Introduction
Immune checkpoint inhibitors are a new class of cancer therapeutics that promote antitumor immune responses. Currently US Food and Drug Administration–approved agents target the coregulatory molecules programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) and show significant activity in multiple cancer types. These medications can induce a variety of cutaneous eruptions, the full spectrum of which is not yet completely characterized. Here, we report a case of bullous pemphigoid (BP), which began shortly after initiating treatment with the PD-1 inhibitor nivolumab.
Case report
A 77-year-old woman with a history of inverse psoriasis presented with a pruritic rash for 3 weeks and a bullous eruption for 1 day. Six weeks before presentation, the patient started treatment with nivolumab (3 mg/kg every 2 weeks) for a T1N2M0 lung adenocarcinoma. She had no prior treatment for the cancer.
Her medical history included diabetes, hypertension, chronic obstructive pulmonary disease, hypothyroidism, and depression. Long-standing medications included morphine, desvenlafaxine, allopurinol, furosemide, clonazepam, pantoprazole, rosuvastatin, aspirin, levothyroxine, and metoclopramide. Her psoriasis was treated previously with topical corticosteroids only. She recently completed a short course of oral prednisone for a chronic obstructive pulmonary disease exacerbation.
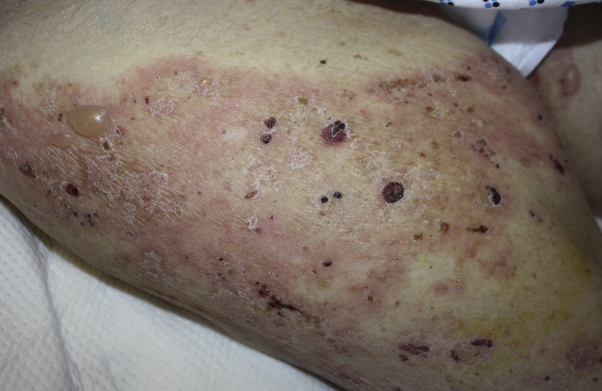
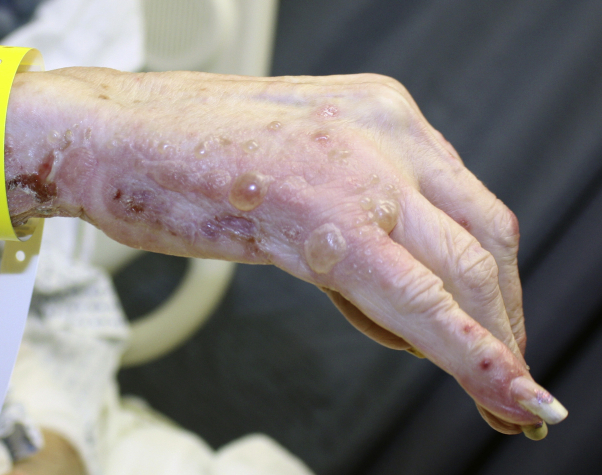
Physical examination found numerous 1- to 20-cm pink plaques studded with dozens of 0.5- to 3.0-cm tense vesicles, bullae, and round erosions (Figs 1 and 2). Lesions were most prominent on the hands, arms, thighs, buttocks, and lower back. Mucous membranes were unaffected. BP disease activity index, a validated BP severity scoring system, was 66.
Fig 1.
Bullous pemphigoid. Representative clinical image from the right thigh.
Fig 2.
Bullous pemphigoid. Representative clinical image from the right hand.
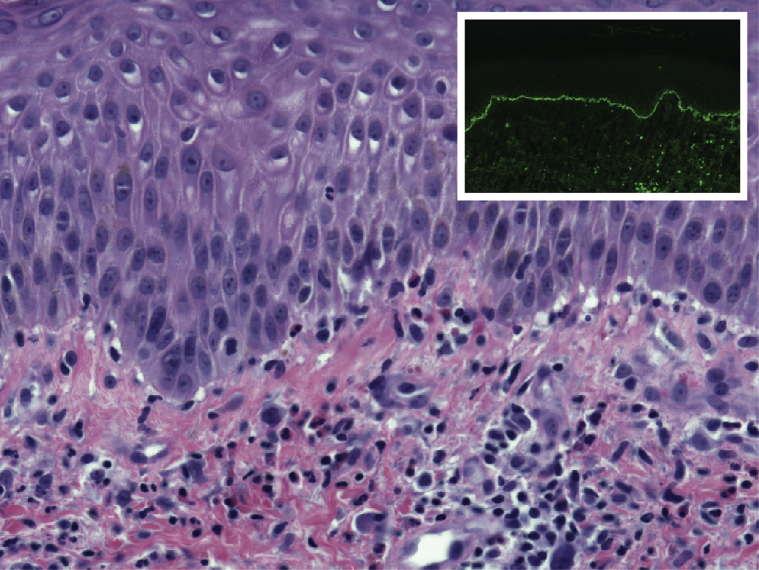
Serum BP180 antibody levels were elevated at 137 U/mL. BP230 antibodies were not detected. Total IgE level was elevated at 210 kU/L. Complete blood count showed an increased absolute eosinophil count of 750/μL. Chart review found a variable but comparably elevated eosinophil count over the prior 3 years. Routine histopathology showed eosinophilic spongiosis and a mixed dermal inflammatory infiltrate with eosinophils. Direct immunofluorescence showed linear IgG and C3 at the dermoepidermal junction (Fig 3). A diagnosis of BP was established.
Fig 3.
Bullous pemphigoid. Hematoxylin-eosin–stained section with paired immunofluorescence for complement C3 (inset).
The patient was initially treated with oral prednisone (1 mg/kg) and clobetasol 0.05% ointment. She improved within 1 week, and the prednisone was rapidly tapered. At this early stage of nivolumab treatment, prednisone and conventional immunosuppressive medications were relatively contraindicated, as they could interfere with the intended antitumor activity of nivolumab. Omalizumab, which was successfully used to treat BP in a small case series,1 was selected as a steroid-sparing agent given her elevated IgE level. The omalizumab was dosed using the asthma nomogram.1 Treatment with omalizumab allowed for complete discontinuation of oral prednisone while maintaining disease control. The nivolumab therapy was restarted.
Discussion
US Food and Drug Administration–approved anti–PD-1 therapies include nivolumab and pembrolizumab. Both medications are reported to induce several cutaneous immune-related adverse events (IRAE), the most common of which are eczematous and lichenoid eruptions, pruritus, and, in melanoma patients, vitiligo.2 At the time of submission, development of BP had been reported in 6 other patients treated with anti–PD-1 or programmed death ligand 1 (PD-L1) therapy.2, 3, 4
B cells secrete pathogenic antibodies and thus play a central role in BP pathogenesis. The effects of PD-1 inhibition are commonly attributed to altered T cell function that results from disruption of the interaction of PD-1 with its ligands (PD-L1 and PD-L2). In the case of nivolumab-associated BP, altered B-cell function likely plays a role as well. B cells also express PD-1 and PD-1 ligands,5 and PD-1 inhibition can directly activate B cells in a T-cell–independent fashion.5 Additionally, immune suppressive B cells, B regulatory cells (Bregs), suppress a variety of autoimmune conditions including the related immunobullous disorder pemphigus vulgaris.6, 7 Bregs express high levels of PD-L1 and can suppress humoral immune responses in a PD-1–dependent fashion.7 Bregs inhibit T-follicular helper cells and activate immunosuppressive T regulatory cells,7 T-cell populations that seem to play a role in BP pathogenesis.8, 9
BP is most common in elderly patients and can be accompanied by peripheral eosinophilia. Based on this patient's age and preexisting peripheral eosinophilia, one could hypothesize that an incipient or forme fruste BP was unmasked by PD-1 blockade. In other reported cases of nivolumab-induced BP, patients also tended to be elderly, and in some patients BP persisted despite discontinuation of anti–PD-1 therapy (Table I). These considerations suggest that some of these patients may have been predisposed to BP formation. An alternative hypothesis is that BP formation is completely de novo in a subset or all patients.
Table I.
Summary of BP cases in the setting of anti–PD-1 and PD-L1 therapy
| Age | Diagnosis | Agent | Prior treatment | Latency | Treatment | Notes | Study |
|---|---|---|---|---|---|---|---|
| 77 | Lung AC | Nivolumab | None | 3 wk | Prednisone taper, clobetasol | Rapid improvement | Current report |
| 75 | Melanoma | Pembrolizumab | Dacarbazine, Ipilimumab | 9 wk | Prednisone taper | Rapid improvement | Hwang et al,3 2016 |
| 68 | Melanoma | Nivolumab | None | 20 mo | Clobetasol | Resolution posttherapy | Hwang et al,3 2016 |
| 72 | Melanoma | Pembrolizumab | None | 7 mo | Prednisone and methotrexate | Good response to therapy | Hwang et al,3 2016 |
| 80 | Melanoma | Nivolumab | Ipilimumab | 24 wk | Topical tacrolimus, oral nicotinamide | No activity after stopping therapy | Naidoo et al,4 2016 |
| 78 | Melanoma | Durvalumab | Ipilimumab | 17.9 wk | Topical corticosteroids | Active despite stopping therapy | Naidoo et al,4 2016 |
| 85 | Lung SCC | Nivolumab | Carboplatin + gemcitabine | 6.1 wk | Prednisone, topical corticosteroids | Active despite stopping therapy | Naidoo et al,4 2016 |
We cannot completely rule out that BP development in this patient was either paraneoplastic, related to another medication, or idiopathic. However, the timing of cancer diagnosis (9 months prior) compared with nivolumab initiation (6 weeks prior) argues against this. Also, her other medications were generally tolerated for years without similar cutaneous symptoms. Further, classic BP serologies were present in this patient and are also generally present in other previously reported cases.4 Many other patients previously received different therapies (including other immune therapies, such as anti–CTLA-4) and did not have BP. The latency for BP development after starting anti–PD-1 therapy in these cases ranged from 3 weeks to 20 months (Table I).
Development of cutaneous IRAE (such as vitiligo in melanoma) may be associated with improved treatment responses to immune checkpoint inhibitors.2, 10 Oncologists are often hesitant to use systemic immune suppressive therapies to treat cutaneous IRAE for fear of suppressing antitumor immunity. In this case, omalizumab was selected as a maintenance therapy based on the theoretical consideration that it should be less inhibitory toward antitumor immune responses than other systemic agents commonly used to treat BP, including prednisone. Previous work has found the potential efficacy of omalizumab in BP, especially in patients with elevated IgE levels.1
Moving forward, it will be important to document cases of BP arising in patients treated with immune checkpoint inhibitors and to develop treatment strategies that do not interfere with antitumor responses. It will also be important to identify any potential relationship of BP development during therapy with anticancer responses.
Acknowledgments
The authors thank Drs C. Ko, M. Bosenberg, A.Galan, and N. Rodic from Yale Dermatopathology.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Yu K.K., Crew A.B., Messingham K.A., Fairley J.A., Woodley D.T. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014;71:468–474. doi: 10.1016/j.jaad.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang S.J., Carlos G., Wakade D. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74:455–461.e1. doi: 10.1016/j.jaad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Hwang S.J., Carlos G., Chou S., Wakade D., Carlino M.S., Fernandez-Penas P. Bullous pemphigoid, an autoantibody-mediated disease, is a novel immune-related adverse event in patients treated with anti-programmed cell death 1 antibodies. Melanoma Res. 2016;26:413–416. doi: 10.1097/CMR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 4.Naidoo J., Schindler K., Querfeld C. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4:383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y., Jeffrey Medeiros L., Young K.H. Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim Biophys Acta. 2016;1865:58–71. doi: 10.1016/j.bbcan.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colliou N., Picard D., Caillot F. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 7.Mauri C., Menon M. The expanding family of regulatory B cells. Int Immunol. 2015;27:479–486. doi: 10.1093/intimm/dxv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q., Liu Z., Dang E. Follicular helper T Cells (Tfh) and IL-21 involvement in the pathogenesis of bullous pemphigoid. PLoS One. 2013;8:e68145. doi: 10.1371/journal.pone.0068145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antiga E., Quaglino P., Volpi W. Regulatory T cells in skin lesions and blood of patients with bullous pemphigoid. J Eur Acad Dermatol Venereol. 2014;28:222–230. doi: 10.1111/jdv.12091. [DOI] [PubMed] [Google Scholar]
- 10.Voskens C.J., Goldinger S.M., Loquai C. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]