Methods and Figure S1). We analyzed the remaining 14 primary infections, which included 763 oral and 52 plasma CMV qPCR measurements. The median age at infection was 70 days (range, 31–205 days). Fifty percent of infants were born to HIV-infected women; none acquired HIV during follow-up. All newborns were healthy and had normal growth and development during the study period. None had symptoms of CMV infection [10].
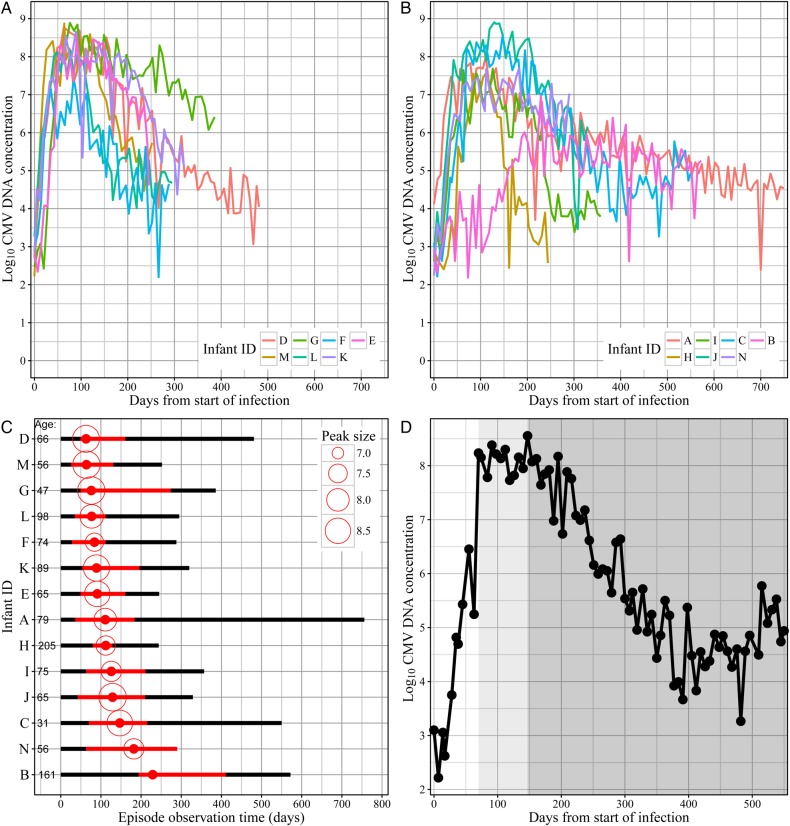
The quantity of CMV in saliva increased rapidly after collection of the first CMV-positive swab (Figure 1A and 1B), and there was extended high-magnitude shedding (within 1 log of the peak), lasting a median of 20.5 weeks (range, 7–32.4 weeks; Figure 1C). We did not observe resolution (defined as two successive negative swabs) during any primary infections; therefore, all infections were right censored. While the true duration of oral shedding episodes could not be determined, minimum durations ranged from 244 to 756 days (Figure 1C).
Figure 1.
Oral shedding of cytomegalovirus (CMV) during primary infection. A and B, Data for all infants are split into 2 groups for visual clarity (ordered by time of peak). Four CMV-negative swabs were removed from the plot. Lines correspond to oral concentrations of CMV DNA. C, Observation periods of primary episodes, ordered by time of peak. Initiations but not resolutions of CMV oral shedding episodes were observed. The time of peak is denoted by a solid red dot, and the size of the peak (log10 CMV DNA concentration) is denoted by size of the open circle. The red portion of the line corresponds to peak shedding levels within 1 log10 of the peak (which begins where the transition phase begins), and the black portion corresponds to lower shedding levels. Infant age at infection (days) is listed on the left side of the bars. D, Example of a primary CMV infection (which occurred in infant C). Three phases are evident: (1) an expansion phase (white shade), (2) a transition phase (shaded light gray), and (3) a clearance phase (shaded gray). This figure is available in black and white in print and in color online.
CMV DNA was detected in plasma from 13 of 14 infants (all except subject K) at least once. Overall, analysis of plasma CMV samples less frequently detected CMV DNA (56% of samples were positive) and revealed lower CMV DNA concentrations (range, 1.8–3.9 log10 copies/mL) than oral samples (Supplementary Figure S2). Plasma and oral measurements were not correlated when comparing all measurements (0.22; 95% CI, −.06–.46) or only positive measurements (−0.04; 95% CI, −.4–.33). A positive plasma CMV sample was not significantly associated with oral CMV load (0.81 log10 increase; 95% CI, −.12–1.73), compared with a negative sample.
Phases of Oral Shedding During Primary CMV Infection
Three distinct phases of oral shedding were apparent during primary CMV infection, based on swab-to-swab stability of viral DNA concentrations (Figure 1C and 1D). We defined these as (1) expansion, which begins when the first positive swab is detected and ends when shedding levels reach a value within 1 log of the peak DNA concentration; (2) transition, which spans from the end of expansion to the time of peak viral DNA concentration; and (3) clearance, which begins after the time of peak viral DNA concentration. Episodes generally followed a similar trajectory within the 3 phases (Figure 1A and 1B); outliers are described below.
Protracted, Exponential Dynamics During Viral Expansion
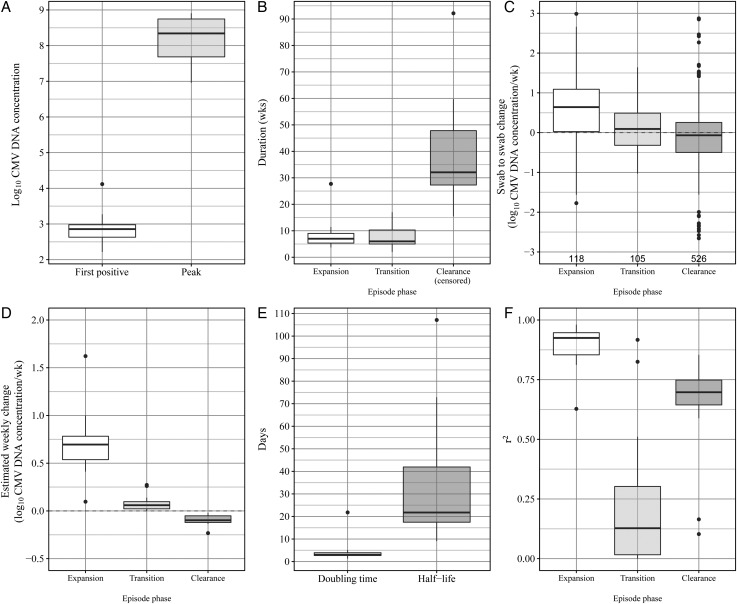
The expansion phase was defined by log-linear growth in viral DNA concentrations (Figure 1D). Expansion kinetics followed a relatively consistent pattern among infants, with narrow ranges of (1) low viral loads detected in first positive samples (mean, 2.9 log10 CMV DNA copies/mL; range, 2.2–4.1 log10 copies/mL; interquartile range [IQR], 2.6–3.0 log10 copies/mL; Figure 2A) and (2) high peak viral levels (mean, 8.2 log10 CMV DNA copies/mL; range, 7–8.9 log10 copies/mL; IQR, 7.7–8.7 log10 copies/mL; Figure 2A). The duration of the expansion phase was a median of 7 weeks (range, 3.7–27.7 weeks; IQR, 5.4–9 weeks; Figure 2B). While the expansion phase was shorter than the clearance phase in all infants, 1 episode (infant B) had a notably long expansion phase (Figure 1B and 1C).
Figure 2.
Primary cytomegalovirus (CMV) episodes are characterized by slow expansion, transition, and clearance phases. Boxes represent the interquartile range (IQR) of the 14 episodes, the whiskers extend to cover all data within 1.5 IQR of the first or third quartile, and dots represent individual data points outside of this range. A, Quantity of CMV in the first CMV-positive swab and the CMV-positive swab obtained at peak CMV DNA load during primary infection. Difference indicates the growth of virus between episode initiation and peak. B, Duration (weeks) for each phase for each episode. C, Change in measured concentration between consecutive swabs for each swab for each phase. Sample size is displayed under the box. D, Estimated weekly change in CMV DNA concentration, based on a regression model for each phase for each episode. E, Estimated doubling time (expansion phase) and half-life (clearance phase) from regression results in panel D. F, r2 value from each regression in model shown in panel D.
During expansion, the CMV DNA quantity in successive saliva specimens collected weekly increased by a median of 0.7 logs (Figure 2C). Using regression modeling, we found a statistically significant change in log concentration over time (Figure 2D), with a median doubling time of 0.4 weeks (range, 0.2–3.1 weeks; IQR, 0.4–0.6 weeks; Figure 2E). For 13 infants, the regression model fit the data closely and confirmed exponential growth (Figure 2F), implying unhindered oral CMV replication and spread during the initial weeks of infection. Infant B had protracted expansion, however, which was not well described by the regression model.
High-Quantity Shedding and Nonlinear Dynamics During Transition Phase
The transition phase was relatively short (median, 6 weeks; range, 2–17 weeks; Figure 2B). During transition, viral expansion slowed substantially, compared with expansion before reaching peak oral viral load, and was poorly characterized by exponential fit (Figure 2C and 2F). Redefining the expansion phase to extend to the peak viral load (ie, eliminating the transition phase) worsened the linear fit of the regression model in 11 of 14 episodes. These findings indicate that our initial definition of the expansion phase best described exponential growth and that the transition phase represents a period of quantitatively different viral replication dynamics prior to reaching peak viral load in saliva.
Extremely Slow, Nonlinear Viral Dynamics of Clearance Phase
We defined the clearance phase as starting at the peak oral viral load (Figure 1C and 1D). Between successive weeks during the clearance phase, the quantity of CMV DNA in saliva decreased slightly (Figure 2C). Log-linear slope estimates demonstrated statistically significant weekly decreases in 13 of 14 episodes (Figure 2D). Despite being right-censored in all infants, clearance was the longest phase of oral shedding during primary CMV infection, lasting a median 32 weeks (range, 15–92 weeks) with an estimated clearance half-life of 3.1 weeks (range, 1.3–15.3 weeks). The lower degree of fit to the data for viral clearance relative to viral expansion (Figure 2F) indicated nonlinear clearance.
We observed rebound in the CMV load of oral shedding during the clearance phase of several primary infections (Figure 1A, 1B, and 1D), suggesting immune escape or reinfection in some infants. To classify CMV shedding rebound, we fit a cubic model of time to the clearance phase. We observed rebound behavior in 5 primary infections, based on analysis of the cubic coefficients (Supplementary Methods and Supplementary Figure S3). There were 3 infants for whom the model predicted rebound shortly after the final sample, based on observed deceleration of clearance. Two additional infants showed signs of decelerating clearance. There was no apparent correlation between rebound time and infection duration, suggesting that rebound shedding is not a universal feature of primary CMV infection (Supplementary Figure S3).
Variability of Oral CMV Shedding Kinetics According to Infant Age and Maternal HIV Status
We found no significant association comparing maternal HIV status with peak magnitude, expansion phase duration, transition phase duration, and clearance phase duration (Supplementary Table S3). Because 2 infants had higher infection ages than the remaining infants (161 and 205 days, compared with <100 days), we performed correlation analyses with and without them. Including the 2 oldest infants, age was negatively correlated with the magnitude of the viral load peak (Spearman correlation, −0.58; 95% CI, −.85 to −.12). The 2 oldest children had the lowest and third-lowest peak magnitudes. We found no other significant correlations in comparisons of age at infection to episode features (Supplementary Table S4).
R0 for CMV-Infected Oral Cells
R0 is the average number of cells infected by a single cell in a fully susceptible population of cells. R0 values of >1 are required to sustain infection. We estimated R0 by fitting a mathematical model that assumed target cell limitation without a time-dependent immune response to the CMV expansion phase (Supplementary Methods). We identified a low R0 for oral CMV infection (median, 1.63; range, 1.09–3.1; IQR, 1.53–1.81), which is consistent with the slow rate of growth during expansion phase.
Target-Cell Limitation Does Not Explain the Dynamics of Oral Shedding During Primary CMV Infection
To understand the kinetics of primary oral infection, we fit several mathematical models to the entire episode for each infant. The first model attempted to capture viral clearance kinetics as a result of target-cell limitation (defined as persistent loss of susceptible cells that sustain viral replication) and excluded immune pressure. Using biologically informed parameter values from the literature (Supplementary Table S1), the model was unable to recapitulate the CMV clearance phase. By relaxing certain assumptions, we achieved better fit to the data with this model (Supplementary Methods and Supplementary Figure S4). However, model fit was contingent upon 2 assumptions that are inconsistent with known CMV virology and oral biology. The best-fit models required slow replenishment of susceptible cells over months or rapid death of CMV-infected cells over hours. Either assumption resulted in a drastic reduction in the total cell population (Supplementary Figure S5), which imply severe denudation of mucosal epithelial cells or destruction of salivary glands, phenomena not observed during CMV infection.
A Cytolytic Host Immune Response Explains the Dynamics of Oral Shedding During Primary CMV Infection
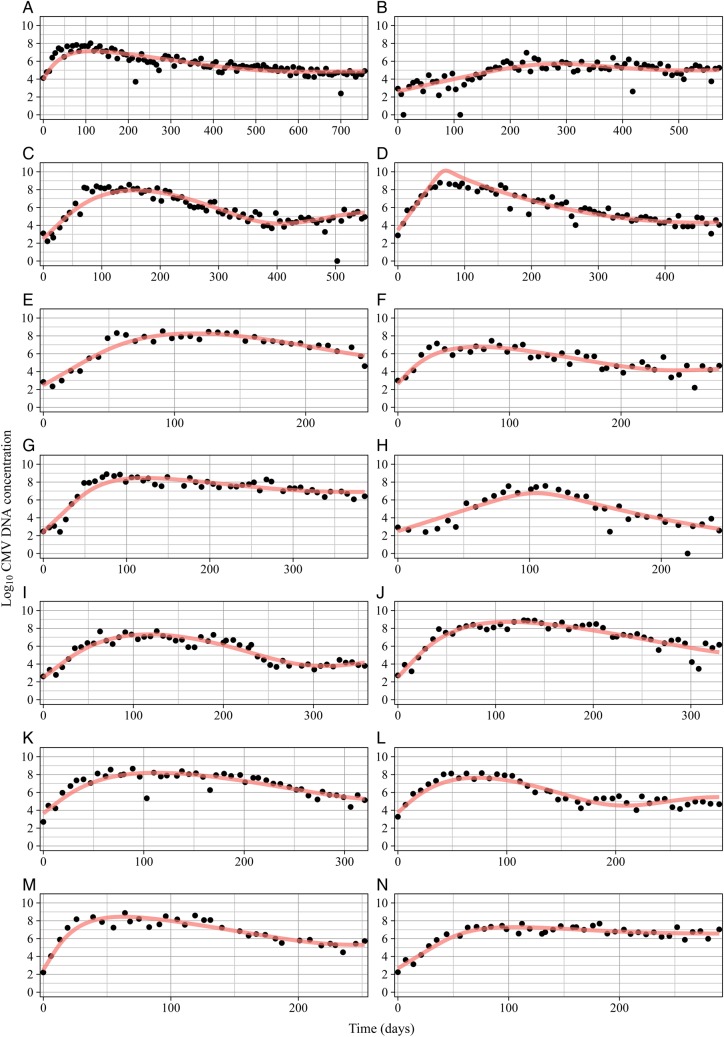
Because target-cell limitation alone failed to capture oral shedding dynamics, we added a cytolytic immune response component to the model (Supplementary Methods). We assumed that (1) immune effector growth depends on the number of CMV-infected cells, (2) the removal rate of infected cells is proportional to the amount of immune effectors, and (3) immune effectors have a limited lifespan. We fit the immune parameters to each episode, assuming fixed values of viral infectivity (β) and the initial infection time estimated with R0. This model demonstrated excellent fit to all 14 primary infections (Figure 3), capturing expansion, transition, and clearance phase kinetics. Unlike the target-cell limitation model, the immunologic model predicted target-cell population stability (13 infants experienced target-cell losses of <1%, and 1 infant had a maximum loss of 15%), which is consistent with the absence of clinically apparent oral pathology during infant CMV infection. This model also accurately simulated viral rebound when present and captured the dynamics of the infant with poorly defined growth and clearance phases (infant B).
Figure 3.
Mathematical modeling of the cytolytic immune pressure during primary cytomegalovirus (CMV) infection accurately predicts the dynamics of oral shedding. Each panel represents an individual infant; the subject identifier (ID) is shown at the top of each graph. Black dots indicate the actual quantity of CMV DNA measured in swabs at each week, and red curves indicate the results of the model. This figure is available in black and white in print and in color online.
Variable Timing and Intensity of the Immune Response
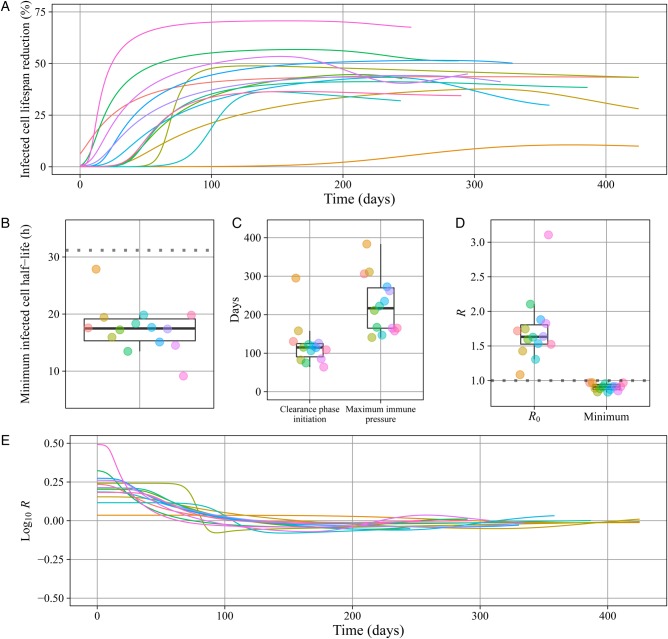
We determined the magnitude of the immune response by calculating the infected cell lifespan during the simulation; the peak immune response corresponds to the shortest infected cell lifespan. At peak response, the infected cell productive lifespan was reduced by a median of 44% (range, 10%–71%; IQR, 39%–52%; Figure 4A), from a 31-hour half-life prior to immune pressure to a median of 17.5 hours (range, 9–28 hours; IQR, 15–19 hours; Figure 4B). Peak immunity occurred a median of 217 days following infection (range, 141–384 days; IQR, 165–270 days; Figure 4C).
Figure 4.
The immune response against primary cytomegalovirus (CMV) infection is slow to develop and only partially effective in suppressing oral shedding. Boxes represent the interquartile range of the data; the whiskers extend to cover all data within 1.5 IQR of the first or third quartile. Individual infant data are represented on the line plots with lines and box plots by dots. A, The percentage reduction in the infected cell lifespan as compared to the baseline lifespan absent immunity, based on the fitted mathematical model for each of the 14 infants. B, The estimated half-life of infected cells at maximum immune pressure for each subject compared to the baseline half-life of 31 hours absent immunity (dotted line). C, Model estimates of clearance phase initiation (maximum viral load) and time of the peak immune response for each infant. D, Estimated maximum basic reproduction number (R0) and minimum effective reproduction number (R) for each infant. E, The estimated R for each infant over time, presented on the log scale to be symmetric around the infection threshold. The region below the dotted line indicates an R of <1, when the infection is clearing.
We characterized the effect of immunologic pressure on oral infection, using R (Supplementary Methods and Figure 4D and 4E). R is determined by the same parameters as R0 but is attenuated by target cell limitation and immune pressure. In the model, the maximum oral viral DNA concentration was reached when the immune response reduced R to 1, thus initiating clearance. The clearance initiation time was heterogeneous across infants, with a median of 115 days (range, 64–295 days; IQR, 91–125 days; Figure 4C).
The model predicted that the peak immune response occurred several months after initiation of the clearance phase (Figure 4C), implying sustained, escalating cytolytic pressure. However, the median minimum R was 0.9 (range, 0.83–0.97; IQR, 0.87–0.94; Figure 4D), barely below the threshold necessary for net elimination of infected cells. The model predicted a gradual reduction in immunologic control corresponding to deceleration of infected oral cell clearance (R increasing toward 1) and cases of rebound (R >1). Among the 5 infants with oral shedding rebound, the estimated immune effector lifespans were shorter than for the other 9 infants (median, 37 vs 66 days).
DISCUSSION
We present a detailed description of oral shedding during primary CMV infection in a cohort of healthy Ugandan infants [10]. While primary CMV infection in infants is notable for protracted shedding with high viral load [3, 5], we were able to identify 3 distinct phases of oral CMV shedding in this cohort of infants, using their known time of infection. The expansion phase occurs over several months and consists of unimpeded exponential growth. CMV expansion is slow as compared to that of other mucosal herpes viruses, such as herpes simplex virus [27] and Epstein-Barr virus [28], which reach peak viral DNA concentrations over days or even hours in young adults. After several months, CMV expansion transitions and decelerates. During the subsequent slow clearance phase, the frequency and duration of viral rebound, which we observed in several infants, are unpredictable. Persistent, high-viral-load oral shedding is not seen during chronic CMV infection [2, 6, 7], including in this cohort [10], which indicates that oral replication ultimately resolves after more than a year after infection and can periodically reactivate.
Our mathematical model identified that clearance of oral shedding results from slowly developing immune pressure. Despite protracted initial viral kinetics and low CMV infectivity, immune pressure takes months to develop and at peak intensity is barely potent enough to induce viral elimination. Oral CMV clearance is slow and inconsistent, marked by substantial week-to-week variability in viral load. The result is at least a year of high-level shedding, which may promote infection of household contacts, including infection or reinfection of the mother during a subsequent pregnancy [29, 30]. Although not observed in all subjects, a rebound in oral shedding appears commonly during CMV clearance [31]. The mechanism may relate to reinfection, increased viral fitness, and/or T-cell exhaustion [32, 33].
Given the low R0 of CMV, even a slight increase in immune pressure would be predicted to eliminate oral viral replication. However, the natural immune response only reduces R to slightly less than 1, resulting in sluggish oral CMV clearance even at the maximum pressure observed. The relative absence of immune control of oral shedding is in contrast to the lack of systemic disease during primary CMV infection in these and other healthy children. These findings are consistent with those for murine CMV models, in which oral shedding is prolonged as compared to systemic viral replication, owing to the compartmentalized and delayed development of an effective immune response in the salivary gland [20, 34]. They are also complemented by findings from studies demonstrating slow development of CMV-specific T-cell responses [35–37].
There are important limitations to our mathematical model. Our parameter values were selected from a limited set of published data regarding CMV replication kinetics in vitro (Supplementary Tables S1 and S2). The model fits well under our assumptions but, because of nonidentifiability across parameters, would also fit under other parameters. However, conclusions from alternative models imply biologically unrealistic outcomes. For instance, the best-fitting target-cell model predicts massive cell death inconsistent with the lack of clinical oral pathology in these children [10]. For our model, we assumed that target cells were oral mucosal and salivary gland epithelium, but CMV also infects endothelial and myeloid cells [12, 38]. The contribution of other cell types to the oral viral DNA concentration during primary infection in humans is unknown and cannot be determined by mathematical modeling without a better understanding of primary CMV biology in humans. Dynamic trends could not be modeled for viremia, owing to the infrequency of blood sampling. Because the cohort was made up of healthy Ugandan infants, the results may not necessarily be applicable to other populations. Multiple studies in other populations identified that oral CMV shedding in young children is persistent [3, 5, 39], but future studies would be needed to establish that the kinetics we identified are universal.
The immunologic component of the model represents an abstraction of complex interactions of immune effector populations where the resulting fitted parameters represent a generalized measure of immune pressure. Therefore, we focused on dynamic trends of the response rather than interpretations of specific parameter values. It is possible that the cell-mediated response against CMV limits viral replication rate or infectivity, rather than infected-cell lifespan. However, this would have equivalent effects on R over time. Studies aimed at simultaneously measuring immune response dynamics and oral viral shedding during primary CMV infection could elucidate more-detailed mathematical models of immunity.
Our detailed kinetic description of oral shedding during primary infant CMV infection highlights important features of CMV replication and the immune response. The duration of shedding is protracted over a year, despite remarkably inefficient viral spread. While immune pressure initiates oral CMV clearance, the response is remarkably delayed and weak. Our analysis highlights the importance of evaluating kinetics through weekly sampling and indicates that future work to measure the correlations between oral shedding, viremia, and effective immune responses would ideally use high-frequency, simultaneous samples. Last, our model suggests that a low threshold of immune pressure is required to interrupt oral CMV replication. Thus, only a slight increase in immune pressure is predicted to eliminate CMV replication in the oral cavity, providing optimism for the development of an effective CMV vaccine.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank all of the study participants, as well as members of the Uganda Cancer Institute, Jackson Orem, Stacy Selke, and Meei-Li Huang.
Financial support. This work was supported by the Roadmap KL2 Clinical Scholar Training Program (KL2 RR025015-01 to S. G.), the University of Washington Center for AIDS Research (new investigator award to S. G. via National Institutes of Health [NIH] grant P30 AI027757), the Child and Family Research Institute (salary award to S. G.), the NIH (grant P30 CA015704), the Canadian Institutes of Health Research (MOP-136825 to S. G.), and the National Institute of Allergy and Infectious Diseases (grant P01 AI030731 to A. W. and J. T. S.).
Potential conflicts of interest. A. W. reports personal fees from Aicuris, Amgen, GSK, UpToDate, and Admedus and research support from Genocea and Vical. C. C. reports grants, personal fees, and nonfinancial support from Janssen Pharmaceuticals and grants and nonfinancial support from GSK and TempTime. L. C. is on the scientific advisory board for and holds stock (<1% of the company) in Immune Design and is a coinventor listed on several patents involving potential HSV vaccine development. S. G. reports nonfinancial support from VBI Vaccines and personal fees from Omeros. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Crumpacker CS II, Zhang JL. Cytomegalovirus. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's principals and practice of infectious diseases. 7th ed Vol 2 Philadelphia, Pennsylvania: Churchill Livingstone, 2010:1971–87. [Google Scholar]
- 2.Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon MJ, Stowell JD, Clark R et al. . Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect Dis 2014; 14:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levinsohn EM, Foy HM, Kenny GE, Wentworth BB, Grayston JT. Isolation of cytomegalovirus from a cohort of 100 infants throughout the first year of life. Proc Soc Exp Biol Med 1969; 132:957–62. [DOI] [PubMed] [Google Scholar]
- 5.Murph JR, Bale JF Jr. The natural history of acquired cytomegalovirus infection among children in group day care. Am J Dis Child 1988; 142:843–6. [DOI] [PubMed] [Google Scholar]
- 6.Ho M. Epidemiology of cytomegalovirus in man. In: Ho M, ed. Cytomegalovirus: biology and infection. 2nd ed New York, New York: Springer US, 1991:155–87. [Google Scholar]
- 7.Stowell JD, Mask K, Amin M et al. . Cross-sectional study of cytomegalovirus shedding and immunological markers among seropositive children and their mothers. BMC Infect Dis 2014; 14:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pass RF, Hutto C, Ricks R, Cloud GA. Increased rate of cytomegalovirus infection among parents of children attending day-care centers. N Engl J Med 1986; 314:1414–8. [DOI] [PubMed] [Google Scholar]
- 9.Taber LH, Frank AL, Yow MD, Bagley A. Acquisition of cytomegaloviral infections in families with young children: a serological study. J Infect Dis 1985; 151:948–52. [DOI] [PubMed] [Google Scholar]
- 10.Gantt S, Orem J, Krantz EM et al. . Prospective characterization of the risk factors for transmission and symptoms of primary human herpesvirus infections among Ugandan infants. J Infect Dis 2016; 214:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston C, Orem J, Okuku F et al. . Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One 2009; 4:e4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeckh M, Huang M, Ferrenberg J et al. . Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol 2004; 42:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stafford MA, Corey L, Cao Y, Daar ES, Ho DD, Perelson AS. Modeling plasma virus concentration during primary HIV infection. J Theor Biol 2000; 203:285–301. [DOI] [PubMed] [Google Scholar]
- 14.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 1996; 271:1582–6. [DOI] [PubMed] [Google Scholar]
- 15.Emery VC, Hassan-Walker AF, Burroughs AK, Griffiths PD. Human cytomegalovirus (HCMV) replication dynamics in HCMV-naive and -experienced immunocompromised hosts. J Infect Dis 2002; 185:1723–8. [DOI] [PubMed] [Google Scholar]
- 16.Heider JA, Bresnahan WA, Shenk TE. Construction of a rationally designed human cytomegalovirus variant encoding a temperature-sensitive immediate-early 2 protein. Proc Natl Acad Sci U S A 2002; 99:3141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawes C. Estimates, from salivary analyses, of the turnover time of the oral mucosal epithelium in humans and the number of bacteria in an edentulous mouth. Arch Oral Biol 2003; 48:329–36. [DOI] [PubMed] [Google Scholar]
- 18.Emery VC, Cope AV, Bowen EF, Gor D, Griffiths PD. The dynamics of human cytomegalovirus replication in vivo. J Exp Med 1999; 190:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapiro M, Duca KA, Lee K et al. . A virtual look at Epstein-Barr virus infection: simulation mechanism. J Theor Biol 2008; 252:633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell AE, Cavanaugh VJ, Slater JS. The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence. Med Microbiol Immunol 2008; 197:205–13. [DOI] [PubMed] [Google Scholar]
- 21.Jonjic S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 1989; 169:1199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8(+) T cells, facilitating protection from local cytomegalovirus infection. Cell Rep 2015; 13:1125–36. [DOI] [PubMed] [Google Scholar]
- 23.Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol 2004; 65:456–64. [DOI] [PubMed] [Google Scholar]
- 24.Miles DJ, Sanneh M, Holder B et al. . Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology 2008; 124:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burg D, Rong L, Neumann AU, Dahari H. Mathematical modeling of viral kinetics under immune control during primary HIV-1 infection. J Theor Biol 2009; 259:751–9. [DOI] [PubMed] [Google Scholar]
- 26.Schiffer JT, Abu-Raddad L, Mark KE et al. . Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A 2010; 107:18973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffer JT, Wald A, Selke S, Corey L, Magaret A. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis 2011; 204:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duca KA, Shapiro M, Delgado-Eckert E et al. . A virtual look at Epstein-Barr virus infection: biological interpretations. PLoS Pathog 2007; 3:1388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26:86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto AY, Mussi-Pinhata MM, Boppana SB et al. . Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010; 202:297 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antoine P, Olislagers V, Huygens A et al. . Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J Immunol 2012; 189:2665–72. [DOI] [PubMed] [Google Scholar]
- 32.Bale JF Jr, Petheram SJ, Souza IE, Murph JR. Cytomegalovirus reinfection in young children. J Pediatr 1996; 128:347–52. [DOI] [PubMed] [Google Scholar]
- 33.Renzette N, Gibson L, Bhattacharjee B et al. . Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PLoS Genet 2013; 9:e1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphreys IR, de Trez C, Kinkade A, Benedict CA, Croft M, Ware CF. Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med 2007; 204:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lidehall AK, Engman ML, Sund F et al. . Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol 2013; 77:135–43. [DOI] [PubMed] [Google Scholar]
- 36.Miles DJ, Sande M, Kaye S et al. . CD4(+) T cell responses to cytomegalovirus in early life: a prospective birth cohort study. J Infect Dis 2008; 197:658–62. [DOI] [PubMed] [Google Scholar]
- 37.Tu W, Chen S, Sharp M et al. . Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol 2004; 172:3260–7. [DOI] [PubMed] [Google Scholar]
- 38.Ho M. Pathology of cytomegalovirus infection. In: Ho M, ed. Cytomegalovirus: biology and infection. 2nd ed New York, New York: Springer US, 1991:189–204. [Google Scholar]
- 39.Pass RF, Hutto SC, Reynolds DW, Polhill RB. Increased frequency of cytomegalovirus infection in children in group day care. Pediatrics 1984; 74:121–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.