Abstract
Pregnancy-induced alterations in immunity may contribute to the increased morbidity associated with influenza A virus infection during pregnancy. We characterized the immune response of monocytes and plasmacytoid dendritic cells (pDCs) to influenza A virus infection in 21 pregnant and 21 nonpregnant women. In pregnant women, monocytes and pDCs exhibit an exaggerated proinflammatory immune response to 2 strains of influenza A virus, compared with nonpregnant women, characterized by increased expression of major histocompatibility complex class II (approximately 2.0-fold), CD69 (approximately 2.2-fold), interferon γ–induced protein 10 (approximately 2.0-fold), and macrophage inflammatory protein 1β (approximately 1.5-fold). This enhanced innate inflammatory response during pregnancy could contribute to pulmonary inflammation following influenza A virus infection.
Keywords: influenza virus, pregnancy, innate immunity, monocytes, plasmacytoid dendritic cells
Pregnant women infected by influenza A virus experience significantly higher morbidity and mortality than influenza A virus–infected nonpregnant women [1]. The enhanced morbidity and mortality are particularly prominent during pandemics, yet they also occur with annual seasonal influenza [2]. The degree to which alterations in the immune system during pregnancy contribute to this increased morbidity and mortality remains unclear.
Some aspects of immunity are suppressed during pregnancy to allow maternal tolerance of fetal antigens. For example, natural killer (NK) and T cells from pregnant women were deficient in interferon γ (IFN-γ) and macrophage inflammatory protein 1β (MIP-1β) production following cytokine stimulation [3]. However, NK and T cells from pregnant women displayed enhanced IFN-γ and MIP-1β responses to influenza A virus, compared with those from nonpregnant women [4]. These discordant results suggest that cellular immune responses are not universally suppressed but instead depend on either the stimulation condition (cytokine vs influenza A virus) or the context of the stimulation. For instance, in the first study NK and T cells were isolated prior to stimulation, whereas in the latter, the NK and T cells were stimulated in the context of a whole peripheral blood mononuclear cell (PBMC) infection, allowing for cell-cell communication.
Monocytes and plasmacytoid dendritic cells (pDCs) produce cytokines and chemokines to activate and recruit NK and T cells and are pivotal for the clearance of influenza A virus [5, 6]. Influenza virus infection increases monocyte and pDC expression of activation markers such as CD69 and the major histocompatibility complex class II molecule HLA-DR, promoting CD4+ T-cell activation [7]. Monocytes and pDCs produce proinflammatory cytokines, such as interferon α (IFN-α), and chemokines, such as IFN-γ–inducible protein 10 (IP-10) and MIP-1β. IP-10 and MIP-1β recruit and activate immune effector cells such as neutrophils, CD8+ T cells, and NK cells, promoting influenza A virus clearance [8, 9]. In one study, PBMCs from pregnant women produced less IFN-α and IFN-λ that did PBMCs from nonpregnant women in response to influenza A virus [10]. Thus, it is possible that differing responses by monocytes and pDCs to influenza A virus infection could contribute to differences in NK and T-cell function.
Here, in light of the potential cross-talk between PBMCs, we evaluated whether pregnancy alters monocyte and pDC responses to A/California/7/2009 (pH1N1) and A/Victoria/361/2011 (H3N2) influenza A virus in humans. Taking advantage of the ability to profile multiple immune parameters in high dimension with mass cytometry, we evaluated the expression of activation markers and production of cytokines and chemokines by monocytes and pDCs in a cohort of 21 pregnant and 21 nonpregnant women.
METHODS
Participants and Study Design
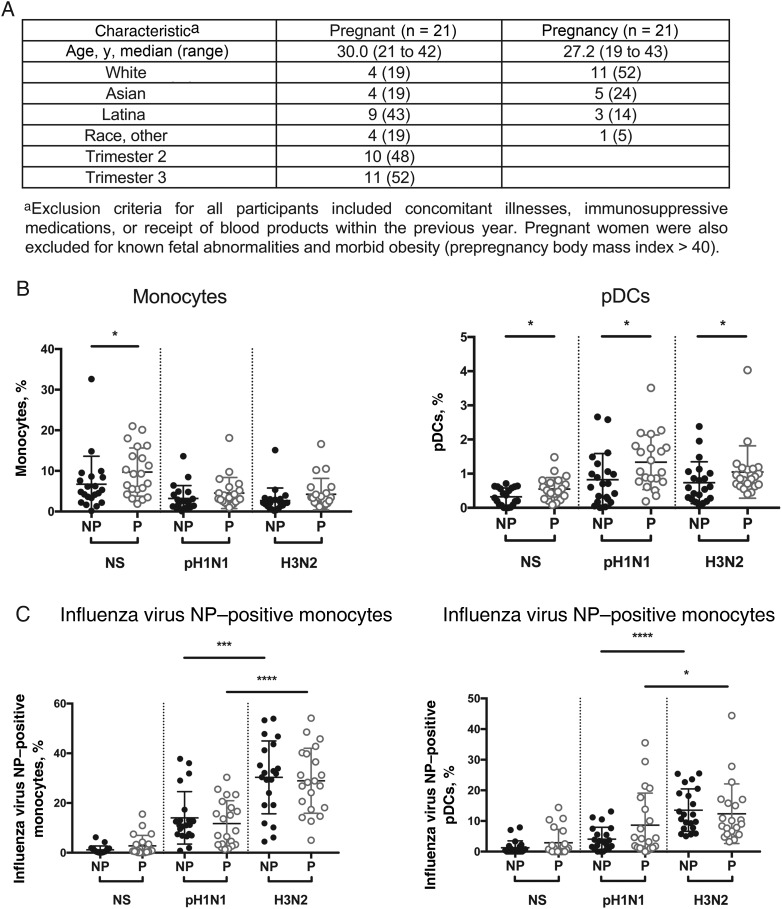
Twenty-one healthy pregnant women were recruited between October 2013 and March 2014 from the Obstetrics Clinic at Lucile Packard Children's Hospital. Twenty-one nonpregnant women were recruited at Stanford's Clinical and Translational Research Unit. Venous blood was collected. Participant criteria are listed in Figure 1A. This study was performed in accordance with the Declaration of Helsinki and approved by the Stanford University Institutional Review Board; written informed consent was obtained from all participants.
Figure 1.
Monocyte and plasmacytoid dendritic cell (pDC) frequency and rate of infection with A/California/7/2009 (pH1N1) and A/Victoria/361/2011 (H3N2) influenza A virus in pregnant (P) and nonpregnant (NP) women. Peripheral blood mononuclear cells (PBMCs) from women in the NP (black dots) and P (gray dots) groups were infected with pH1N1 or H3N2 influenza A virus at a multiplicity of infection of 3 for 7 hours or were mock infected (NS). A, Cohort demographic characteristics. B, Frequency of monocytes and pDCs. C, Frequency of influenza virus nucleoprotein (NP)–positive monocytes and pDCs, respectively, as detected by intracellular staining. Each dot represents the cell frequency from 1 patient. Bars represent the mean of each population. *P < .05, ***P < .001, and ****P < .0001 for differences between the P and NP groups.
PBMC Isolation, Virus Preparation, and Infection
PBMCs were isolated from whole blood by Ficoll-Paque (GE Healthcare) and cryopreserved in 90% fetal bovine serum (Thermo Scientific)/10% dimethyl sulfoxide (Sigma-Aldrich). pH1N1 and H3N2 wild-type influenza A viruses were propagated in embryonated chicken eggs. Cryopreserved PBMCs were thawed, washed, and resuspended in serum-free medium before infection with pH1N1 or H3N2 at a multiplicity of infection of 3 for 1 hour at 37°C, after which serum was added to reach 10% fetal bovine serum. After 2 hours, the cells were resuspended with 2 μM monensin and 3 μg/mL brefeldin A for 4 hours, yielding a total stimulation duration of 7 hours [4].
Cell Staining and CyTOF Acquisition
Detailed staining protocols have been described elsewhere [11]. Staining panels are described in Supplementary Table 1. All antibodies were conjugated using MaxPar X8 labeling kits (DVS Sciences). Stained cells were analyzed on a CyTOF-1 (Fluidigm) and analyzed using FloJo v10 (Treestar).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism, version 7.0a (GraphPad Software). Pregnant and control participant characteristics were compared using Mann–Whitney tests. A P value of < .05 was considered statistically significant.
RESULTS
Frequency and Influenza A Virus Infection Levels in Monocytes and pDCs
We quantified the frequencies of CD14+ monocytes and CD4+CD123+ pDCs within thawed PBMCs obtained from 21 pregnant and 21 nonpregnant women (see Supplementary Figure 1 for gating scheme). In mock-infected (unstimulated) samples, pregnant women have a 1.4-fold higher frequency of monocytes (P = .04) and a 1.7-fold higher frequency of pDCs (P = .03), compared with controls (Figure 1B and Supplementary Tables 2 and 3), in accordance with prior studies [12]. Following infection with pH1N1 and H3N2 influenza A virus, there was no significant difference in monocyte frequency. However, the frequency of pDCs remained significantly higher in pregnant women after pH1N1 (2.1-fold higher; P = .02) or H3N2 (1.4-fold higher; P = .04) infection (Figure 1B and Supplementary Tables 2 and 3). There were no significant differences in the infection rate of monocytes or pDCs from pregnant and nonpregnant women infected by pH1N1 or H3N2 influenza A virus, based on staining for intracellular influenza A virus nucleoprotein (Figure 1C).
Effects of Influenza A Virus on Monocyte and pDC Activation During Pregnancy
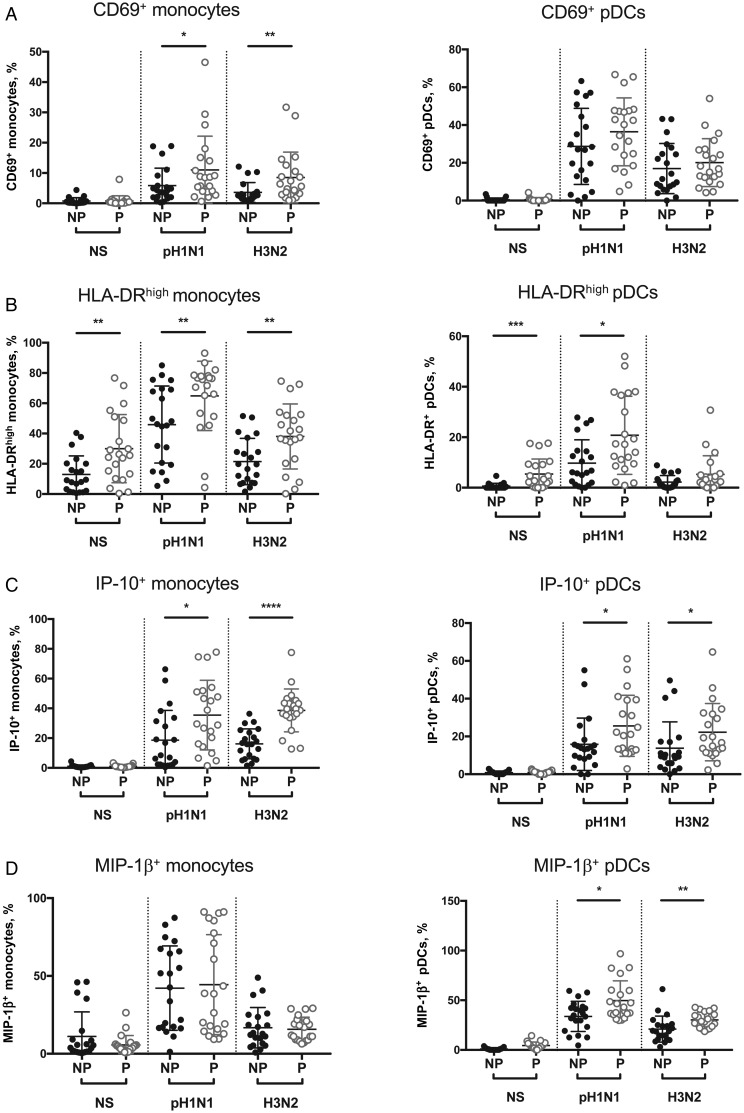
To evaluate whether the immune response of monocytes and pDCs to influenza A virus is altered in pregnancy, we measured the expression of the activation markers HLA-DR and CD69. Infection with pH1N1 and H3N2 influenza A virus significantly increased CD69 expression in monocytes and pDCs in both pregnant and control women (Figure 2A, Supplementary Figure 4, and Supplementary Tables 3 and 4). However, monocyte activation was significantly more dramatic in pregnancy. CD69 expression was 1.9-fold higher in H1N1-infected monocytes (P = .05) and 2.4-fold higher in H3N2-infected monocytes (P = .005) from pregnant women, compared with those from controls (Figure 2A). Similarly, HLA-DR expression on infected monocytes was 1.4-fold (for pH1N1; P = .01) and 1.8-fold (for H3N2; P = .01) higher in pregnant women (Figure 2B and Supplementary Figure 4B). In pDCs, there were no significant differences in CD69 upregulation between pregnant and control women (Figure 2A and Supplementary Figure 4A). However, HLA-DR expression was 8.9-fold higher at baseline (P = .0001) and 2.1-fold higher following H1N1 infection (P = .01) in pregnant women (Figure 2A and 2B and Supplementary Figure 4B). There were no differences in the frequency of HLA-DR– and CD69-expressing monocytes or pDCs in the second and third trimesters of pregnancy (Supplementary Figure 3).
Figure 2.
Monocyte and plasmacytoid dendritic cell (pDC) immune responses to A/California/7/2009 (pH1N1) and A/Victoria/361/2011 (H3N2) influenza A virus infection in pregnant (P) and nonpregnant (NP) women. Peripheral blood mononuclear cells (PBMCs) from women in the NP (black dots) and P (gray dots) groups were infected with pH1N1 or H3N2 influenza A virus at a multiplicity of infection of 3 for 7 hours or were mock infected (NS). A, Frequency of CD69-expressing monocytes (left) and pDCs (right). B, Frequency of HLA-DR expression on monocytes and pDCs. C and D, Frequency of monocytes and pDCs producing interferon γ–induced protein 10 (IP-10) and macrophage inflammatory protein 1β (MIP-1β), respectively. Each dots represents the cell frequency for 1 patient. *P < .05, **P < .01, ***P < .001, and ****P < .0001 for differences between the P and NP groups.
Effects of Influenza A Virus on Monocyte and pDC Function During Pregnancy
To assess the functional responses of monocytes and pDCs to influenza A virus in pregnant and nonpregnant women, we assessed intracellular levels of the proinflammatory cytokine IFN-α and the chemokines MIP-1β and IP-10. In both pregnant and nonpregnant women, influenza A virus infection led to a significantly higher frequency of monocytes and pDCs producing IFN-α, IP-10, and MIP-1β (Figure 2C and 2D, Supplementary Figures 2 and 4, and Supplementary Tables 3 and 4). There were no significant differences in the frequency of IFN-α–producing monocytes and pDCs between pregnant and nonpregnant women (Supplementary Figure 3), although there was a significant increase in the IFN-α median signal intensity (MSI) among pregnant women in response to influenza A virus (Supplementary Figure 4). Following pH1N1 and H3N2 infection, pregnant women had a significantly higher frequency of monocytes and pDCs producing IP-10 than did nonpregnant women, with a 2.0-fold increased frequency of IP-10–producing monocytes and 1.6-fold increased frequency of IP-10–producing pDCs (Figure 2C). In addition, pregnant women had a 1.5-fold increase in the percentage of pDCs producing MIP-1β after infection with both pH1N1 and H3N2 influenza A virus (Figure 2D). Thus, the monocyte and pDC responses to pH1N1 and H3N2 influenza A virus are enhanced during pregnancy.
DISCUSSION
During pregnancy, the immune system is faced with the challenge of avoiding fetal rejection while protecting against pathogens. This challenge may contribute to the unusual susceptibility of pregnant women to complications from influenza A virus infection. Monocytes and pDCs are pivotal innate immune cells in the defense against influenza A virus infection. Here, we evaluated the immune phenotype and function of monocytes and pDCs from pregnant women upon pH1N1 and H3N2 influenza virus infection of PBMCs. Monocytes and pDCs cells exhibit enhanced expression of HLA-DR and CD69 activation markers, exaggerated IP-10 production, and increased MIP-1β production by pDCs in pregnant women. These data support the idea that monocytes and pDCs exhibit an enhanced activation and proinflammatory phenotype upon influenza A virus infection during pregnancy. This excessive activation could distort the immune response away from a healthy response to a damaging if not fatal reaction.
These observations are in line with the critical role that the activation status of monocytes and pDCs plays in the immune response to influenza A virus [5, 6]. The increased frequency and activation status of monocytes and pDCs in response to influenza A virus infection may increase priming and activation of other immune cells, particularly CD4+ T cells recognizing HLA-DR. This could explain the enhanced CD4+ T-cell response of pregnant women to influenza virus infection of mixed PBMC cultures that we reported previously [4].
Even more dramatic than the elevated activation status was the elevation in IP-10 and MIP-1β production noted in monocytes and pDCs from pregnant women in response to influenza A virus infection. Chemokines such as IP-10 and MIP-1β play a major role in the recruitment of effector cells such as neutrophils, NK cells, and T cells to promote the clearance of influenza virus. However, several lines of evidence support the idea that an increased abundance of chemokines during influenza virus infection is linked to elevated levels of morbidity and mortality [13]. For example, systemic IP-10 levels were significantly elevated in serum of patients with acute respiratory distress syndrome (ARDS) induced by the seasonal 2009 influenza A(H1N1) virus, compared with patients without complications [14]. Also, in a murine model, the IP-10 and CXCR3 axis enhances the development of ARDS via the recruitment of neutrophils after pH1N1 influenza virus infection [15]. Our results and these previous discoveries suggest, that in pregnancy, influenza A virus infection leads to acute and exaggerated production of IP-10 and MIP-1β by monocytes and pDCs that could contribute to ARDS and other respiratory complications, increasing the risk of influenza-associated mortality among pregnant women.
Monocytes and pDCs are the most potent IFN-α producers within human PBMCs. Forbes et al reported that IFN-α production is impaired within PBMCs in pregnant women as compared to controls upon influenza virus infection [10]. Here, we did not find a difference in the frequency of IFN-α–producing monocytes and pDCs between pregnant women and controls. The reasons for this discrepancy are not clear, but it is important to note that there are several differences in the study design that could explain the results. First, after performing time course analysis to identify the time of peak innate immune cell activity, we used mass cytometry to assess the frequency of cytokine-producing cells 7 hours after infection. The prior studies examined cytokine concentration after 48 hours of culture, a time when we observed significant levels of cell death within monocytes and pDCs in infected cultures. Thus, it is possible that monocytes and pDCs in pregnant women produce smaller cytokine levels per cell or that differences in the response timing influenced results. Further investigations are needed to elucidate specific cell type(s) and mechanisms defective in IFN-α production from total PBMCs during pregnancy during influenza A virus infection. In addition, all of the studies involved relatively small cohorts of subjects, with varying prior immunization status and differences in race distribution, which could further influence results.
An effective antiviral response is driven by the interaction between immune cells as a language to achieve an appropriate and healthy immune response. Of note, several prior studies of influenza immunity during pregnancy, particularly those focused on NK and T cells, studied these cells in isolation and found their responses to be suppressed [3]. Such studies excluded cross-talk with other immune cells. Our finding that monocytes and pDCs have elevated chemokine responses to influenza A virus during pregnancy may explain our prior finding, using mixed infections, that NK-cell and T-cell responses were enhanced, as the lymphocytes could have been activated by the monocytes and pDCs [4]. The extent to which innate and adaptive immune cells interact during influenza virus infection may provide further insight into influenza pathogenesis.
In summary, we report here that monocytes and pDCs in pregnancy display a pronounced proinflammatory phenotype during influenza A virus infection in vitro. These data, together with our previous work highlighting the enhanced T-cell and NK-cell responsiveness to pH1N1 influenza A virus infection, provide further evidence supporting an enhanced and disproportionate inflammatory response to influenza A virus during pregnancy. This enhanced response could contribute to elevated morbidity and mortality during pregnancy and could present opportunities for therapeutic intervention.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the pregnant and control study volunteers, for their participation; Kanta Subbarao, MBBS, MPH (National Institutes of Health), for providing the influenza virus; Joanne Lau, for her outstanding assistance with pregnant patient enrollment; and Sally Mackey (regulatory and data manager), Sue Swope, RN (clinic manager), Tony Trela and Nancy Mastman (research nurses), Ashima Goel, Raquel Fleischmann, Kyrsten Spann, and Sushil Batra (clinical research assistants, who provided regulatory support, scheduling, and data entry), and Michele Ugur (phlebotomist; all of the Stanford–LPCH Vaccine Program), for enrolling the control volunteers.
Financial support. This work was supported by the Doris Duke Charitable Foundation (2013 Clinical Scientist Development Award 2013099 to C. A. B.), the Stanford Child Heath Research Institute (Talia and John Morgridge Faculty Scholar award to C. A. B.), the McCormick Faculty Award (to C. A. B.), the Stanford Human Immunology Project Consortium (Infrastructure and Opportunity Fund to C. A. B. and grants U19AI090019 and U19A1057229 to M. M. D.), the Stanford Child Health Research Institute (Stanford clinical and translational science award UL1 TR000093 to A. W. K.), the National Institutes of Health (NIH; training grant [viral infections in children] T32 AI78896-05 to A. W. K.), a Smith Family Stanford Graduate Fellowship (to N. L. B.), and the NIH/National Center for Research Resources (clinical and translational science award UL1 RR025744).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med 2014; 371:1075–7. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis 2008; 14:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraus TA, Engel SM, Sperling RS et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol 2012; 32:300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kay AW, Fukuyama J, Aziz N et al. Enhanced natural killer-cell and T-cell responses to influenza A virus during pregnancy. Proc Natl Acad Sci U S A 2014; 111:14506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen IC, Scull MA, Moore CB et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009; 30:556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol 2010; 11:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GeurtsvanKessel CH, Willart MAM, van Rijt LS et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med 2008; 205:1621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussfeld D, Kaufmann A, Meyer RG, Gemsa D, Sprenger H. Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactivated influenza A virus. Cell Immunol 1998; 186:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 2002; 168:3195–204. [DOI] [PubMed] [Google Scholar]
- 10.Forbes RL, Wark PAB, Murphy VE, Gibson PG. Pregnant women have attenuated innate interferon responses to 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2012; 206:646–53. [DOI] [PubMed] [Google Scholar]
- 11.Strauss-Albee DM, Fukuyama J, Liang EC et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med 2015; 7:297ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraus TA, Sperling RS, Engel SM et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol 2010; 64:411–26. [DOI] [PubMed] [Google Scholar]
- 13.de Jong MD, Simmons CP, Thanh TT et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To KKW, Hung IFN, Li IWS et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa A, Kuba K, Morita M et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am J Respir Crit Care Med 2013; 187:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.